








生物技术通报 ›› 2021, Vol. 37 ›› Issue (5): 212-220.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0828
龚晓惠1( ), 杨敏1, 李舒婷1, 林晟豪1, 许文涛1,2(
), 杨敏1, 李舒婷1, 林晟豪1, 许文涛1,2( )
)
收稿日期:2020-07-05
出版日期:2021-05-26
发布日期:2021-06-11
作者简介:龚晓惠,女,研究方向:功能核酸生物传感器;E-mail: 基金资助:
GONG Xiao-hui1( ), YANG Min1, LI Shu-ting1, LIN Sheng-hao1, XU Wen-tao1,2(
), YANG Min1, LI Shu-ting1, LIN Sheng-hao1, XU Wen-tao1,2( )
)
Received:2020-07-05
Published:2021-05-26
Online:2021-06-11
摘要:
银纳米簇是一类粒径介于银原子和银纳米颗粒之间,由几个到几十个银原子组成,核心尺寸小于2 nm的银纳米材料。近年来银纳米簇以其固有的广谱抑菌性、不致耐药性、良好的生物相容性、低剂量有效性等优势在抗菌应用领域受到了较高的关注。综述了银纳米簇的抗菌机理及抗菌活性的影响因素,探讨其细胞毒性,并对目前已有的抗菌应用进行总结,展望了银纳米簇未来的应用方向及要突破的问题,着重突出DNA银纳米簇的广泛应用前景。
龚晓惠, 杨敏, 李舒婷, 林晟豪, 许文涛. 银纳米簇抗菌机理、活性及其应用的研究进展[J]. 生物技术通报, 2021, 37(5): 212-220.
GONG Xiao-hui, YANG Min, LI Shu-ting, LIN Sheng-hao, XU Wen-tao. Progress on Antibacterial Mechanism,Activity and Application of Silver Nanoclusters[J]. Biotechnology Bulletin, 2021, 37(5): 212-220.
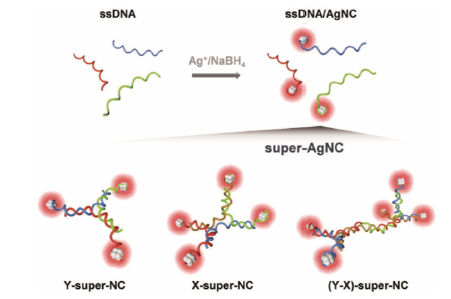
图4 Y-super-NC、X-super-NC和(Y-X)-super-NC合成过程及结构示意图[26]
Fig. 4 Schematic diagram of synthetic process and struc-ture of Y-super-NC, X-super-NC, and (Y-X)-super-NC
| [1] |
Zheng J, Nicovich PR, Dickson RM . Highly fluorescent noble-metal quantum dots[J]. Annual Review of Physical Chemistry, 2007,58(1):409-431.
doi: 10.1146/annurev.physchem.58.032806.104546 URL |
| [2] |
Zheng KY, Setyawati MI, Leong DT, et al. Antimicrobial silver nanomaterials[J]. Coordination Chemistry Reviews, 2018,357:1-17.
doi: 10.1016/j.ccr.2017.11.019 URL |
| [3] |
Xu H, Suslick KS. Water-soluble fluorescent silver nanoclusters[J]. Advanced Materials, 2010,22(10):1078-1082.
doi: 10.1002/adma.200904199 URL |
| [4] | Díez I, Ras RHA. Few-atom silver clusters as fluorescent reporters[M]. Berlin, Heidelberg:Springer, 2010. |
| [5] |
Jin R. Quantum sized, thiolate-protected gold nanoclusters[J]. Nanoscale, 2010,2(3):343-362.
doi: 10.1039/B9NR00160C URL |
| [6] |
Luo Z, Zheng K, Xie J. Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications[J]. Chemical Communications, 2014,50(40):5143-5155.
doi: 10.1039/C3CC47512C URL |
| [7] | 李艳乐. 基于DNA-银纳米簇的荧光生物传感技术及纳米银抗菌性能研究[D]. 长沙:湖南大学, 2016. |
| Li YL. Fluorescent biosensor based on DNA-templated silver nanoclusters and antibacterial properties of silver nanoclusters[D]. Changsha:Hunan University, 2016. | |
| [8] |
Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent:a case study on E. coli as a model for Gram-negative bacteria[J]. Journal of Colloid and Interface Science, 2004,275(1):177-182.
doi: 10.1016/j.jcis.2004.02.012 URL |
| [9] |
Li WR, Xie XB, Shi QS, et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli[J]. Applied Microbiology and Biotechnology, 2010,85(4):1115-1122.
doi: 10.1007/s00253-009-2159-5 URL |
| [10] |
Hwang ET, Lee JH, Chae YJ, et al. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria[J]. Small, 2008,4(6):746-750.
doi: 10.1002/smll.200700954 pmid: 18528852 |
| [11] |
Gogoi SK, Gopinath P, Paul A, et al. Green fluorescent protein-expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles[J]. Langmuir, 2006,22(22):9322-9328.
doi: 10.1021/la060661v URL |
| [12] |
Kaviya S, Santhanalakshmi J, Viswanathan B, et al. Biosynjournal of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2011,79(3):594-598.
doi: 10.1016/j.saa.2011.03.040 URL |
| [13] | Dakal TC, Kumar A, Majumdar RS, et al. Mechanistic basis of antimicrobial actions of silver nanoparticles[J]. Frontiers in Microbiology, 2016,7:1831. |
| [14] |
Li X, Fu T, Li B, et al. Riboflavin-protected ultrasmall silver nanoclusters with enhanced antibacterial activity and the mechanisms[J]. RSC Advances, 2019,9(23):13275-13282.
doi: 10.1039/C9RA02079A URL |
| [15] |
Jin JC, Wu XJ, Xu J, et al. Ultrasmall silver nanoclusters:Highly efficient antibacterial activity and their mechanisms[J]. Biomaterials Science, 2017,5(2):247-257.
doi: 10.1039/C6BM00717A URL |
| [16] |
Wang X, Gao W, Xu W, et al. Fluorescent Ag nanoclusters templated by carboxymethyl-β-cyclodextrin(CM-β-CD)and their in vitro antimicrobial activity[J]. Materials Science and Engineering C, 2013,33(2):656-662.
doi: 10.1016/j.msec.2012.10.012 URL |
| [17] |
Xu D, Wang Q, Yang T, et al. Polyethyleneimine capped silver nanoclusters as efficient antibacterial agents[J]. International Journal of Environmental Research And Public Health, 2016,13(3):334.
doi: 10.3390/ijerph13030334 URL |
| [18] | 刘亚, 程博闻, 韦媛辉. 纳米银PP抗菌纺粘布的开发[J]. 纺织学报, 2006,27(2):78-80. |
| Liu Y, Cheng BW, Wei YH. Development of anti-bacterial spun-bonded polypropylene(PP)fabric treated with nanometer silver anti-bacterial agent[J]. Journal of Textile Research, 2006,27(2):78-80. | |
| [19] |
Yuan X, Setyawati MI, Leong DT, et al. Ultrasmall Ag+-rich nanoclusters as highly efficient nanoreservoirs for bacterial killing[J]. Nano Research, 2014,7(3):301-307.
doi: 10.1007/s12274-013-0395-6 URL |
| [20] |
Farrag M, Mohamed RA. Ecotoxicity of~ 1 nm silver and palladium nanoclusters protected by l-glutathione on the microbial growth under light and dark conditions[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2016,330:117-125.
doi: 10.1016/j.jphotochem.2016.07.027 URL |
| [21] |
Fayaz AM, Balaji K, Girilal M, et al. Biogenic synjournal of silver nanoparticles and their synergistic effect with antibiotics:a study against gram-positive and gram-negative bacteria[J]. Bulletin of the Chemical Society of Japan, 2010,6(1):103-109.
doi: 10.1246/bcsj.6.103 URL |
| [22] | Kim KJ, Sung WS, Moon SK, et al. Antifungal effect of silver nanoparticles on dermatophytes[J]. J Microbiol Biotechnol, 2008,18(8):1482-1484. |
| [23] |
Díez I, Ras RHA. Fluorescent silver nanoclusters[J]. Nanoscale, 2011,3(5):1963-19700.
doi: 10.1039/c1nr00006c URL |
| [24] | Javani S, Lorca R, Latorre A, et al. Antibacterial activity of DNA-stabilized silver nanoclusters tuned by oligonucleotide sequence[J]. ACS Applied Materials & Interfaces, 2016,8(16):10147-10154. |
| [25] |
Eun H, Kwon WY, Kalimuthu K, et al. Melamine-promoted formation of bright and stable DNA-silver nanoclusters and their antimicrobial properties[J]. Journal of Materials Chemistry B, 2019,7(15):2512-2517.
doi: 10.1039/C8TB03166E URL |
| [26] | Yang L, Yao C, Li F, et al. Synjournal of branched DNA scaffolded super-nanoclusters with enhanced antibacterial performance[J]. Small, 2018,14(16):e1800185. |
| [27] |
Huma ZE, Gupta A, Javed I, et al. Cationic silver nanoclusters as potent antimicrobials against multidrug-resistant bacteria[J], ACS Omega, 2018,3(12):16721-16727.
doi: 10.1021/acsomega.8b02438 URL |
| [28] |
Chakraborty I, Udayabhaskararao T, Deepesh GK, et al. Sunlight mediated synjournal and antibacterial properties of monolayer protected silver clusters[J]. Journal of Materials Chemistry B, 2013,1(33):4059-4064.
doi: 10.1039/c3tb20603c pmid: 32260958 |
| [29] |
Yuan X, Setyawati MI, Tan AS, et al. Highly luminescent silver nanoclusters with tunable emissions:cyclic reduction-decomposition synjournal and antimicrobial properties[J]. NPG Asia Materials, 2013,5(2):e39.
doi: 10.1038/am.2013.3 URL |
| [30] |
Padmos JD, Boudreau RTM, Weaver DF, et al. Structure of tiopronin-protected silver nanoclusters in a one-dimensional assembly[J]. The Journal of Physical Chemistry C, 2015,119(43):24627-24635.
doi: 10.1021/acs.jpcc.5b07426 URL |
| [31] |
Sangsuwan A, Kawasaki H, Matsumura Y, et al. Antimicrobial silver nanoclusters bearing biocompatible phosphorylcholine-based zwitterionic protection[J]. Bioconjugate Chemistry, 2016,27(10):2527-2533.
doi: 10.1021/acs.bioconjchem.6b00455 pmid: 27689806 |
| [32] |
Nakal-Chidiac A, García O, García-Fernández L, et al. Chitosan-stabilized silver nanoclusters with luminescent, photothermal and antibacterial properties[J]. Carbohydrate Polymers, 2020,250:116973.
doi: 10.1016/j.carbpol.2020.116973 URL |
| [33] | Wang SS, Wang YY, Peng Y, et al. Exploring the antibacteria performance of multicolor Ag, Au and Cu nanoclusters[J]. ACS Applied Materials & Interfaces, 2019,11(8):8461-8469. |
| [34] |
Zheng K, Setyawati MI, Lim TP, et al. Antimicrobial cluster bombs:silver nanoclusters packed with daptomycin[J]. ACS nano, 2016,10(8):7934-7942.
doi: 10.1021/acsnano.6b03862 URL |
| [35] |
Shitomi K, Miyaji H, Miyata S, et al. Photodynamic inactivation of oral bacteria with silver nanoclusters/rose bengal nanocomposite[J]. Photodiagnosis and Photodynamic Therapy, 2020,30:101647.
doi: S1572-1000(19)30592-7 pmid: 31904554 |
| [36] | Takenaka S, Karg E, Roth C, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats[J]. Environmental Health Perspectives, 2001,109(4):547-551. |
| [37] |
Tang J, Xiong L, Wang S, et al. Distribution, translocation and accumulation of silver nanoparticles in rats[J]. Journal of Nanoscience and Nanotechnology, 2009,9(8):4924-4932.
doi: 10.1166/jnn.2009.1269 URL |
| [38] |
Sung JH, Ji JH, Yoon JU, et al. Lung function changes in sprague-dawley rats after prolonged inhalation exposure to silver nanoparticles[J]. Inhalation Toxicology, 2008,20(6):567-574.
doi: 10.1080/08958370701874671 URL |
| [39] |
Lee H, Choi Y, Jung E, et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation[J]. Journal of Nanoparticle Research, 2010,12(5):1567-1578.
doi: 10.1007/s11051-009-9666-2 URL |
| [40] |
Hussain SM, Hess KL, Gearhart JM, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells[J]. Toxicology in Vitro, 2005,19(7):975-983.
pmid: 16125895 |
| [41] |
Braydichstolle LK, Hussain SM, Schlager JJ, et al. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells[J]. Toxicological Sciences, 2005,88(2):412-419.
doi: 10.1093/toxsci/kfi256 URL |
| [42] |
Ahamed M, Karns M, Goodson MS, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells[J]. Toxicology and Applied Pharmacology, 2008,233(3):404-410.
doi: 10.1016/j.taap.2008.09.015 URL |
| [43] |
Arora S, Jain J, Rajwade JM, et al. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells[J]. Toxicology and Applied Pharmacology, 2009,236(3):310-318.
doi: 10.1016/j.taap.2009.02.020 pmid: 19269301 |
| [44] |
Foldbjerg R, Olesen PL, Hougaard M, et al. PVP- coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes[J]. Toxicology Letters, 2009,190(2):156-162.
doi: 10.1016/j.toxlet.2009.07.009 pmid: 19607894 |
| [45] |
Gopinath P, Gogoi SK, Sanpui P, et al. Signaling gene cascade in silver nanoparticle induced apoptosis[J]. Colloids and Surfaces B:Biointerfaces, 2010,77(2):240-245.
doi: 10.1016/j.colsurfb.2010.01.033 URL |
| [46] |
Samberg ME, Oldenburg SJ, Monteiroriviere NA, et al. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro[J]. Environmental Health Perspectives, 2009,118(3):407-413.
doi: 10.1289/ehp.0901398 URL |
| [47] |
Khandelwal P, Poddar P. Fluorescent metal quantum clusters:an updated overview of the synjournal, properties, and biological applications[J]. Journal of Materials Chemistry B, 2017,5(46):9055-9084.
doi: 10.1039/c7tb02320k pmid: 32264589 |
| [48] | Chandirasekar S, Chandrasekaran C, Muthukumarasamyvel T, et al. Sodium cholate-templated blue light-emitting Ag subnanoclusters:in vivo toxicity and imaging in zebrafish embryos[J]. ACS Applied Materials & Interfaces, 2015,7(3):1422-1430. |
| [49] |
Díez I, Eronen P, Österberg M, et al. Functionalization of nanofibrillated cellulose with silver nanoclusters:fluorescence and antibacterial activity[J]. Macromolecular Bioscience, 2011,11(9):1185-1191.
doi: 10.1002/mabi.201100099 URL |
| [50] |
Wang X, Gao W, Xu S, et al. Luminescent fibers:In situ synjournal of silver nanoclusters on silk via ultraviolet light-induced reduction and their antibacterial activity[J]. Chemical Engineering Journal, 2012,210:585-589.
doi: 10.1016/j.cej.2012.09.034 URL |
| [51] |
Balagna C, Irfan M, Perero S, et al. Characterization of antibacterial silver nanocluster/silica composite coating on high performance Kevlar® textile[J]. Surface and Coatings Technology, 2017,321:438-447.
doi: 10.1016/j.surfcoat.2017.05.009 URL |
| [52] |
Mishra SK, Raveendran S, Ferreira JMF, et al. In situ impregnation of silver nanoclusters in microporous Chitosan-PEG membranes as an antibacterial and drug delivery percutaneous device[J]. Langmuir, 2016,32(40):10305-10316.
doi: 10.1021/acs.langmuir.6b02844 URL |
| [53] |
Guo Q, Li J, Chen T, et al. Antimicrobial thin-film composite membranes with chemically decorated ultrasmall silver nanoclusters[J]. ACS Sustainable Chem Eng, 2019,7(17):14848-14855.
doi: 10.1021/acssuschemeng.9b02929 URL |
| [54] | Mei L, Teng Z, Zhu G, et al. Silver nanocluster-embedded zein films as antimicrobial coating materials for food packaging[J]. ACS Applied Materials & Interfaces, 2017,9(40):35297-35304. |
| [55] | 石璐. 银纳米簇水凝胶在餐具消毒及药物载体方面的应用[D]. 无锡:江南大学, 2016. |
| SHI L. Silver nanoclusters hydrogel for tableware disinfection and drug delivery[D]. Wuxi:Jiangnan University, 2016. | |
| [56] | 马芸. 银纳米簇水凝胶的制备及其应用[D]. 无锡:江南大学, 2014. |
| MA Y. Preparation of Ag nanoclusters hydrogel and its applications[D]. Wuxi:Jiangnan University, 2014. | |
| [57] | 银娜, 梁俊, 高志贤. 银纳米簇用于食品包装污染物的检测及其抗菌性能的应用[J]. 包装工程, 2019,40(21):44-50. |
| Yin N, Liang J, Gao ZX. Silver nanoclusters for the detection of food packaging contaminants and their application in antibacterial properties[J]. Packaging Engineering, 2019,40(21):44-50. | |
| [58] |
Lu R, Zou W, Du H, et al. Antimicrobial activity of Ag nanoclusters encapsulated in porous silica nanospheres[J]. Ceramics International, 2014,40(2):3693-3698.
doi: 10.1016/j.ceramint.2013.09.055 URL |
| [59] | Patil AG, Bafna HR, More MP, et al. Green synjournal of graphene based silver nanocomposite for enhanced antibacterial activity against dental pathogens[J]. JSM Nanotechnology and Nanomedicine, SciMed Central, USA, 2017,5(3):1-7. |
| [60] | 杜丽娜, 金义光. 核酸药物纳米制剂的设计及递送新技术[J]. 国际药学研究杂志, 2017,44(11):1052-1068. |
| Du LN, Jin YG. Design of nucleic acid-loaded nanoscale formulations and relevant novel delivery techniques[J]. Journal of International Pharmaceutical Research, 2017,44(11):1052-1068. |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 温晓蕾, 李建嫄, 李娜, 张娜, 杨文香. 小麦叶锈菌与小麦互作的酵母双杂交cDNA文库构建与应用[J]. 生物技术通报, 2023, 39(9): 136-146. |
| [3] | 韩浩章, 张丽华, 李素华, 赵荣, 王芳, 王晓立. 盐碱胁迫诱导的猴樟酵母cDNA文库构建及CbP5CS上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 236-245. |
| [4] | 李英, 岳祥华. DNA甲基化在解析毛竹自然变异中的应用[J]. 生物技术通报, 2023, 39(7): 48-55. |
| [5] | 姚近东, 汤华妹, 杨文霄, 张丽珊, 林向民. 恩诺沙星胁迫下嗜水气单胞菌的比较蛋白质组学研究[J]. 生物技术通报, 2023, 39(4): 288-296. |
| [6] | 李天顺, 李宸葳, 王佳, 朱龙佼, 许文涛. 功能核酸筛选过程中次级文库的有效制备[J]. 生物技术通报, 2023, 39(3): 116-122. |
| [7] | 祝瑛萱, 李克景, 何敏, 郑道琼. 酵母模型揭示胁迫因子驱动基因组变异的研究进展[J]. 生物技术通报, 2023, 39(11): 191-204. |
| [8] | 段敏杰, 李怡斐, 杨小苗, 王春萍, 黄启中, 黄任中, 张世才. 辣椒锌指蛋白DnaJ-Like基因家族鉴定及对高温胁迫的表达响应[J]. 生物技术通报, 2023, 39(1): 187-198. |
| [9] | 刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105. |
| [10] | 张淼, 杨露露, 贾岩龙, 王天云. DNA甲基化和组蛋白甲基化修饰的表观遗传调控作用研究进展[J]. 生物技术通报, 2022, 38(7): 23-30. |
| [11] | 王晨晨, 张凡丽, 陈珮琪, 翁思瑶, 王慧芳, 崔小娟. 哺乳动物DNA甲基转移酶DNMT1和DNMT3结构与功能的研究进展[J]. 生物技术通报, 2022, 38(7): 31-39. |
| [12] | 申恒, 刘思慧, 李跃, 李敬涛, 梁文星. 一种用于PCR的番茄DNA快速粗提方法[J]. 生物技术通报, 2022, 38(6): 74-80. |
| [13] | 易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190. |
| [14] | 张雨函, 范熠, 李婷婷, 庞爽, 刘为, 白可喻, 张西美. 基于宏基因组测序的植物叶表微生物富集及DNA提取方法[J]. 生物技术通报, 2022, 38(3): 256-263. |
| [15] | 谢田朋, 柳娜, 刘越敏, 曲馨, 薄双琴, 景明. 化肥减量配施中药源植物生长调节剂对当归质量和根际土壤细菌群落的影响[J]. 生物技术通报, 2022, 38(3): 79-91. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||