








生物技术通报 ›› 2022, Vol. 38 ›› Issue (9): 191-197.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0570
收稿日期:2022-05-09
出版日期:2022-09-26
发布日期:2022-10-11
作者简介:史光珍,女,硕士研究生,研究方向:遗传学;E-mail: 基金资助:
SHI Guang-zhen( ), WANG Zhao-ye, SUN Qi, ZHU Xin-xia(
), WANG Zhao-ye, SUN Qi, ZHU Xin-xia( )
)
Received:2022-05-09
Published:2022-09-26
Online:2022-10-11
摘要:
旨在克隆雪莲(Saussurea involucrata)SikCDPK1 启动子并分析其活性,为进一步解析雪莲 SikCDPK1基因的转录调控机制奠定基础。通过TAIL-PCR技术从雪莲中克隆SikCDPK1启动子,利用PlantCARE分析启动子区顺式作用元件,构建全长启动子或5'端缺失启动子驱动的GUS重组表达载体 P0∷GUS、P1∷GUS、P2∷GUS和P3∷GUS,转入根癌农杆菌中进行瞬时转化试验,通过GUS组织化学染色分析不同长度启动子的活性,分别测定低温和干旱胁迫下的GUS酶活。结果显示,获得了1 042 bp的SikCDPK1启动子序列,pSikCDPK1具有真核生物启动子核心元件TATA-box和CAAT-box,还含有多个与逆境、激素、光响应等相关的顺式作用元件。转化的烟草叶片经GUS染色之后均显蓝色,启动活性依次为P0>P1>P2>P3,低温、干旱处理后GUS酶活性发生变化。SikCDPK1启动子被成功克隆,具有驱动下游报告基因表达的活性。
史光珍, 王兆晔, 孙琦, 朱新霞. 雪莲SikCDPK1启动子的克隆和活性分析[J]. 生物技术通报, 2022, 38(9): 191-197.
SHI Guang-zhen, WANG Zhao-ye, SUN Qi, ZHU Xin-xia. Cloning and Activity Analysis of SikCDPK1 Promoter from Saussurea involucrata[J]. Biotechnology Bulletin, 2022, 38(9): 191-197.
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| AD1 | NTCGASTWTSGWGTT | TAIL-PCR |
| AD2 | NGTCGASWGANAWGAAAA | TAIL-PCR |
| AD3 | WGTCNACWANCANACA | TAIL-PCR |
| AD4 | TGWGNAGWANCANAGA | TAIL-PCR |
| AD5 | AGWGNAGWANCAWAGG | TAIL-PCR |
| SP1 | CGTTATCCCAAACTGCCCTTGTCCTA | TAIL-PCR |
| SP2 | TCCGTTAGGGGTAGGTGGGGTATCTT | TAIL-PCR |
| SP3 | TTCGGTCCAACACAAGTATTCCCCAT | TAIL-PCR |
| pSikCDPK1-F1 | CCCAAGCTTCCTTAGCATCTATGAGGGTCG | 启动子克隆 Promoter cloning |
| pSikCDPK1-R1 | GACTAGTTCCAACACAAGTATTCCCCATG | 启动子克隆 Promoter cloning |
| pSikCDPK1-F2 | CCCAAGCTTCTCTTCTTATGGGTTCAAAGGGTCA | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F3 | CCCAAGCTTAAGCGTCATGCCAGTCAAGC | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F4 | CCCAAGCTTTTTCAATGGAGAAGCGACGAGC | 5'端缺失分析 5'-end deletion analysis |
表1 本研究所用引物
Table 1 Primers used in this study
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| AD1 | NTCGASTWTSGWGTT | TAIL-PCR |
| AD2 | NGTCGASWGANAWGAAAA | TAIL-PCR |
| AD3 | WGTCNACWANCANACA | TAIL-PCR |
| AD4 | TGWGNAGWANCANAGA | TAIL-PCR |
| AD5 | AGWGNAGWANCAWAGG | TAIL-PCR |
| SP1 | CGTTATCCCAAACTGCCCTTGTCCTA | TAIL-PCR |
| SP2 | TCCGTTAGGGGTAGGTGGGGTATCTT | TAIL-PCR |
| SP3 | TTCGGTCCAACACAAGTATTCCCCAT | TAIL-PCR |
| pSikCDPK1-F1 | CCCAAGCTTCCTTAGCATCTATGAGGGTCG | 启动子克隆 Promoter cloning |
| pSikCDPK1-R1 | GACTAGTTCCAACACAAGTATTCCCCATG | 启动子克隆 Promoter cloning |
| pSikCDPK1-F2 | CCCAAGCTTCTCTTCTTATGGGTTCAAAGGGTCA | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F3 | CCCAAGCTTAAGCGTCATGCCAGTCAAGC | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F4 | CCCAAGCTTTTTCAATGGAGAAGCGACGAGC | 5'端缺失分析 5'-end deletion analysis |
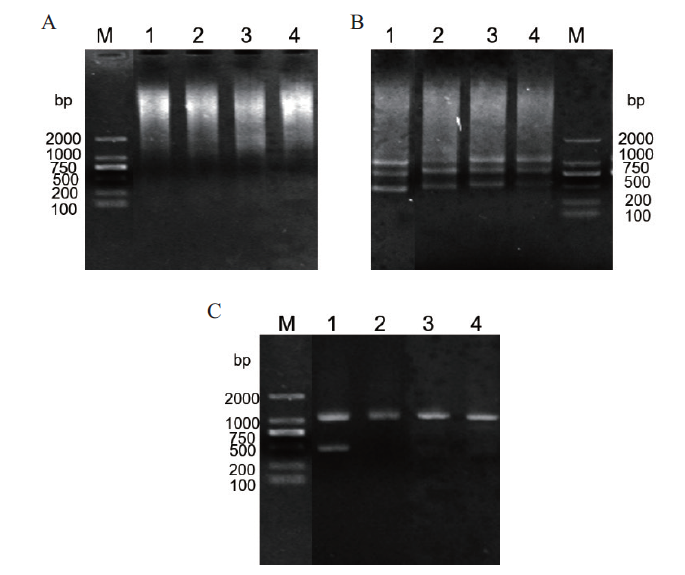
图2 SikCDPK1基因启动子的克隆 A:第一轮PCR扩增;B:第二轮PCR扩增;C:第三轮PCR扩增。M:DNA2000 marker;1:AD1;2:AD2;3:AD3;4:AD4
Fig. 2 Cloning of SikCDPK1 promoter A:The first round of PCR amplification. B:The second round of PCR amplification. C:The third round of PCR amplification. M:DNA2000 marker. 1:AD1. 2:AD2. 3:AD3. 4:AD4
| 顺式作用元件名称 Name of cis-acting element | 序列 Sequence(5'-3') | 功能 Function | 位置 Location/ nt |
|---|---|---|---|
| O2-site | GATGA(C/T)(A/G)TG(A/G)或GATGATGTGG | 玉米醇溶蛋白代谢调节 Zein metabolism regulation | -56 to -48,-596 to -587 |
| CGTCA-motif | CGTCA | 茉莉酸甲酯响应 MeJA-responsiveness | -94 to -89,-562 to -557,-479 to -474 |
| CBFHV | RYCGAC | 低温反应元件 Cis-acting element for cold | -103 to -97,-379 to -373,-672 to -666 |
| GT1GMSCAM4 | GAAAAA | 盐诱导响应相关元件 NaCl-induced | -159 to -153 |
| LTRECOREATCOR15 | CCGAC | 冷诱导低温反应元件 Cis-acting element for cold induction | -215 to -210,-459 to -454 |
| GC-motif | GCCCCC | 参与缺氧特异性诱导的类增强子Enhancer-like element involved in anoxic specific inducibility | -287 to -281 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应 MeJA-responsiveness | -310 to -305,-499 to -494 |
| ABRE | TACGGTC | ABA响应Abscisic acid responsiveness | -327 to -320 |
| W-box | TTGACC | WRKY转录因子的结合位点WRKY transcription factor binding site | -387 to -381 |
| box-S | AGCCACC | 无功能No function | -571 to -564 |
| MYC | CAATTG | 无功能No function | -533 to -539 |
| G-box | CACGTC | 光响应 Light responsiveness | -689 to -683 |
| MYB | CAACCA | MYB 顺式作用元件MYB Cis-acting element | -759 to -753 |
| OSE2ROOTNODULE | CTCTT | 在根瘤的感染细胞中被激活 Activated in infected cells of root nodules | -821 to -816 |
| MYB-Core | CGTTAG | 脱水胁迫反应元件Responsive to dehydration | -958 to -952 |
| DPBFCOREDCDC3 | ACACNNG | 脱落酸响应元件ABA-responsive elements | -975 to -967 |
| ACGTATERD1 | ACGT | 脱水诱导反应元件 Early responsive to dehydration | -1 038 to -1 034 |
表2 启动子 pSikCDPK1序列中的顺式作用元件及功能
Table 2 Cis-elements and functions in the promoter pSikCDPK1 sequence
| 顺式作用元件名称 Name of cis-acting element | 序列 Sequence(5'-3') | 功能 Function | 位置 Location/ nt |
|---|---|---|---|
| O2-site | GATGA(C/T)(A/G)TG(A/G)或GATGATGTGG | 玉米醇溶蛋白代谢调节 Zein metabolism regulation | -56 to -48,-596 to -587 |
| CGTCA-motif | CGTCA | 茉莉酸甲酯响应 MeJA-responsiveness | -94 to -89,-562 to -557,-479 to -474 |
| CBFHV | RYCGAC | 低温反应元件 Cis-acting element for cold | -103 to -97,-379 to -373,-672 to -666 |
| GT1GMSCAM4 | GAAAAA | 盐诱导响应相关元件 NaCl-induced | -159 to -153 |
| LTRECOREATCOR15 | CCGAC | 冷诱导低温反应元件 Cis-acting element for cold induction | -215 to -210,-459 to -454 |
| GC-motif | GCCCCC | 参与缺氧特异性诱导的类增强子Enhancer-like element involved in anoxic specific inducibility | -287 to -281 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应 MeJA-responsiveness | -310 to -305,-499 to -494 |
| ABRE | TACGGTC | ABA响应Abscisic acid responsiveness | -327 to -320 |
| W-box | TTGACC | WRKY转录因子的结合位点WRKY transcription factor binding site | -387 to -381 |
| box-S | AGCCACC | 无功能No function | -571 to -564 |
| MYC | CAATTG | 无功能No function | -533 to -539 |
| G-box | CACGTC | 光响应 Light responsiveness | -689 to -683 |
| MYB | CAACCA | MYB 顺式作用元件MYB Cis-acting element | -759 to -753 |
| OSE2ROOTNODULE | CTCTT | 在根瘤的感染细胞中被激活 Activated in infected cells of root nodules | -821 to -816 |
| MYB-Core | CGTTAG | 脱水胁迫反应元件Responsive to dehydration | -958 to -952 |
| DPBFCOREDCDC3 | ACACNNG | 脱落酸响应元件ABA-responsive elements | -975 to -967 |
| ACGTATERD1 | ACGT | 脱水诱导反应元件 Early responsive to dehydration | -1 038 to -1 034 |
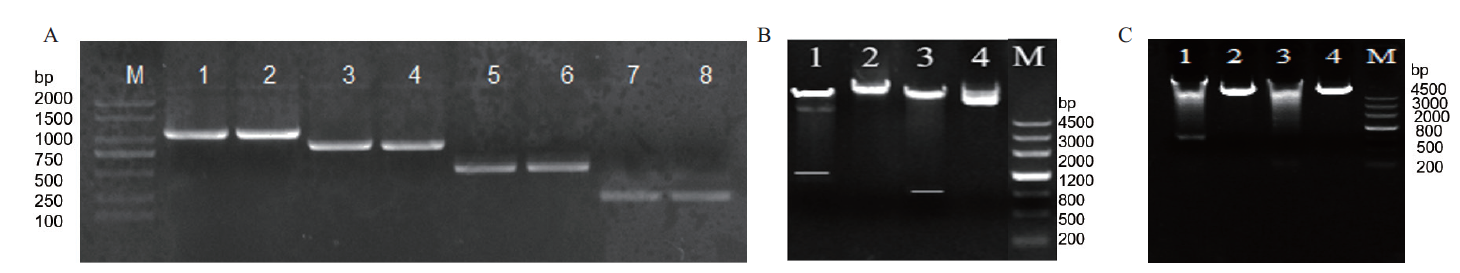
图3 SikCDPK1启动子的PCR扩增及表达载体双酶切鉴定 A:PCR扩增SikCDPK1启动子(M:DNA2000 marker;1,2:P0的PCR产物;3,4:P1的PCR产物;5,6:P2的PCR产物;7,8:P3的PCR产物);B,C:表达载体双酶切鉴定(B1:P0的双酶切;B2:P0的质粒;B3:P1的双酶切;B4:P1的质粒;C1:P2的双酶切;C2:P2的质粒;C3:P3的双酶切;C4:P3的质粒;M:DNA marker III)
Fig. 3 PCR amplification of promoter SikCDPK1 and double restriction identification of expression vector A:PCR amplification SikCDPK1 promoter(M:DNA2000 marker. 1,2:PCR product of P0. 3,4:PCR product of P1. 5,6:PCR product of P2. 7,8:PCR product of P3). B,C:Double digestion identification of expression vector(B1:P0 double digestion. B2:P0 plasmid. B3:P1 double digestion. B4:P1 plasmid. C1:P2 double digestion. C2:P2 plasmid. C3:P3 double digestion. C4:P3 plasmid. M:DNA marker III)
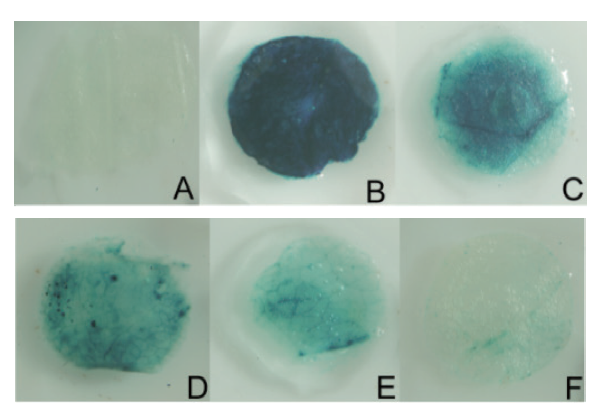
图4 SikCDPK1启动子瞬时转化烟草GUS染色分析 A:阴性对照(非转基因型);B:阳性对照(35S∷GUS);C:P0;D:P1;E:P2;F:P3
Fig. 4 GUS staining analysis of transient transformed tobacco with SikCDPK1 promoter A:Negative control(Non-transgenic). B:Positive control(35S∷GUS). C:P0. D:P1. E:P2. F:P3
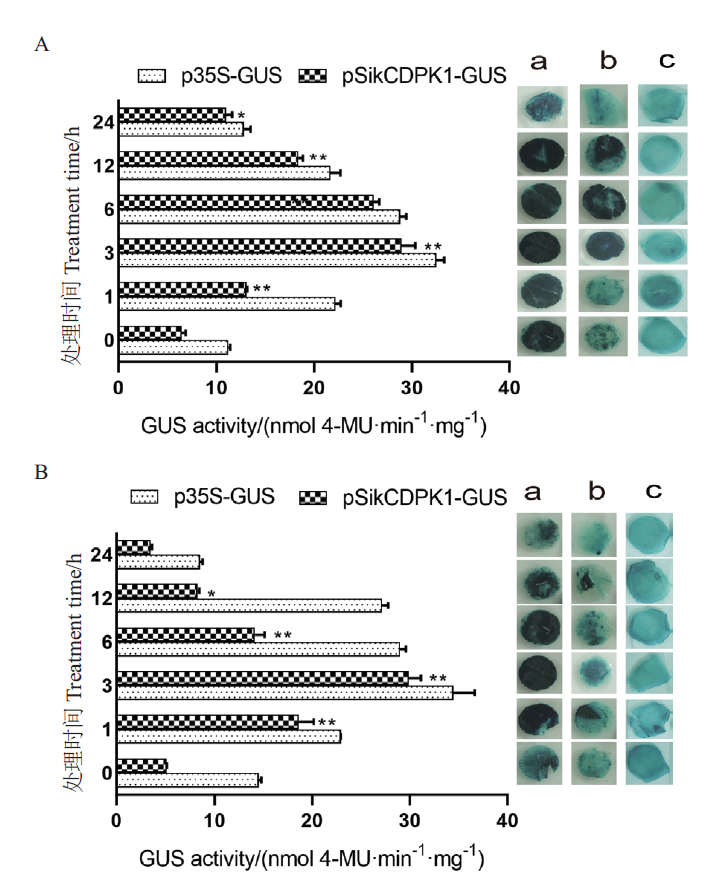
图5 胁迫处理下GUS染色分析及酶活力的测定 A:低温胁迫后的 GUS 染色和酶活力情况;B:PEG胁迫后的 GUS 染色和酶活力情况。a:p35S-GUS-1304阳性对照;b:pSikCDPK1-GUS-1304实验组;c:常温下实验组对照
Fig. 5 Gus staining analysis and enzyme activity determin-ation under stress treatment A:Gus staining and enzyme activity after low temperature stress. B:Gus staining and enzyme activity after PEG stress. a:Positive control p35S-GUS-1304. b:Experimental group pSikCDPK1-GUS-1304. c:Experimental group control at room temperature
| [1] |
Li J, Liu HL, Xia WW, et al. De novo transcriptome sequencing and the hypothetical cold response mode of Saussurea involucrata in extreme cold environments[J]. Int J Mol Sci, 2017, 18(6):1155.
doi: 10.3390/ijms18061155 URL |
| [2] |
Singh A, Sagar S, Biswas DK. Calcium dependent protein kinase, a versatile player in plant stress management and development[J]. Crit Rev Plant Sci, 2017, 36(5/6):336-352.
doi: 10.1080/07352689.2018.1428438 URL |
| [3] |
Smale ST, Kadonaga JT. The RNA polymerase II core promoter[J]. Annu Rev Biochem, 2003, 72:449-479.
pmid: 12651739 |
| [4] | 田晓涵. 两种不同生境植物CDPK1基因的克隆及功能初探[D]. 石河子: 石河子大学, 2016. |
| Tian XH. Cloning and functional analysis of CDPK1 genes in two different habitats plants[D]. Shihezi: Shihezi University, 2016 | |
| [5] |
Liu YG, Chen YL. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences[J]. BioTechniques, 2007, 43(5):649-650, 652, 654 passim.
doi: 10.2144/000112601 URL |
| [6] |
Shi SJ, Li SG, Asim M, et al. The Arabidopsis calcium-dependent protein kinases(CDPKs)and their roles in plant growth regulation and abiotic stress responses[J]. Int J Mol Sci, 2018, 19(7):1900.
doi: 10.3390/ijms19071900 URL |
| [7] | Danino YM, Even D, Ideses D, et al. The core promoter:at the heart of gene expression[J]. Biochim Biophys Acta, 2015, 1849(8):1116-1131. |
| [8] | 李旭娟, 林秀琴, 字秋艳, 等. 甘蔗ScMOC1基因启动子的克隆与瞬时表达分析[J]. 植物遗传资源学报, 2019, 20(3):709-717. |
| Li XJ, Lin XQ, Zi QY, et al. Cloning and transient expression analysis of ScMOC1 promoter in sugarcane[J]. J Plant Genet Resour, 2019, 20(3):709-717. | |
| [9] |
Dang FF, Wang YN, She JJ, et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection[J]. Physiol Plant, 2014, 150(3):397-411.
doi: 10.1111/ppl.12093 URL |
| [10] |
Dang FF, Wang YN, Yu L, et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection[J]. Plant Cell Environ, 2013, 36(4):757-774.
doi: 10.1111/pce.12011 URL |
| [11] |
Eulgem T. Dissecting the WRKY web of plant defense regulators[J]. PLoS Pathog, 2006, 2(11):e126.
doi: 10.1371/journal.ppat.0020126 pmid: 17121464 |
| [12] |
Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling[J]. Curr Opin Plant Biol, 2007, 10(4):366-371.
pmid: 17644023 |
| [13] |
Knoth C, Ringler J, Dangl JL, et al. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica[J]. Mol Plant Microbe Interact, 2007, 20(2):120-128.
doi: 10.1094/MPMI-20-2-0120 URL |
| [14] |
Wang YN, Dang FF, Liu ZQ, et al. CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection[J]. Mol Plant Pathol, 2013, 14(2):131-144.
doi: 10.1111/j.1364-3703.2012.00836.x URL |
| [15] |
Bahn SC, Bae MS, Park YB, et al. Molecular cloning and characterization of a novel low temperature-induced gene, blti2, from barley(Hordeum vulgare L.)[J]. Biochim Biophys Acta, 2001, 1522(2):134-137.
pmid: 11750066 |
| [16] |
Chen A, Gusta LV, Brûlé-Babel A, et al. Varietal and chromosome 2H locus-specific frost tolerance in reproductive tissues of barley(Hordeum vulgare L. )detected using a frost simulation chamber[J]. Theor Appl Genet, 2009, 119(4):685-694.
doi: 10.1007/s00122-009-1079-1 URL |
| [17] |
Chen A, Reinheimer J, Brûlé-Babel A, et al. Genes and traits associated with chromosome 2H and 5H regions controlling sensitivity of reproductive tissues to frost in barley[J]. Theor Appl Genet, 2009, 118(8):1465-1476.
doi: 10.1007/s00122-009-0995-4 pmid: 19277599 |
| [18] |
Dunn MA, Goddard NJ, Zhang L, et al. Low-temperature-responsive barley genes have different control mechanisms[J]. Plant Mol Biol, 1994, 24(6):879-888.
pmid: 8204825 |
| [19] |
Ivashuta S, Naumkina M, Gau M, et al. Genotype-dependent transcriptional activation of novel repetitive elements during cold acclimation of alfalfa(Medicago sativa)[J]. Plant J, 2002, 31(5):615-627.
pmid: 12207651 |
| [20] |
Conforte AJ, Guimarães-Dias F, Neves-Borges AC, et al. Isolation and characterization of a promoter responsive to salt, osmotic and dehydration stresses in soybean[J]. Genet Mol Biol, 2017, 40(1 suppl 1):226-237.
doi: S1415-47572017000200226 pmid: 28350037 |
| [21] |
Alok A, Kaur J, Tiwari S. Functional characterization of wheat myo-inositol oxygenase promoter under different abiotic stress conditions in Arabidopsis thaliana[J]. Biotechnol Lett, 2020, 42(10):2035-2047.
doi: 10.1007/s10529-020-02967-1 URL |
| [22] |
Qian WJ, Xiao B, Wang L, et al. CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis[J]. BMC Plant Biol, 2018, 18(1):228.
doi: 10.1186/s12870-018-1456-5 URL |
| [1] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [2] | 陈中元, 王玉红, 代为俊, 张艳敏, 叶倩, 刘旭平, 谭文松, 赵亮. 柠檬酸铁铵对悬浮HEK293细胞转染的影响机制探究[J]. 生物技术通报, 2023, 39(9): 311-318. |
| [3] | 蒋铭轩, 李康, 罗亮, 刘建祥, 芦海平. 植物表达外源蛋白研究进展及展望[J]. 生物技术通报, 2023, 39(11): 110-122. |
| [4] | 董亚茹, 赵东晓, 耿兵, 李云芝, 王照红. 桑树MnERF2的表达分析[J]. 生物技术通报, 2022, 38(11): 112-121. |
| [5] | 林艳丽, 覃建兵, 伍翔, 王岩岩, 潘佑找, 柳忠玉. 虎杖PcMYB1启动子的克隆及其活性分析[J]. 生物技术通报, 2021, 37(5): 48-55. |
| [6] | 刘文浩, 王瑞丰, 刘琬琳, 许杰. 不同调控元件及组合对烟草外源蛋白瞬时表达的效果分析[J]. 生物技术通报, 2020, 36(7): 62-71. |
| [7] | 刘兆书, 刘元元, 秦玉杰, 毕权, 王赛赛, 祝建波. 天山雪莲SikCML7的克隆及表达分析[J]. 生物技术通报, 2019, 35(6): 48-54. |
| [8] | 胡积祥, 曹雅倩, 朱秀梅, 余超, 田芳, 杨凤环, 陈华民, 何晨阳. 基于瞬时表达系统的水稻miRNA靶基因快速验证系统的建立[J]. 生物技术通报, 2019, 35(10): 57-63. |
| [9] | 沈子又, 张超, 董彬, 付建新, 胡绍庆, 赵宏波. 桂花OfLCYB和OfLCYE启动子的克隆和活性分析[J]. 生物技术通报, 2018, 34(1): 137-143. |
| [10] | 张晓慧,韩榕. 两种瞬时表达体系研究拟南芥Profilin-1的亚细胞定位[J]. 生物技术通报, 2017, 33(5): 57-62. |
| [11] | 席海秀,艾可筠,佟少明. 普通小麦幼苗叶肉细胞原生质体分离方法的优化[J]. 生物技术通报, 2016, 32(4): 68-73. |
| [12] | 黎玉顺, 刘逸泠, 刘步仓, 穆建强, 祝建波. 新疆雪莲水孔蛋白sikPIP1基因的克隆与功能分析[J]. 生物技术通报, 2015, 31(9): 97-105. |
| [13] | 宿明星,孙颖颢,施鹤,李秋莉. 植物非生物胁迫相关转录因子研究方法[J]. 生物技术通报, 2015, 31(1): 51-60. |
| [14] | 池俊杰, 戴绍军, 魏建华, 王宏芝. 拟南芥原生质体瞬时表达快速验证重组酶外源基因删除[J]. 生物技术通报, 2014, 0(7): 86-92. |
| [15] | 赵欣梅, 吴树彪, 王亦学, 崔贵梅, 王晓清, 杜建中, 王小丽, 尚勇进, 孙毅. 花青素合成调控转录基因作为直观标记的载体构建及其在玉米幼胚中的瞬时表达[J]. 生物技术通报, 2014, 0(4): 77-82. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||