








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 252-260.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1422
王雨辰1,2( ), 丁尊丹2, 关菲菲2, 田健2, 刘国安1(
), 丁尊丹2, 关菲菲2, 田健2, 刘国安1( ), 伍宁丰2(
), 伍宁丰2( )
)
收稿日期:2021-11-13
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:王雨辰,女,硕士研究生,研究方向:细胞生物学和环保酶工程;E-mail: 基金资助:
WANG Yu-chen1,2( ), DING Zun-dan2, GUAN Fei-fei2, TIAN Jian2, LIU Guo-an1(
), DING Zun-dan2, GUAN Fei-fei2, TIAN Jian2, LIU Guo-an1( ), WU Ning-feng2(
), WU Ning-feng2( )
)
Received:2021-11-13
Published:2022-08-26
Online:2022-09-14
摘要:
漆酶(EC 1.10.3.2)是一种氧化还原酶,在有毒和致癌化合物的氧化降解方面具有应用价值。通过序列分析,从UniParc数据库中筛选到耐热的漆酶基因ba4,其全长1 860 bp,编码620个氨基酸。通过最适反应温度回归预测模型(PMT)预测出BA4是耐热的漆酶,并在NCBI蛋白数据库中进行比对分析,其与来源于Klebsiella michiganensis的铜抗性系统多铜氧化酶(STW26195.1)相似性为58.75%,证明漆酶ba4是Copper_res_A超家族新的漆酶基因。将其全序列合成并在大肠杆菌BL21(DE3)中异源表达并纯化,性质测定结果表明该酶在温度45-65℃之间均有较高的酶活,最适温度为50℃,最适pH为5.5。以ABTS为底物测定米氏常数(Km)(2 144.5±358.5)μmol/L,kcat为(44.06±3.14)min-1,最大反应速率(Vmax)为623.2 μmol/(min·g),kcat/Km为(0.020 9±0.002)L/(μmol·min)。漆酶BA4(60-70 U/L)在50℃条件下与玉米赤霉烯酮(0.1 mg/mL)反应2 h,降解率达到了90%以上;漆酶BA4(70-80 U/L)在40℃和50℃条件下与棉酚反应(1 mg/mL)1 h,降解率均为30%。漆酶BA4良好的酶学性质以及对玉米赤霉烯酮和棉酚有效降解为酶的应用奠定了良好的基础。
王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260.
WANG Yu-chen, DING Zun-dan, GUAN Fei-fei, TIAN Jian, LIU Guo-an, WU Ning-feng. Identification of the Thermostable Laccase Gene ba4 and Characterization of Its Enzymatic Properties[J]. Biotechnology Bulletin, 2022, 38(8): 252-260.
| 溶液 Solution | 加入量 Added amount/μL |
|---|---|
| A液:0.2 mol/L Na2HPO4-0.1 mol/L 柠檬酸(pH 6.0) | 750 |
| B液:5 mmol/L ABTS | 200 |
| BA4蛋白 | 50 |
表1 漆酶酶活测定体系
Table 1 Determination system for laccase enzyme activity
| 溶液 Solution | 加入量 Added amount/μL |
|---|---|
| A液:0.2 mol/L Na2HPO4-0.1 mol/L 柠檬酸(pH 6.0) | 750 |
| B液:5 mmol/L ABTS | 200 |
| BA4蛋白 | 50 |
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 棉酚Gossypol/(mg·mL-1) | 0.00 | 0.05 | 0.1 | 0.15 | 0.2 |
表2 标定棉酚所用浓度
Table 2 Concentrations of gossypol in marking the standa-rd curve
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 棉酚Gossypol/(mg·mL-1) | 0.00 | 0.05 | 0.1 | 0.15 | 0.2 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 玉米赤霉烯酮浓度 ZEN/(mg·mL-1) | 0.0000 | 0.0625 | 0.1250 | 0.2500 | 0.5000 | 1.0000 |
表3 标定玉米赤霉烯酮所用浓度
Table 3 Concentrations of ZEN in marking the standard curve
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 玉米赤霉烯酮浓度 ZEN/(mg·mL-1) | 0.0000 | 0.0625 | 0.1250 | 0.2500 | 0.5000 | 1.0000 |

图3 漆酶BA4微好氧发酵20 h检测 M:蛋白质分子质量标准;1:IPTG诱导漆酶BA4破碎上清;2:IPTG诱导漆酶BA4破碎沉淀;3:NTA-200洗脱液
Fig. 3 Expression of BA4 after micro aerobic fermentation for 20 h M:Protein marker;1:supernatant of crushed IPTG-induced laccase BA4;2:precipitation of crushed IPTG-induced laccase BA4;3:NTA-200 eluant
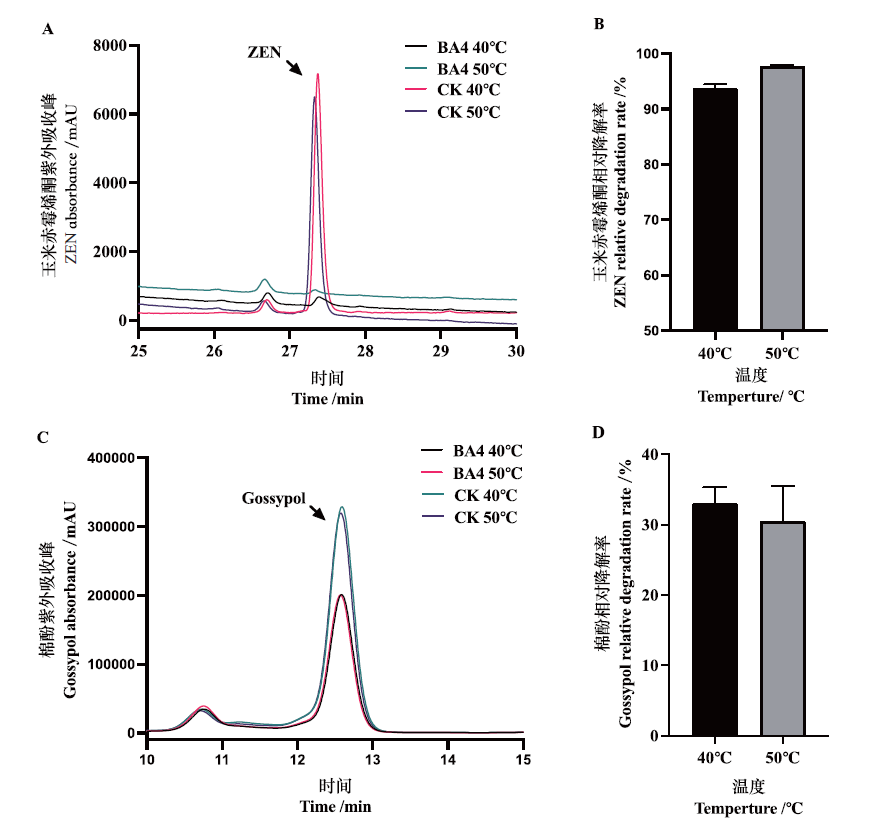
图6 漆酶BA4对ZEN和棉酚的降解作用 A:色谱图,CK:ZEN对照品;处理组:漆酶BA4分别在 40℃和50℃条件下处理ZEN;B:ZEN相对降解率,漆酶BA4分别在40℃和50℃条件下处理ZEN以色谱图峰面积计算相对降解率;C:色谱图,CK:棉酚对照品;处理组:漆酶BA4分别在 40℃和50℃条件下处理棉酚;D:棉酚相对降解率,漆酶BA4分别在40℃和50℃条件下处理棉酚以色谱图峰面积计算相对降解率
Fig. 6 Degradation of ZEN and gossypol by laccase BA4 A:Chromatogram. CK:ZEN reference substance;treatment group:laccase BA4 treating ZEN at 40℃ and 50℃,respectively. B:Relative degradation rate of ZEN. Treating ZEN with laccase BA4 at 40℃ and 50℃,respectively,and the relative degradation rate was calculated based on the peak area of the chromatogram. C:Chromatogram. CK:Gossypol reference substance. Treatment group:Laccase BA4 treated gossypol at 40℃ and 50℃,respectively. D:Relative degradation rate of gossypol. Laccase BA4 was treated with gossypol at 40℃ and 50℃,respectively,and the relative degradation rate was calculated based on the peak area of the chromatogram
| [1] |
Giardina P, Faraco V, Pezzella C, et al. Laccases:a never-ending story[J]. Cell Mol Life Sci, 2010, 67(3):369-385.
doi: 10.1007/s00018-009-0169-1 pmid: 19844659 |
| [2] |
Singh G, Bhalla A, Kaur P, et al. Laccase from prokaryotes:a new source for an old enzyme[J]. Rev Environ Sci Bio/technology, 2011, 10(4):309-326.
doi: 10.1007/s11157-011-9257-4 URL |
| [3] |
Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases[J]. Chem Rev, 1996, 96(7):2563-2606.
pmid: 11848837 |
| [4] |
Hidayat A, Yanto DHY. Biodegradation and metabolic pathway of phenanthrene by a new tropical fungus, Trametes hirsuta D7[J]. J Environ Chem Eng, 2018, 6(2):2454-2460.
doi: 10.1016/j.jece.2018.03.051 URL |
| [5] |
Dwivedi UN, Singh P, Pandey VP, et al. Structure-function relationship among bacterial, fungal and plant laccases[J]. J Mol Catal B Enzym, 2011, 68(2):117-128.
doi: 10.1016/j.molcatb.2010.11.002 URL |
| [6] |
Sharma B, Dangi AK, Shukla P. Contemporary enzyme based technologies for bioremediation:a review[J]. J Environ Manage, 2018, 210:10-22.
doi: 10.1016/j.jenvman.2017.12.075 URL |
| [7] |
Rodríguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases:a review[J]. Biotechnol Adv, 2006, 24(5):500-513.
pmid: 16716556 |
| [8] |
Galai S, Limam F, Marzouki MN. A new Stenotrophomonas maltophilia strain producing laccase. use in decolorization of synthetics dyes[J]. Appl Biochem Biotechnol, 2009, 158(2):416-431.
doi: 10.1007/s12010-008-8369-y pmid: 18931956 |
| [9] |
Knutson K, Ragauskas A. Laccase-mediator biobleaching applied to a direct yellow dyed paper[J]. Biotechnol Prog, 2004, 20(6):1893-1896.
doi: 10.1021/bp049833+ URL |
| [10] |
Santhanam N, Vivanco JM, Decker SR, et al. Expression of industrially relevant laccases:prokaryotic style[J]. Trends Biotechnol, 2011, 29(10):480-489.
doi: 10.1016/j.tibtech.2011.04.005 URL |
| [11] |
Alberts JF, Gelderblom WCA, Botha A, et al. Degradation of aflatoxin B(1)by fungal laccase enzymes[J]. Int J Food Microbiol, 2009, 135(1):47-52.
doi: 10.1016/j.ijfoodmicro.2009.07.022 pmid: 19683355 |
| [12] |
Zeinvand-Lorestani H, Sabzevari O, Setayesh N, et al. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products[J]. Chemosphere, 2015, 135:1-6.
doi: 10.1016/j.chemosphere.2015.03.036 pmid: 25876029 |
| [13] |
Zinedine A, Soriano JM, Moltó JC, et al. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of Zearalenone:an oestrogenic mycotoxin[J]. Food Chem Toxicol, 2007, 45(1):1-18.
doi: 10.1016/j.fct.2006.07.030 URL |
| [14] |
Maragos C. Zearalenone occurrence and human exposure[J]. World Mycotoxin J, 2010, 3(4):369-383.
doi: 10.3920/WMJ2010.1240 URL |
| [15] |
Richard JL. Some major mycotoxins and their mycotoxicoses—an overview[J]. Int J Food Microbiol, 2007, 119(1/2):3-10.
doi: 10.1016/j.ijfoodmicro.2007.07.019 URL |
| [16] |
Wu N, Ou W, Zhang ZD, et al. Recent advances in detoxification strategies for Zearalenone contamination in food and feed[J]. Chin J Chem Eng, 2021, 30:168-177.
doi: 10.1016/j.cjche.2020.11.011 URL |
| [17] |
Xu JH, Liu T, Chi JX, et al. Online high-efficient specific detection of Zearalenone in rice by using high-loading aptamer affinity hydrophilic monolithic column coupled with HPLC[J]. Talanta, 2020, 219:121309.
doi: 10.1016/j.talanta.2020.121309 URL |
| [18] |
Pack E, Stewart J, Rhoads M, et al. Quantification of Zearalenone and α-Zearalenol in swine liver and reproductive tissues using GC-MS[J]. Toxicon X, 2020, 8:100058.
doi: 10.1016/j.toxcx.2020.100058 URL |
| [19] |
Li CL, Deng CL, Zhou S, et al. High-throughput and sensitive determination of urinary Zearalenone and metabolites by UPLC-MS/MS and its application to a human exposure study[J]. Anal Bioanal Chem, 2018, 410(21):5301-5312.
doi: 10.1007/s00216-018-1186-4 URL |
| [20] |
Liu ZW, Hua QC, Wang J, et al. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals[J]. Biosens Bioelectron, 2020, 158:112178.
doi: 10.1016/j.bios.2020.112178 URL |
| [21] |
Wang L, Chen M, Luo XC, et al. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein[J]. Front Chem, 2020, 8:583176.
doi: 10.3389/fchem.2020.583176 URL |
| [22] |
Sunilkumar G, Campbell LM, Puckhaber L, et al. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol[J]. PNAS, 2006, 103(48):18054-18059.
pmid: 17110445 |
| [23] |
Adams R, Geissman TA, Edwards JD. Gossypol, a pigment of cottonseed[J]. Chem Rev, 1960, 60:555-574.
doi: 10.1021/cr60208a002 URL |
| [24] |
Yang J, Zhang F, Li JR, et al. Synthesis and antiviral activities of novel gossypol derivatives[J]. Bioorg Med Chem Lett, 2012, 22(3):1415-1420.
doi: 10.1016/j.bmcl.2011.12.076 URL |
| [25] |
Dodou K, Anderson RJ, Small DAP, et al. Investigations on gossypol:past and present developments[J]. Expert Opin Investig Drugs, 2005, 14(11):1419-1434.
doi: 10.1517/13543784.14.11.1419 URL |
| [26] |
Zhang L, Jiang HX, Cao XX, et al. Chiral gossypol derivatives:evaluation of their anticancer activity and molecular modeling[J]. Eur J Med Chem, 2009, 44(10):3961-3972.
doi: 10.1016/j.ejmech.2009.04.025 pmid: 19447525 |
| [27] |
Widsten P, Kandelbauer A. Laccase applications in the forest products industry:a review[J]. Enzyme Microb Technol, 2008, 42(4):293-307.
doi: 10.1016/j.enzmictec.2007.12.003 URL |
| [28] |
Fabbrini M, Galli C, Gentili P. Comparing the catalytic efficiency of some mediators of laccase[J]. J Mol Catal B Enzym, 2002, 16(5/6):231-240.
doi: 10.1016/S1381-1177(01)00067-4 URL |
| [29] | 罗爽, 谢天, 刘忠川, 等. 漆酶/介体系统研究进展[J]. 应用与环境生物学报, 2015, 21(6):987-995. |
| Luo S, Xie T, Liu ZC, et al. Laccase-mediator system:a reviewcase-mediator system:a review[J]. Chin J Appl Environ Biol, 2015, 21(6):987-995. | |
| [30] | 关菲菲, 田健, 伍宁丰, 等. 漆酶及其突变体和应用:CN113388591B[P], 2021-11-09. |
| Guan FF, Tian J, Wu NF, et al. Application of laccase and its mutants:CN113388591B[P], 2021-11-09. | |
| [31] | 国家卫生和计划生育委员会. 食品安全国家标准植物性食品中游离棉酚的测定:GB 5009. 148—2014[S]. 北京: 中国标准出版社, 2015. |
| National Food Safety Standard. Determination of Free Gossypol in Plant Food:GB 5009. 148—2014[S]. Beijing: Standards Press of China, 2015. | |
| [32] |
Lovley DR. Anaerobic benzene degradation[J]. Biodegradation, 2000, 11(2/3):107-116.
doi: 10.1023/A:1011191220463 URL |
| [33] |
Bosch R, García-Valdés E, Moore ER. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10[J]. Gene, 1999, 236(1):149-157.
pmid: 10433976 |
| [34] | Soana F, Sturini M, Cermenati L, et al. Titanium dioxide photocatalyzed oxygenation of naphthalene and some of its derivatives[J]. J Chem Soc, Perkin Trans 2, 2000(4):699-704. |
| [35] |
Wang XL, Bai YG, Huang HQ, et al. Degradation of aflatoxin B1 and Zearalenone by bacterial and fungal laccases in presence of structurally defined chemicals and complex natural mediators[J]. Toxins, 2019, 11(10):609.
doi: 10.3390/toxins11100609 URL |
| [36] |
Camarero S, Ibarra D, Martínez MJ, et al. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes[J]. Appl Environ Microbiol, 2005, 71(4):1775-1784.
doi: 10.1128/AEM.71.4.1775-1784.2005 URL |
| [1] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [2] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [3] | 贾晨波, 苏一黄, 马秀梅, 王春利, 徐春燕. 端梗霉Z45产漆酶培养基的优化及其对染料的脱色[J]. 生物技术通报, 2022, 38(6): 252-260. |
| [4] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [5] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [6] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [7] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [8] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [9] | 陈明雨, 倪烜, 司友斌, 孙凯. 固定化真菌漆酶在环境有机污染修复中的应用研究进展[J]. 生物技术通报, 2021, 37(6): 244-258. |
| [10] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [11] | 熊雪, 李鹏, 张贵合, 向准, 陶文广, 周光燕, 和耀威. 不同栽培基质诱导对香菇液体发酵产漆酶活性的影响[J]. 生物技术通报, 2021, 37(12): 50-59. |
| [12] | 王豪, 唐禄鑫, 马鸿飞, 钱坤, 司静, 崔宝凯. 东方栓孔菌漆酶的固定化及其对不同类型染料的脱色作用[J]. 生物技术通报, 2021, 37(11): 142-157. |
| [13] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [14] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| [15] | 王惠兰, 吴金勇, 陈祥松, 袁丽霞, 朱薇薇, 姚建铭. N-乙酰神经氨酸醛缩酶的固定化及固定化酶性质研究[J]. 生物技术通报, 2020, 36(6): 165-173. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||