








生物技术通报 ›› 2022, Vol. 38 ›› Issue (2): 34-43.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1077
收稿日期:2021-08-21
出版日期:2022-02-26
发布日期:2022-03-09
作者简介:刘萌萌,女,硕士研究生,研究方向:作物遗传育种;E-mail: 基金资助:
LIU Meng-meng( ), HAN Li-jun, LIU Bao-ling, XUE Jin-ai(
), HAN Li-jun, LIU Bao-ling, XUE Jin-ai( ), LI Run-zhi(
), LI Run-zhi( )
)
Received:2021-08-21
Published:2022-02-26
Online:2022-03-09
摘要:
三酰基甘油脂肪酶(SDP1)是催化三酰甘油降解的关键酶,在植物油脂代谢调控中起着重要作用。克隆棉花SDP1并研究其在3种胁迫下的表达分析,为解析棉花SDP1的生物学功能提供依据。以陆地棉品种冀丰1271为试材,克隆GhSDP1编码序列和上游启动子序列;利用PlantCARE分析GhSDP1启动子区顺式作用元件;qRT-PCR检测逆境胁迫下GhSDP1的表达谱;通过烟草瞬时表达pGhSDP1启动子+GUS载体检测启动子活性。结果表明,GhSDP1的编码序列为2 541 bp,其在盐、低温和干旱胁迫下呈差异表达模式。pGhSDP1除具有启动子所必需的TATA-box和CAAT-box等基本顺式作用元件外,还含有多个与光响应、激素响应及逆境应答等相关的顺式作用元件。棉花pGhSDP1启动子能驱动GUS蛋白高效表达,具有较强的启动子活性。研究揭示了棉花GhSDP1参与胁迫应答的新功能。
刘萌萌, 韩立军, 刘宝玲, 薛金爱, 李润植. 陆地棉GhSDP1及其启动子的克隆与表达分析[J]. 生物技术通报, 2022, 38(2): 34-43.
LIU Meng-meng, HAN Li-jun, LIU Bao-ling, XUE Jin-ai, LI Run-zhi. Cloning and Expression Analysis of GhSDP1 and Its Promoter in Gossypium hirsutum[J]. Biotechnology Bulletin, 2022, 38(2): 34-43.
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 用途Application |
|---|---|---|
| GhSDP1-F | ATGGATATAACTAATGAGGCCAGTGT | GhSDP1引物 |
| GhSDP1-R | TTAGGCATCTACAACACCACCCTG | Primer pairs for GhSDP1 gene |
| pGhSDP1-F | TTGGAGTTAGATTGTAAATAAT | GhSDP1启动子引物 |
| pGhSDP1-R | AGCTAGTTTCTAATAGGGAATG | Primer pairs for the promoter of GhSDP1 |
| 1301-pGhSDP1-F | tcctctagagtcgacctgcagTTGGAGTTAGATTGTAAATAAT | GUS染色载体引物 |
| 1301-pGhSDP1-R | ttaccctcagatctaccatggAGCTAGTTTCTAATAGGGAATG | Primer pairs for GUS staining vector |
| qGhSDP1-F | CTTCGGCAACCAGATTCTTCC | GhSDP1荧光定量引物 |
| qGhSDP1-R | AGCGACTCCACCACCTACT | Primer pairs for qRT-PCR of GhSDP1 |
| histone-F | AAGCCTCATCGATACCGTCC | 内参基因引物 |
| histone-R | GGCTCTGGAAACGCAAATCA | Primer pairs for internal reference gene |
表1 本研究所用引物
Table 1 Primers used in this study
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 用途Application |
|---|---|---|
| GhSDP1-F | ATGGATATAACTAATGAGGCCAGTGT | GhSDP1引物 |
| GhSDP1-R | TTAGGCATCTACAACACCACCCTG | Primer pairs for GhSDP1 gene |
| pGhSDP1-F | TTGGAGTTAGATTGTAAATAAT | GhSDP1启动子引物 |
| pGhSDP1-R | AGCTAGTTTCTAATAGGGAATG | Primer pairs for the promoter of GhSDP1 |
| 1301-pGhSDP1-F | tcctctagagtcgacctgcagTTGGAGTTAGATTGTAAATAAT | GUS染色载体引物 |
| 1301-pGhSDP1-R | ttaccctcagatctaccatggAGCTAGTTTCTAATAGGGAATG | Primer pairs for GUS staining vector |
| qGhSDP1-F | CTTCGGCAACCAGATTCTTCC | GhSDP1荧光定量引物 |
| qGhSDP1-R | AGCGACTCCACCACCTACT | Primer pairs for qRT-PCR of GhSDP1 |
| histone-F | AAGCCTCATCGATACCGTCC | 内参基因引物 |
| histone-R | GGCTCTGGAAACGCAAATCA | Primer pairs for internal reference gene |

图1 构建的pCAMBIA1301-proGhSDP1植物表达载体简图 A:pCAMBIA1301植物表达载体;B:pCAMBIA1301-proGhSDP1载体
Fig.1 Diagram of constructing pCAMBIA1301-proGhS-DP1 plant expression vector A:pCAMBIA1301 plant expression vector. B:pCAMBIA1301-proGhSDP1 plant expression vector
| 试剂Reagent | 母液浓度 Mother liquor | 终浓度 Final concentration | 使用量 Volume of use |
|---|---|---|---|
| 乙酰丁香酮(AS) | 100 μmol/mL | 100 μmol/L | 100 μL |
| 硫酸镁(MgSO4) | 1 mmol/mL | 10 mmol/L | 1 mL |
| 2-(N-吗啡啉)- 乙磺酸(MES) | 1 mmol/mL | 10 mmol/L | 1 mL |
| 无菌水(ddH2O) | 定容至100 mL |
表2 烟草农杆菌侵染液配置
Table 2 Preparation of tobacco infecting solution
| 试剂Reagent | 母液浓度 Mother liquor | 终浓度 Final concentration | 使用量 Volume of use |
|---|---|---|---|
| 乙酰丁香酮(AS) | 100 μmol/mL | 100 μmol/L | 100 μL |
| 硫酸镁(MgSO4) | 1 mmol/mL | 10 mmol/L | 1 mL |
| 2-(N-吗啡啉)- 乙磺酸(MES) | 1 mmol/mL | 10 mmol/L | 1 mL |
| 无菌水(ddH2O) | 定容至100 mL |

图3 棉花幼苗在不同胁迫处理下的表型观察 A:NaCl处理(200 mmol/L);B:低温处理(4℃);C:PEG处理(15%)
Fig.3 Phenotype observation of cotton seedlings under dif-ferent stress treatments A:NaCl treatment(200 mmol/L). B:Cold stress treatment(4℃). C:PEG treatment(15%)
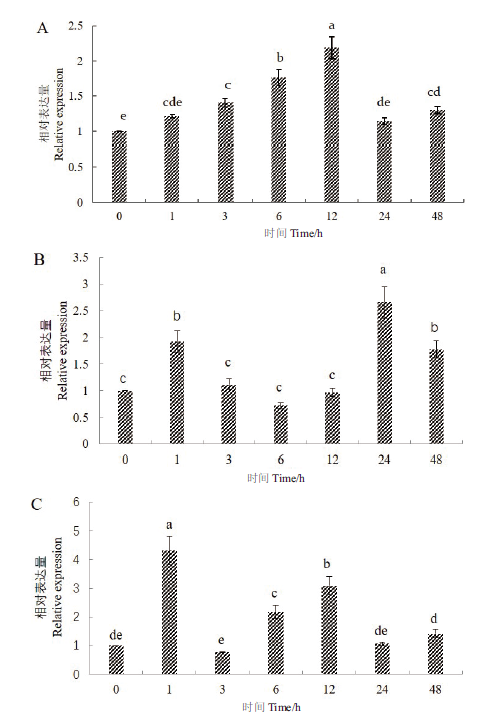
图4 GhSDP1在不同胁迫处理下的表达情况 A:NaCl处理;B:低温处理;C:PEG处理。小写字母表示差异达到(P<0.05)显著水平
Fig.4 Relative expressions of GhSDP1 gene under different stress treatments A:NaCl treatment. B:Cold stress treatment. C:PEG treatment. The lowercase letters indicate that the difference reached a significant level(P<0.05)

图5 陆地棉GhSDP1启动子的PCR扩增产物 M:DL2000 DNA marker;1-2:目的片段
Fig.5 PCR amplified product of GhSDP1 promoter in G. hirsutum M:DL2000 DNA marker. 1-2:Target fragment
| 元件名称 Cis- element | 核心序列 Sequence | 功能 Function | 分布 Position | 数量Number |
|---|---|---|---|---|
| GATABOX | GATA | 光应答元件 | 138(+),680(-),686(-),773(+),870(-),1116(-),1194(-),1304(-),1397(+),1577(+),1626(-),1680(+),1765(-) | 13 |
| ARR1AT | NGATT | 应答调控因子ARR1结合元件 | 9(+),55(+),111(-),116(-),132(+),382(-),707(-),715(-),806(-),845(-),1111(-),1150(-),1170(-),1439(+),1509(-),1538(+),1638 | 21 |
| ACGTATERD1 | ACGT | 脱水相关顺式作用元件 | (-),1690(-),1801(-),817(-),883(-) 141(+),851(+) | 2 |
| MYB1AT | WAACCA | 干旱响应相关元件 | 1308(-),1381(-),1480(-),1566(-) | 4 |
| MYBCORE | CNGTTR | 干旱应答 | 947(+),1203(-),1389(-),1828(+) | 4 |
| MYCCONSENSUSAT | CANNTG | 干旱及低温响应元件 | 947(+),1673(-) | 2 |
| GT1CONSENSUS | GRWAAW | 盐、水杨酸及光响应元件 | 773(+),818(+),819(+),1223(-),1316(-),1326(-),1371(+),1397(+),1450(+),1451(+),1461(+),1649(+),1663(-),1700(-),1718(+),1763(-),1823(-) | 17 |
| GAREAT | TAACAAR | 赤霉素应答元件 | 634(-),830(-) | 2 |
| WRKY71OS | TGAC | W-box 核心区,参与赤霉素信号途径 | 224(-),734(-),793(-),849(+),1581(-) | 5 |
| BIHD1OS | TGTCA | 抗病响应相关元件 | 1351(+) | 1 |
| WBOXNTERF3 | TGACY | ERF3结合位点,损伤响应元件 | 792(-),1580(-) | 2 |
| POLLEN1LELAT52 | AGAAA | 花粉特异表达相关元件 | 50(-),261(+),415(-),475(-),570(+),704(-),748(+),788(+),933(-),1050(+),1255(+) | 11 |
表3 棉花GhSDP1基因启动子部分顺式作用元件
Table 3 Part of putative cis-acting elements and their positions in the GhSDP1 promoter
| 元件名称 Cis- element | 核心序列 Sequence | 功能 Function | 分布 Position | 数量Number |
|---|---|---|---|---|
| GATABOX | GATA | 光应答元件 | 138(+),680(-),686(-),773(+),870(-),1116(-),1194(-),1304(-),1397(+),1577(+),1626(-),1680(+),1765(-) | 13 |
| ARR1AT | NGATT | 应答调控因子ARR1结合元件 | 9(+),55(+),111(-),116(-),132(+),382(-),707(-),715(-),806(-),845(-),1111(-),1150(-),1170(-),1439(+),1509(-),1538(+),1638 | 21 |
| ACGTATERD1 | ACGT | 脱水相关顺式作用元件 | (-),1690(-),1801(-),817(-),883(-) 141(+),851(+) | 2 |
| MYB1AT | WAACCA | 干旱响应相关元件 | 1308(-),1381(-),1480(-),1566(-) | 4 |
| MYBCORE | CNGTTR | 干旱应答 | 947(+),1203(-),1389(-),1828(+) | 4 |
| MYCCONSENSUSAT | CANNTG | 干旱及低温响应元件 | 947(+),1673(-) | 2 |
| GT1CONSENSUS | GRWAAW | 盐、水杨酸及光响应元件 | 773(+),818(+),819(+),1223(-),1316(-),1326(-),1371(+),1397(+),1450(+),1451(+),1461(+),1649(+),1663(-),1700(-),1718(+),1763(-),1823(-) | 17 |
| GAREAT | TAACAAR | 赤霉素应答元件 | 634(-),830(-) | 2 |
| WRKY71OS | TGAC | W-box 核心区,参与赤霉素信号途径 | 224(-),734(-),793(-),849(+),1581(-) | 5 |
| BIHD1OS | TGTCA | 抗病响应相关元件 | 1351(+) | 1 |
| WBOXNTERF3 | TGACY | ERF3结合位点,损伤响应元件 | 792(-),1580(-) | 2 |
| POLLEN1LELAT52 | AGAAA | 花粉特异表达相关元件 | 50(-),261(+),415(-),475(-),570(+),704(-),748(+),788(+),933(-),1050(+),1255(+) | 11 |

图6 表达载体重组子双酶切电泳图 M:DNA marker DL10 000;1:双酶切结果;2:质粒
Fig.6 Electrophoresis of double-digested expression vector M:DNA marker DL10 000;1:result of enzyme digestion;2:plasmid
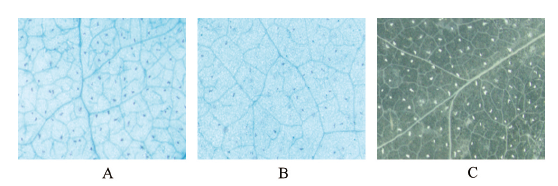
图7 携带有pGhSDP1∷GUS载体的农杆菌侵染烟草叶片GUS染色 A:pGhSDP1∷GUS;B:CaMV35S∷GUS(阳性对照);C:GV3101(阴性对照)
Fig.7 GUS staining of the tobacco leaves infected by agrob-acterium containing the pGhSDP1∷GUS vector A:pGhSDP1∷GUS. B:CaMV35S∷GUS(positive control). C:GV3101(negative control)
| [1] | 陶芬芳, 邢蔓, 岳宁燕, 等. 植物三酰甘油合成相关基因研究进展[J]. 作物研究, 2017, 31(3):330-336. |
| Tao FF, Xing M, Yue NY, et al. Research advances of genes related to plant triacylglycerol synjournal[J]. Crop Res, 2017, 31(3):330-336. | |
| [2] | Arunachalam Sivagurulingam AP, Sivanandi P, Pandian S, et al. Optimization and kinetic studies on biodiesel production from microalgae(Euglena sanguinea)using calcium methoxide as catalyst[J]. Energy Sources A:Recovery Util Environ Eff, 2019, 41(12):1497-1507. |
| [3] |
Yang Y, Benning C. Functions of triacylglycerols during plant development and stress[J]. Curr Opin Biotechnol, 2018, 49:191-198.
doi: 10.1016/j.copbio.2017.09.003 URL |
| [4] |
Graham IA, Eastmond PJ. Pathways of straight and branched chain fatty acid catabolism in higher plants[J]. Prog Lipid Res, 2002, 41(2):156-181.
pmid: 11755682 |
| [5] |
Penfield S, Pinfield-Wells HM, Graham IA. Storage reserve mobilisation and seedling establishment in Arabidopsis[J]. Arabidopsis Book, 2006, 4:e0100.
doi: 10.1199/tab.0100 URL |
| [6] |
Eastmond PJ, Germain V, Lange PR, et al. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle[J]. PNAS, 2000, 97(10):5669-5674.
pmid: 10805817 |
| [7] |
Poxleitner M, Rogers SW, Lacey Samuels A, et al. A role for caleosin in degradation of oil-body storage lipid during seed germination[J]. Plant J, 2006, 47(6):917-933.
pmid: 16961733 |
| [8] |
Eastmond PJ. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds[J]. Plant Cell, 2006, 18(3):665-675.
pmid: 16473965 |
| [9] |
Matsui K, Fukutomi S, Ishii M, et al. A tomato lipase homologous to DAD1(LeLID1)is induced in post-germinative growing stage and encodes a triacylglycerol lipase[J]. FEBS Lett, 2004, 569(1/2/3):195-200.
doi: 10.1016/j.febslet.2004.05.064 URL |
| [10] |
Kelly AA, Quettier AL, Shaw E, et al. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis[J]. Plant Physiol, 2011, 157(2):866-875.
doi: 10.1104/pp.111.181784 URL |
| [11] |
Kelly AA, Shaw E, Powers SJ, et al. Suppression of the SUGAR-DEPENDENT1 triacylglycerol lipase family during seed development enhances oil yield in oilseed rape(Brassica napus L.)[J]. Plant Biotechnol J, 2013, 11(3):355-361.
doi: 10.1111/pbi.12021 URL |
| [12] |
Vanhercke T, Divi UK, El Tahchy A, et al. Step changes in leaf oil accumulation via iterative metabolic engineering[J]. Metab Eng, 2017, 39:237-246.
doi: S1096-7176(16)30091-X pmid: 27993560 |
| [13] |
Thazar-Poulot N, Miquel M, Fobis-Loisy I, et al. Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies[J]. PNAS, 2015, 112(13):4158-4163.
doi: 10.1073/pnas.1403322112 pmid: 25775518 |
| [14] | Kanai M, Yamada T, Hayashi M, et al. Soybean(Glycine max L.)triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil[J]. Sci Rep, 2019, 9(1):1-10. |
| [15] | Suman SK, Kumar M, Sharma VK, et al. A modified and efficient CTAB genomic DNA extraction method from maize leaf for PCR analysis[J]. J Pharmacogn Phytochem, 2020, 9(4):3418-3420. |
| [16] |
Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves[J]. Plant J, 2000, 22(6):543-551.
pmid: 10886774 |
| [17] | 张丽华, 程智慧, 李霞. GUS基因分泌型植物表达载体的构建及对烟草的转化[J]. 西北农林科技大学学报:自然科学版, 2004, 32(S1):94-96. |
| Zhang LH, Cheng ZH, Li X. Secretory plant expressing vector construction of GUS gene and transformation to tobacco[J]. J Northwest Sci Tech Univ Agric For, 2004, 32(S1):94-96. | |
| [18] |
Connors BJ, Miller M, Maynard CA, et al. Cloning and characterization of promoters from American chestnut capable of directing reporter gene expression in transgenic Arabidopsis plants[J]. Plant Sci, 2002, 163(4):771-781.
doi: 10.1016/S0168-9452(02)00214-5 URL |
| [19] | 姜骋, 张曦, 田晴, 等. 白桦BpbHLH112基因克隆及其启动子表达特性分析[J]. 植物研究, 2020, 40(4):583-592. |
| Jiang C, Zhang X, Tian Q, et al. Isolation of the BpbHLH112 gene and expression analysis of its promoter in Betula platyphylla[J]. Bull Bot Res, 2020, 40(4):583-592. | |
| [20] |
Liu J, Wang F, Yu G, et al. Functional analysis of the maize C-repeat/DRE motif-binding transcription factor CBF3 promoter in response to abiotic stress[J]. Int J Mol Sci, 2015, 16(6):12131-12146.
doi: 10.3390/ijms160612131 URL |
| [21] | 王成慧. 薄皮甜瓜CmLOX08基因的克隆及其在干旱和盐胁迫中的功能分析[D]. 沈阳:沈阳农业大学, 2019. |
| Wang CH. Cloning and functional analysis of CmLOX08 in drought and salt stresses from oriental melon(Cucumis melo var. makuwa makino)[D]. Shenyang:Shenyang Agricultural University, 2019. | |
| [22] | 秦琳琳, 张曦, 姜骋, 等. 白桦BpZFP4基因启动子克隆和逆境响应元件功能分析[J]. 植物研究, 2019, 39(6):917-926. |
| Qin LL, Zhang X, Jiang C, et al. Cloning and functional analysis of BpZFP4 promoter from birch(Betula platyphylla)[J]. Bull Bot Res, 2019, 39(6):917-926. | |
| [23] |
Ullah A, Manghwar H, Shaban M, et al. Phytohormones enhanced drought tolerance in plants:a coping strategy[J]. Environ Sci Pollut Res Int, 2018, 25(33):33103-33118.
doi: 10.1007/s11356-018-3364-5 URL |
| [24] |
Hu EM, Liu M, Zhou R, et al. Relationship between melatonin and abscisic acid in response to salt stress of tomato[J]. Sci Hortic, 2021, 285:110176.
doi: 10.1016/j.scienta.2021.110176 URL |
| [25] |
Jia T, Hou J, Iqbal MZ, et al. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana[J]. Plant Physiol Biochem, 2021, 165:147-160.
doi: 10.1016/j.plaphy.2021.05.018 URL |
| [26] |
Miri M, Ghooshchi F, Tohidi-Moghadam HR, et al. Ameliorative effects of foliar spray of Glycine betaine and gibberellic acid on cowpea(Vigna unguiculata L. Walp. )yield affected by drought stress[J]. Arab J Geosci, 2021, 14(10):1-9.
doi: 10.1007/s12517-020-06304-8 URL |
| [27] | 崔力勃. 脱落酸(ABA)对十字花科油籽油分积累的影响与作用机制[D]. 杭州:浙江大学, 2017. |
| Cui LB. The mechanism of abscisic acid(ABA)on seed oil accumulation in cruciferous oilseeds[D]. Hangzhou:Zhejiang University, 2017. | |
| [28] | 李志兰, 华水金, 蒋立希. 植物GDSL脂酶家族基因的研究进展[J]. 农业生物技术学报, 2014, 22(7):916-924. |
| Li ZL, Hua SJ, Jiang LX. Advances in studying plant GDSL-type lipase genes[J]. J Agric Biotechnol, 2014, 22(7):916-924. | |
| [29] |
Rushton PJ, Reinstädler A, Lipka V, et al. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling[J]. Plant Cell, 2002, 14(4):749-762.
pmid: 11971132 |
| [30] | 阳淑金, 宋爱萍, 何深颖, 等. CaMV 35S启动子在菊花中驱动GUS外源基因的表达分析[J]. 南京农业大学学报, 2015, 38(4):554-559. |
| Yang SJ, Song AP, He SY, et al. Expression analysis of CaMV 35S promoting GUS exogenous genes in transgenic Chrysanthemum[J]. J Nanjing Agric Univ, 2015, 38(4):554-559. | |
| [31] | 王映红, 张蓓, 张楠, 等. 魔芋AaHSFB1基因及其启动子的克隆与功能分析[J]. 生物工程学报, 2021, 37(12):1-12. |
| Wang YH, Zhang B, Zhang N, et al. Cloning and functional analysis of AaHSFB1 and its promoter in Amorphophallus[J]. Chin J Biotech, 2021, 37(12):1-12. | |
| [32] |
Cai G, Kim SC, Li J, et al. Transcriptional regulation of lipid catabolism during seedling establishment[J]. Mol Plant, 2020, 13(7):984-1000.
doi: 10.1016/j.molp.2020.04.007 URL |
| [33] |
Xu X, Vanhercke T, Shrestha P, et al. Upregulated lipid biosynjournal at the expense of starch production in potato(Solanum tuberosum)vegetative tissues via simultaneous downregulation of ADP-glucose pyrophosphorylase and sugar Dependent1 expressions[J]. Front Plant Sci, 2019, 10:1444.
doi: 10.3389/fpls.2019.01444 URL |
| [1] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [2] | 王一帆, 候林慧, 常永春, 杨亚杰, 陈天, 赵祝跃, 荣二花, 吴玉香. 陆地棉与拟似棉异源六倍体的合成与性状鉴定[J]. 生物技术通报, 2023, 39(5): 168-176. |
| [3] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [4] | 杨岚, 张晨曦, 樊学伟, 王阳光, 王春秀, 李文婷. 鸡 BMP15 基因克隆、表达模式及启动子活性分析[J]. 生物技术通报, 2023, 39(4): 304-312. |
| [5] | 史光珍, 王兆晔, 孙琦, 朱新霞. 雪莲SikCDPK1启动子的克隆和活性分析[J]. 生物技术通报, 2022, 38(9): 191-197. |
| [6] | 陈光, 李佳, 杜瑞英, 王旭. pOsHAK1:OsFLN2提高水稻的糖代谢水平和抗旱性[J]. 生物技术通报, 2022, 38(8): 92-100. |
| [7] | 聂立斌, 易铃欣, 邓妍, 盛琦, 吴晓玉, 张斌. 途径工程改造谷氨酸棒杆菌产莽草酸[J]. 生物技术通报, 2022, 38(6): 93-102. |
| [8] | 镐青青, 姚圣, 刘佳禾, 陈佩珍, 张梦洋, 季孔庶. 马尾松NAC转录因子基因PmNAC8的克隆及表达分析[J]. 生物技术通报, 2022, 38(4): 202-216. |
| [9] | 叶鹏林, KwasiKyere-Yeboah, 高恶斌. 启动子PpetE与Pcpc560对集胞藻PCC 6803生物合成乙醇的影响[J]. 生物技术通报, 2022, 38(2): 141-149. |
| [10] | 时雅倩, 申亚茹, 陈漫影, 何淑敏, 刘予涵, 何天楠, 陈清西, 文志丰. 黄毛草莓F-box蛋白基因FnFBOX1及其启动子的克隆和表达分析[J]. 生物技术通报, 2022, 38(2): 44-56. |
| [11] | 马麒, 李吉莲, 徐守振, 陈红, 刘文豪, 宁新柱, 林海. 陆地棉果枝夹角性状的主基因+多基因混合遗传模型分析[J]. 生物技术通报, 2022, 38(10): 148-158. |
| [12] | 陈臣, 黄芝阳, 于海燕, 袁海彬, 田怀香. 原核生物转录调控研究技术及进展[J]. 生物技术通报, 2022, 38(10): 54-65. |
| [13] | 余婧, 杨慧, 余世洲, 赵会纳, 郑庆霞, 王兵, 雷波. 烟草NtCBT基因启动子酵母单杂诱饵载体构建及互作蛋白筛选[J]. 生物技术通报, 2022, 38(10): 73-79. |
| [14] | 胡子曜, 代培红, 刘超, 玛迪娜·木拉提, 王倩, 吾尕力汗·阿不都维力, 赵燚, 孙玲, 徐诗佳, 李月. 陆地棉小GTP结合蛋白基因GhROP3的克隆、表达及VIGS载体的构建[J]. 生物技术通报, 2021, 37(9): 106-113. |
| [15] | 沈雅丽, 潘阳阳, 王靖雷, 马睿, 赵改红, 王桂荣, 张倩, 王萌. 一种基于PXR启动子报告基因药物筛选方法的构建及其应用[J]. 生物技术通报, 2021, 37(9): 274-284. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||