








生物技术通报 ›› 2023, Vol. 39 ›› Issue (4): 201-211.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0961
李怡君1( ), 吴晨晨1, 李睿1, 王喆2, 何山文3, 韦善君1(
), 吴晨晨1, 李睿1, 王喆2, 何山文3, 韦善君1( ), 张晓霞2(
), 张晓霞2( )
)
收稿日期:2022-08-08
出版日期:2023-04-26
发布日期:2023-05-16
通讯作者:
韦善君,女,博士,副教授,研究方向:农业资源微生物学;E-mail: wei.s.j@163.com;作者简介:李怡君,女,硕士研究生,研究方向:微生物学;E -mail:1624968646@qq.com吴晨晨为本文共同第一作者
基金资助:
LI Yi-jun1( ), WU Chen-chen1, LI Rui1, WANG Zhe2, HE Shan-wen3, WEI Shan-jun1(
), WU Chen-chen1, LI Rui1, WANG Zhe2, HE Shan-wen3, WEI Shan-jun1( ), ZHANG Xiao-xia2(
), ZHANG Xiao-xia2( )
)
Received:2022-08-08
Published:2023-04-26
Online:2023-05-16
摘要:
为了探究适用于分离水稻内生细菌的方案以获得水稻内细菌新资源,本研究基于培养组的方法,以水稻3个品种种子及幼苗为材料,从材料表面消毒、菌悬液的制备和培养基配比3个方面进行探究,并对分离获得代表性纯培养物的16S rRNA基因进行测序鉴定。结果表明,75%乙醇和含2%有效氯的次氯酸钠溶液组合对种子先后处理90 s和12 min,对植株组织先后处理90 s和5 min即可获得良好的消毒效果。水稻种子内生细菌量为105-106 CFU/g,其中90%-95%分布于颖壳内,不同品种或同一品种的不同生境来源的种子之间内生细菌群落组成有明显差异;在制备种子菌悬液时需注意控制研磨程度以获得种类丰富的菌落,剥去颖壳有利于分离培养糙米中的内生菌。对于根、茎、叶内生细菌的分离,需要根据材料不同控制接种菌悬液的浓度以获得其中微量的内生细菌;在培养基中添加组织浸提液和延长培养时间至两周以上,可促进根、茎、叶内生细菌的分离培养。采用调整后的方案,本研究对3个水稻品种的种子和它们的植株根、茎、叶内生细菌进行分离培养,分别完成了71、14、10和2个代表性纯培养物的16S rRNA基因测序鉴定,其中有8株与已知类群模式菌株同源序列相似度在97.22%-98.74%,新种获得率为8.25%。研究结果为分离培养水稻内生细菌新资源提供了方法参考。
李怡君, 吴晨晨, 李睿, 王喆, 何山文, 韦善君, 张晓霞. 水稻内生细菌新资源分离培养方案探究[J]. 生物技术通报, 2023, 39(4): 201-211.
LI Yi-jun, WU Chen-chen, LI Rui, WANG Zhe, HE Shan-wen, WEI Shan-jun, ZHANG Xiao-xia. Exploring Cultivation Approaches for New Endophytic Bacterial Resource in Oryza sativa[J]. Biotechnology Bulletin, 2023, 39(4): 201-211.
| 材料 Materials | 处理时间 Treatment time/min | ||
|---|---|---|---|
| 75%乙醇 75% ethanol | NaClO溶液 NaClO solution | ||
| 种子、植株组织Seeds, and plant tissues | 1.5 | 12 | |
| 种子 Seeds | 1.5 | 15 | |
| 种子 Seeds | 3 | 12 | |
| 种子 Seeds | 3 | 15 | |
| 植株组织 Plant tissues | 1.5 | 5 | |
表1 材料表面消毒方案
Table 1 Surface sterilization protocol
| 材料 Materials | 处理时间 Treatment time/min | ||
|---|---|---|---|
| 75%乙醇 75% ethanol | NaClO溶液 NaClO solution | ||
| 种子、植株组织Seeds, and plant tissues | 1.5 | 12 | |
| 种子 Seeds | 1.5 | 15 | |
| 种子 Seeds | 3 | 12 | |
| 种子 Seeds | 3 | 15 | |
| 植株组织 Plant tissues | 1.5 | 5 | |
| 菌落类型 Colony type | 形状和色泽 Shape and color | 直径 Diameter/mm |
|---|---|---|
| I | 黄色,圆形Yellow and round | 3-5 |
| Ⅱ | 黄色,圆形Yellow and round | 1-3 |
| Ⅲ | 中间黄色,边缘浅白Yellow in the middle, and light white on the edge | 3-5 |
| Ⅳ | 白色,圆形White and round | 3-5 |
| Ⅴ | 白色,圆形White and round | 1-3 |
| Ⅵ | 粉红色,圆形Pink and round | 1-3 |
| Ⅶ | 橙色,圆形Orange and round | 3-5 |
表2 水稻种子内生细菌菌落类型
Table 2 Colony types of endophytic bacteria in rice seeds
| 菌落类型 Colony type | 形状和色泽 Shape and color | 直径 Diameter/mm |
|---|---|---|
| I | 黄色,圆形Yellow and round | 3-5 |
| Ⅱ | 黄色,圆形Yellow and round | 1-3 |
| Ⅲ | 中间黄色,边缘浅白Yellow in the middle, and light white on the edge | 3-5 |
| Ⅳ | 白色,圆形White and round | 3-5 |
| Ⅴ | 白色,圆形White and round | 1-3 |
| Ⅵ | 粉红色,圆形Pink and round | 1-3 |
| Ⅶ | 橙色,圆形Orange and round | 3-5 |
| 品种 Cultivar | 种子部位 Seed part | 稀释度 Dilution | 菌落数/皿* CFU/ dish * | 内生细菌总数 Total number of endophytic bacteria/(CFU·g-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Ⅱ | Ⅲ | Ⅳ | Ⅴ | Ⅵ | Ⅶ | 总数 Total | ||||
| 日本晴 Nipponbare | 完整种子 Intact seeds | 10-2 | 26 | 69 | 31 | 9 | 227 | 83 | 0 | 445 | 3.2×105 |
| 10-3 | 10 | 8 | 14 | 1 | 7 | 23 | 0 | 63 | |||
| 糙米 Brown rice | 10-1 | 78 | 0 | 0 | 0 | 4 | 178 | 0 | 260 | 1.3×104 | |
| 10-2 | 24 | 6 | 0 | 0 | 1 | 63 | 0 | 94 | |||
| 日本晴-LF Nipponbare-LF | 完整种子 Intact seeds | 10-2 | 24 | 0 | 0 | 9 | 260 | 1 | 0 | 293 | 1.5×105 |
| 10-3 | 9 | 0 | 0 | 0 | 68 | 0 | 0 | 77 | |||
| 糙米 Brown rice | 10-1 | 1 | 2 | 0 | 1 | 19 | 139 | 6 | 168 | 8.5×103 | |
| 10-2 | 6 | 0 | 0 | 9 | 0 | 35 | 0 | 50 | |||
| 培矮64 Peiai 64 | 完整种子 Intact seeds | 10-2 | 220 | 40 | 0 | 0 | 376 | 2 | 0 | 638 | 1.6×106 |
| 10-3 | 119 | 35 | 0 | 0 | 147 | 13 | 0 | 314 | |||
| 糙米 Brown rice | 10-1 | 240 | 40 | 0 | 10 | 64 | 0 | 0 | 354 | 1.2×105 | |
| 10-2 | 143 | 37 | 0 | 30 | 21 | 1 | 0 | 232 | |||
| Kashlath | 完整种子 Intact seeds | 10-2 | 44 | 144 | 0 | 43 | 653 | 0 | 0 | 884 | 8.0×105 |
| 10-3 | 24 | 5 | 0 | 8 | 121 | 0 | 0 | 158 | |||
| 糙米 Brown rice | 10-1 | 530 | 9 | 0 | 0 | 0 | 25 | 0 | 564 | 9.0×103 | |
| 10-2 | 120 | 10 | 0 | 0 | 0 | 60 | 0 | 180 | |||
表3 水稻种子内生菌数量
Table 3 Amount of endophytic bacteria in rice seeds
| 品种 Cultivar | 种子部位 Seed part | 稀释度 Dilution | 菌落数/皿* CFU/ dish * | 内生细菌总数 Total number of endophytic bacteria/(CFU·g-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Ⅱ | Ⅲ | Ⅳ | Ⅴ | Ⅵ | Ⅶ | 总数 Total | ||||
| 日本晴 Nipponbare | 完整种子 Intact seeds | 10-2 | 26 | 69 | 31 | 9 | 227 | 83 | 0 | 445 | 3.2×105 |
| 10-3 | 10 | 8 | 14 | 1 | 7 | 23 | 0 | 63 | |||
| 糙米 Brown rice | 10-1 | 78 | 0 | 0 | 0 | 4 | 178 | 0 | 260 | 1.3×104 | |
| 10-2 | 24 | 6 | 0 | 0 | 1 | 63 | 0 | 94 | |||
| 日本晴-LF Nipponbare-LF | 完整种子 Intact seeds | 10-2 | 24 | 0 | 0 | 9 | 260 | 1 | 0 | 293 | 1.5×105 |
| 10-3 | 9 | 0 | 0 | 0 | 68 | 0 | 0 | 77 | |||
| 糙米 Brown rice | 10-1 | 1 | 2 | 0 | 1 | 19 | 139 | 6 | 168 | 8.5×103 | |
| 10-2 | 6 | 0 | 0 | 9 | 0 | 35 | 0 | 50 | |||
| 培矮64 Peiai 64 | 完整种子 Intact seeds | 10-2 | 220 | 40 | 0 | 0 | 376 | 2 | 0 | 638 | 1.6×106 |
| 10-3 | 119 | 35 | 0 | 0 | 147 | 13 | 0 | 314 | |||
| 糙米 Brown rice | 10-1 | 240 | 40 | 0 | 10 | 64 | 0 | 0 | 354 | 1.2×105 | |
| 10-2 | 143 | 37 | 0 | 30 | 21 | 1 | 0 | 232 | |||
| Kashlath | 完整种子 Intact seeds | 10-2 | 44 | 144 | 0 | 43 | 653 | 0 | 0 | 884 | 8.0×105 |
| 10-3 | 24 | 5 | 0 | 8 | 121 | 0 | 0 | 158 | |||
| 糙米 Brown rice | 10-1 | 530 | 9 | 0 | 0 | 0 | 25 | 0 | 564 | 9.0×103 | |
| 10-2 | 120 | 10 | 0 | 0 | 0 | 60 | 0 | 180 | |||
| 品种 Cultivar | 部位 Seed part | 成功鉴定菌株数量 Number of identified strains | 种的数量 Number of species | 部位共有种数量 Number of site-common species | 部位特有种数量 Number of site-specific species |
|---|---|---|---|---|---|
| 日本晴 Nipponbare | 完整种子 Intact seed | 24 | 6 | 1 | 5 |
| 糙米 Brown rice | 2 | 2 | 1 | ||
| 日本晴-LF Nipponbare-LF | 完整种子 Intact seed | 7 | 4 | 1 | 3 |
| 糙米 Brown rice | 2 | 1 | 0 | ||
| 培矮64 Peiai 64 | 完整种子 Intact seed | 19 | 6 | 3 | 3 |
| 糙米 Brown rice | 6 | 4 | 1 | ||
| Kashlath | 完整种子 Intact seed | 6 | 3 | 1 | 2 |
| 糙米 Brown rice | 5 | 3 | 2 |
表4 代表性水稻种子内生菌鉴定结果概况
Table 4 An overview of the representative endophytic bacteria in rice seeds
| 品种 Cultivar | 部位 Seed part | 成功鉴定菌株数量 Number of identified strains | 种的数量 Number of species | 部位共有种数量 Number of site-common species | 部位特有种数量 Number of site-specific species |
|---|---|---|---|---|---|
| 日本晴 Nipponbare | 完整种子 Intact seed | 24 | 6 | 1 | 5 |
| 糙米 Brown rice | 2 | 2 | 1 | ||
| 日本晴-LF Nipponbare-LF | 完整种子 Intact seed | 7 | 4 | 1 | 3 |
| 糙米 Brown rice | 2 | 1 | 0 | ||
| 培矮64 Peiai 64 | 完整种子 Intact seed | 19 | 6 | 3 | 3 |
| 糙米 Brown rice | 6 | 4 | 1 | ||
| Kashlath | 完整种子 Intact seed | 6 | 3 | 1 | 2 |
| 糙米 Brown rice | 5 | 3 | 2 |
| 植物组织Plant tissue | 培养基Culture medium | 菌悬液浓度Concentration of bacterial suspension | 菌落数/皿CFU/dish |
|---|---|---|---|
| 根Root | 基础培养基Basic culture medium | 10-1 | 0-5 |
| 5×10-1 | 20-50 | ||
| 组织浸提液Tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 20-50 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 5-10 | |
| 5×10-1 | 20-50 | ||
| 茎Stem | 基础培养基Basic culture medium | 10-1 | 0 |
| 5×10-1 | 5-20 | ||
| 组织浸提液Tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 5-20 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 5-10 | |
| 5×10-1 | 20-50 | ||
| 叶Leaf | 基础培养基Basic culture medium | 10-1 | 0 |
| 5×10-1 | 0 | ||
| 原浆 Protoplasm | 0-2 | ||
| 组织浸提液Tissue extract | 10-1 | 0 | |
| 5×10-1 | 0 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 5-10 |
表5 培养基和菌悬液浓度对植株内生菌分离效果的影响
Table 5 Effects of culture medium and bacterial suspension concentration on the isolation of endophytic bacteria in plant tissues
| 植物组织Plant tissue | 培养基Culture medium | 菌悬液浓度Concentration of bacterial suspension | 菌落数/皿CFU/dish |
|---|---|---|---|
| 根Root | 基础培养基Basic culture medium | 10-1 | 0-5 |
| 5×10-1 | 20-50 | ||
| 组织浸提液Tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 20-50 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 5-10 | |
| 5×10-1 | 20-50 | ||
| 茎Stem | 基础培养基Basic culture medium | 10-1 | 0 |
| 5×10-1 | 5-20 | ||
| 组织浸提液Tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 5-20 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 5-10 | |
| 5×10-1 | 20-50 | ||
| 叶Leaf | 基础培养基Basic culture medium | 10-1 | 0 |
| 5×10-1 | 0 | ||
| 原浆 Protoplasm | 0-2 | ||
| 组织浸提液Tissue extract | 10-1 | 0 | |
| 5×10-1 | 0 | ||
| 基础培养基+组织浸提液 Basic culture medium + tissue extract | 10-1 | 0-5 | |
| 5×10-1 | 5-10 |
| 菌落类型 Colony type | 形状和色泽 Shape and color | 直径 Diameter/mm |
|---|---|---|
| A | 橙色,圆形 Orange and round | 1-3 |
| B | 白色,圆形 White and round | 1-3 |
| C | 青色,圆形 Cyan and round | 1-3 |
| D | 黄棕色,圆形 Yellowish brown and round | 3-5 |
| E | 透明,圆形 Transparent and round | 1-3 |
| F | 白色,圆形,表面干燥 White, round, and dry surface | 1-3 |
| G | 黄色,圆形 Yellow and round | 1-3 |
| H | 中央黄色,边缘透明,圆形 Yellow center, transparent edge, and round | >5 |
表6 水稻植株内生菌菌落分类
Table 6 Colony types of endophyic bacteria from rice plants
| 菌落类型 Colony type | 形状和色泽 Shape and color | 直径 Diameter/mm |
|---|---|---|
| A | 橙色,圆形 Orange and round | 1-3 |
| B | 白色,圆形 White and round | 1-3 |
| C | 青色,圆形 Cyan and round | 1-3 |
| D | 黄棕色,圆形 Yellowish brown and round | 3-5 |
| E | 透明,圆形 Transparent and round | 1-3 |
| F | 白色,圆形,表面干燥 White, round, and dry surface | 1-3 |
| G | 黄色,圆形 Yellow and round | 1-3 |
| H | 中央黄色,边缘透明,圆形 Yellow center, transparent edge, and round | >5 |
| 分离物编号 Isolate ID | 来源 Source | 最高匹配种 Top-hit taxon | 模式菌株编号 Top-hit strain | 相似率 Similarity/% | 菌落类型 Colony type |
|---|---|---|---|---|---|
| SD-7 | 根、茎 Roots and stems | Chitinophaga ginsengihumi | SR18 | 98.18 | B |
| SD-9 | 根、茎 Roots and stems | Sphingomonas Canadensis | FWC47(T) | 97.35 | G |
| SD-10 | 茎 Stems | Diaminobutyricibacter tongyongensis | KIS66-7(T) | 98.74 | G |
| SD-11 | 茎Stems | Hephaestia caeni | DSM 25527(T) | 97.59 | B |
| SD-22 | 根Roots | Phycicoccus endophyticus | IP6SC6 | 98.41 | B |
| SD-26 | 茎Stems | Sphingomonas astaxanthinifaciens | DSM 22298 | 97.56 | A |
表7 水稻植株内生细菌潜在新种
Table 7 Potential new species of endophytic bacteria in rice plants
| 分离物编号 Isolate ID | 来源 Source | 最高匹配种 Top-hit taxon | 模式菌株编号 Top-hit strain | 相似率 Similarity/% | 菌落类型 Colony type |
|---|---|---|---|---|---|
| SD-7 | 根、茎 Roots and stems | Chitinophaga ginsengihumi | SR18 | 98.18 | B |
| SD-9 | 根、茎 Roots and stems | Sphingomonas Canadensis | FWC47(T) | 97.35 | G |
| SD-10 | 茎 Stems | Diaminobutyricibacter tongyongensis | KIS66-7(T) | 98.74 | G |
| SD-11 | 茎Stems | Hephaestia caeni | DSM 25527(T) | 97.59 | B |
| SD-22 | 根Roots | Phycicoccus endophyticus | IP6SC6 | 98.41 | B |
| SD-26 | 茎Stems | Sphingomonas astaxanthinifaciens | DSM 22298 | 97.56 | A |
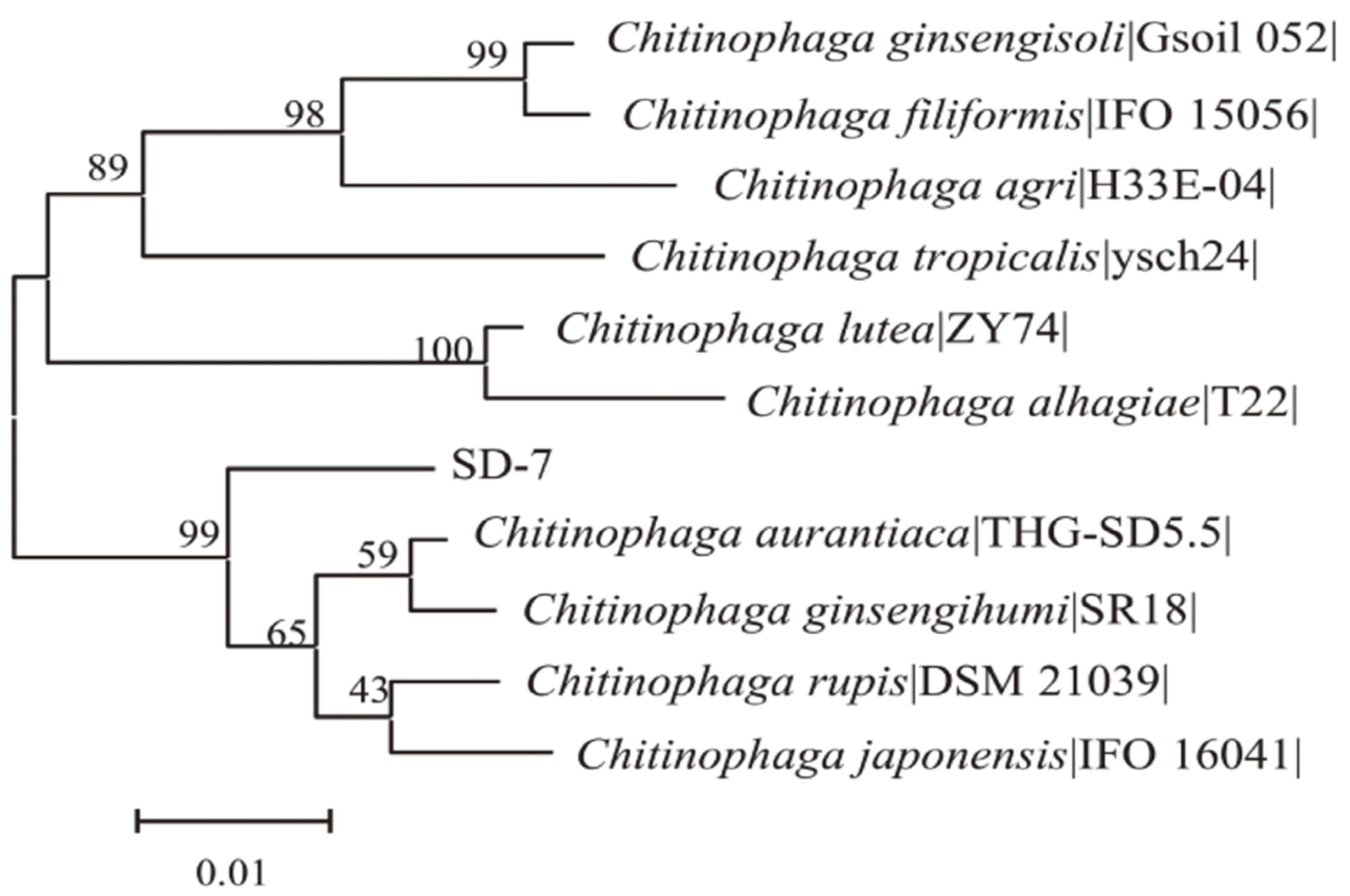
图3 依据 16S rRNA 基因序列构建的SD-7相关菌株的N-J系统发育树
Fig. 3 Neighbor-joining phylogenetic tree of bacterial stra-ins related to isolates SD-7 based on 16S rRNA gene sequences

图4 依据 16S rRNA 基因序列构建的SD-10相关菌株的N-J系统发育树
Fig. 4 Neighbor-joining phylogenetic tree of bacterial stra-ins related to isolates SD-10 based on 16S rRNA gene sequences

图5 依据 16S rRNA 基因序列构建的SD-11相关菌株的N-J系统发育树
Fig. 5 Neighbor-joining phylogenetic tree of bacterial stra-ins related to isolates SD-11 based on 16S rRNA gene sequences

图6 依据 16S rRNA 基因序列构建的SD-22相关菌株的N-J系统发育树
Fig. 6 Neighbor-joining phylogenetic tree of bacterial str-ains related to isolates SD-22 based on 16S rRNA gene sequences

图7 依据16S rRNA 基因序列构建的SD-9、SD-26、SD-29、SD-30相关菌株的N-J 系统发育树
Fig. 7 Neighbor-joining phylogenetic tree of bacterial str-ains related to isolates SD-9, SD-26, SD-29 and SD-30 based on 16S rRNA gene sequences
| [1] |
Baedke J, et al. The holobiont concept before Margulis[J]. J Exp Zool B Mol Dev Evol, 2020, 334(3): 149-155.
doi: 10.1002/jez.b.v334.3 URL |
| [2] |
Ali M, Ali Q, Sohail MA, et al. Diversity and taxonomic distribution of endophytic bacterial community in the rice plant and its prospective[J]. Int J Mol Sci, 2021, 22(18): 10165.
doi: 10.3390/ijms221810165 URL |
| [3] |
Lyu DM, Zajonc J, Pagé A, et al. Plant holobiont theory: the phytomicrobiome plays a central role in evolution and success[J]. Microorganisms, 2021, 9(4): 675.
doi: 10.3390/microorganisms9040675 URL |
| [4] |
Puente ME, Li CY, Bashan Y. Endophytic bacteria in cacti seeds can improve the development of cactus seedlings[J]. Environ Exp Bot, 2009, 66(3): 402-408.
doi: 10.1016/j.envexpbot.2009.04.007 URL |
| [5] |
ALKahtani MDF, Fouda A, Attia KA, et al. Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture[J]. Agronomy, 2020, 10(9): 1325.
doi: 10.3390/agronomy10091325 URL |
| [6] |
Mahgoub HAM, Fouda A, Eid AM, et al. Biotechnological application of plant growth-promoting endophytic bacteria isolated from halophytic plants to ameliorate salinity tolerance of Vicia faba L[J]. Plant Biotechnol Rep, 2021, 15(6): 819-843.
doi: 10.1007/s11816-021-00716-y |
| [7] |
Afzal I, Shinwari ZK, Sikandar S, et al. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants[J]. Microbiol Res, 2019, 221: 36-49.
doi: S0944-5013(18)30459-2 pmid: 30825940 |
| [8] |
祖国蔷, 胡哲, 王琪, 等. Burkholderia sp. GD17对水稻幼苗镉耐受的调节[J]. 生物技术通报, 2022, 38(4): 153-162.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0915 |
| Zu GQ, Hu Z, Wang Q, et al. Regulatory role of Burkholderia sp. GD17 in rice seedling’s responses to cadmium stress[J]. Biotechnol Bull, 2022, 38(4): 153-162. | |
| [9] | 刘江苇, 刘颖, 徐婷, 等. 水稻内生菌研究进展及展望[J]. 生命科学研究, 2021, 25(3): 232-239. |
| Liu JW, Liu Y, Xu T, et al. Advances and prospects of rice endophytes[J]. Life Sci Res, 2021, 25(3): 232-239. | |
| [10] |
王志山, 黎妮, 等. 水稻种子内生细菌研究进展[J]. 生物技术通报, 2022, 38(1): 236-246.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0437 |
| Wang ZS, Li N, et al. Research progress in endophytic bacteria in rice seeds[J]. Biotechnol Bull, 2022, 38(1): 236-246. | |
| [11] | 刘云霞, 张青文, 周明牂. 水稻体内细菌的动态研究[J]. 应用生态学报, 1999, 10(6): 735-738. |
| Liu YX, Zhang QW, Zhou MZ. Population dynamics of endophytic bacteria in symptom free rice plants[J]. Chin J Appl Ecol, 1999, 10(6): 735-738. | |
| [12] | 沙月霞. 不同水稻组织内生细菌的群落多样性[J]. 微生物学报, 2018, 58(12): 2216-2228. |
| Sha YX. Diversity of bacterial endophytic community in different rice tissues[J]. Acta Microbiol Sin, 2018, 58(12): 2216-2228. | |
| [13] | 王雪君, 等. 水稻4个生长时期茎部可培养内生菌多样性分析[J]. 热带作物学报, 2015, 36(6): 1078-1085. |
| Wang XJ, et al. Diversity of culturable endobacterial communities in rice(Oryza sativa L.) stem at different growth stages[J]. Chin J Trop Crops, 2015, 36(6): 1078-1085. | |
| [14] | 李南南, 等. 3个杂交水稻亲本成熟期种子内生细菌多样性研究[J]. 食品科学技术学报, 2017, 35(4): 56-64. |
| Li NN, et al. Diversity of endophytic bacterial communities in three parental seeds of hybrid rice(Oryza sativa L.) at maturity stage[J]. J Food Sci Technol, 2017, 35(4): 56-64. | |
| [15] |
Walitang DI, Kim CG, et al. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars[J]. BMC Plant Biol, 2018, 18(1): 51.
doi: 10.1186/s12870-018-1261-1 pmid: 29587643 |
| [16] |
Ferrando L, et al. Strong shift in the diazotrophic endophytic bacterial community inhabiting rice(Oryza sativa)plants after flooding[J]. FEMS Microbiol Ecol, 2015, 91(9): fiv104.
doi: 10.1093/femsec/fiv104 URL |
| [17] |
Bertani I, Abbruscato P, Piffanelli P, et al. Rice bacterial endophytes: isolation of a collection, identification of beneficial strains and microbiome analysis[J]. Environ Microbiol Rep, 2016, 8(3): 388-398.
doi: 10.1111/1758-2229.12403 URL |
| [18] |
Verma SK, Kingsley K, Irizarry I, et al. Seed-vectored endophytic bacteria modulate development of rice seedlings[J]. J Appl Microbiol, 2017, 122(6): 1680-1691.
doi: 10.1111/jam.13463 pmid: 28375579 |
| [19] |
Walitang DI, et al. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice[J]. BMC Microbiol, 2017, 17(1): 209.
doi: 10.1186/s12866-017-1117-0 pmid: 29073903 |
| [20] |
Shahzad R, Waqas M, Khan AL, et al. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa[J]. Plant Physiol Biochem, 2016, 106: 236-243.
doi: 10.1016/j.plaphy.2016.05.006 URL |
| [21] |
Krishnamoorthy A, Agarwal T, Kotamreddy JNR, et al. Impact of seed-transmitted endophytic bacteria on intra- and inter-cultivar plant growth promotion modulated by certain sets of metabolites in rice crop[J]. Microbiol Res, 2020, 241: 126582.
doi: 10.1016/j.micres.2020.126582 URL |
| [22] |
Alain K, Querellou J. Cultivating the uncultured: limits, advances and future challenges[J]. Extremophiles, 2009, 13(4): 583-594.
doi: 10.1007/s00792-009-0261-3 pmid: 19548063 |
| [23] |
Sun L, Qiu FB, Zhang XX, et al. Endophytic bacterial diversity in rice(Oryza sativa L.) roots estimated by 16S rDNA sequence analysis[J]. Microb Ecol, 2008, 55(3): 415-424.
doi: 10.1007/s00248-007-9287-1 URL |
| [24] |
Eevers N, Gielen M, et al. Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media[J]. Microb Biotechnol, 2015, 8(4): 707-715.
doi: 10.1111/1751-7915.12291 pmid: 25997013 |
| [25] | 孟炯放. 功能性内生菌的筛选及其对水稻幼苗生长的影响[D]. 保定: 河北农业大学, 2019. |
| Meng JF. Screening of functional endophytic bacteria and effects on the growth of rice seedlings[D]. Baoding: Hebei Agricultural University, 2019. | |
| [26] |
Kim M, Oh HS, Park SC, et al. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes[J]. Int J Syst Evol Microbiol, 2014, 64(Pt 2): 346-351.
doi: 10.1099/ijs.0.059774-0 URL |
| [27] | 赵霞, 等. 长期稻-稻-紫云英轮作对水稻种子内生细菌的影响及促生功能评价[J]. 中国土壤与肥料, 2020(1): 208-215. |
| Zhao X, et al. Effects of long-term rice-rice-milk vetch rotation on rice seeds endophytic bacteria and evaluation of growth promoting function[J]. Soil Fertil Sci China, 2020(1): 208-215. | |
| [28] |
Mano H, et al. Culturable surface and endophytic bacterial flora of the maturing seeds of rice plants(Oryza sativa)cultivated in a paddy field[J]. Microb Environ, 2006, 21(2): 86-100.
doi: 10.1264/jsme2.21.86 URL |
| [29] |
Mano H, et al. Culturable endophytic bacterial flora of the maturing leaves and roots of rice plants(Oryza sativa)cultivated in a paddy field[J]. Microb Environ, 2007, 22(2): 175-185.
doi: 10.1264/jsme2.22.175 URL |
| [30] | 王伯勋, 等. 水稻不同生长时期不同组织中抗砷内生菌的筛选与鉴定[J]. 环境科学, 2018, 39(5): 2464-2471. |
| Wang BX, et al. Screening and identification of arsenic-resistant endophytic bacteria from different rice tissues(Oryza sativa L.) in different growth stages[J]. Environ Sci, 2018, 39(5): 2464-2471. | |
| [31] |
Yu YE, Chen ZM, Xie HT, et al. Overhauling the effect of surface sterilization on analysis of endophytes in tea plants[J]. Front Plant Sci, 2022, 13: 849658.
doi: 10.3389/fpls.2022.849658 URL |
| [32] |
Prakamhang J, Minamisawa K, Teamtaisong K, et al. The communities of endophytic diazotrophic bacteria in cultivated rice(Oryza sativa L.)[J]. Appl Soil Ecol, 2009, 42(2): 141-149.
doi: 10.1016/j.apsoil.2009.02.008 URL |
| [33] | 王伟平, 李南南, 黎妮, 等. 基于微生物分离培养的超级杂交水稻深两优5814种子内生细菌多样性研究[J]. 杂交水稻, 2016, 31(4): 61-66. |
| Wang WP, Li NN, Li N, et al. Diversity of endophytic bacterial communities in seeds of super hybrid rice shenliangyou 5814 by traditional microbial culture technique[J]. Hybrid Rice, 2016, 31(4): 61-66. | |
| [34] |
Nalin R, Simonet P, Vogel TM, et al. Rhodanobacter lindaniclasticus gen. nov., sp. nov., a lindane-degrading bacterium[J]. Int J Syst Bacteriol, 1999, 49 Pt 1: 19-23.
pmid: 10028243 |
| [35] |
Cho GY, Lee JC, Whang KS. Rhodanobacter rhizosphaerae sp. nov., isolated from soil of ginseng rhizosphere[J]. Int J Syst Evol Microbiol, 2017, 67(5): 1387-1392.
doi: 10.1099/ijsem.0.001825 URL |
| [36] |
Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes[J]. Appl Soil Ecol, 2012, 61: 217-224.
doi: 10.1016/j.apsoil.2011.09.011 URL |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [3] | 李雪琪, 张素杰, 于曼, 黄金光, 周焕斌. 基于CRISPR/CasX介导的水稻基因组编辑技术的建立[J]. 生物技术通报, 2023, 39(9): 40-48. |
| [4] | 吴元明, 林佳怡, 柳雨汐, 李丹婷, 张宗琼, 郑晓明, 逄洪波. 基于BSA-seq和RNA-seq挖掘水稻株高相关QTL[J]. 生物技术通报, 2023, 39(8): 173-184. |
| [5] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [6] | 姚莎莎, 王晶晶, 王俊杰, 梁卫红. 植物激素信号通路调控水稻粒型的分子机制[J]. 生物技术通报, 2023, 39(8): 80-90. |
| [7] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [8] | 任沛东, 彭健玲, 刘圣航, 姚姿婷, 朱桂宁, 陆光涛, 李瑞芳. 沙福芽孢杆菌GX-H6的分离鉴定及对水稻细菌性条斑病的防病效果[J]. 生物技术通报, 2023, 39(5): 243-253. |
| [9] | 卢振万, 李雪琪, 黄金光, 周焕斌. 利用胞嘧啶碱基编辑技术创制耐草甘膦水稻[J]. 生物技术通报, 2023, 39(2): 63-69. |
| [10] | 杨茂, 林宇丰, 戴阳朔, 潘素君, 彭伟业, 严明雄, 李魏, 王冰, 戴良英. OsDIS1通过抗氧化途径负调控水稻耐旱性[J]. 生物技术通报, 2023, 39(2): 88-95. |
| [11] | 蒋铭轩, 李康, 罗亮, 刘建祥, 芦海平. 植物表达外源蛋白研究进展及展望[J]. 生物技术通报, 2023, 39(11): 110-122. |
| [12] | 邹兰, 王茜, 李慕仪, 叶坤浩, 黄晶. 乌头内生细菌JY-3-1R的鉴定及其生防和促生能力研究[J]. 生物技术通报, 2023, 39(10): 246-255. |
| [13] | 姜南, 石杨, 赵志慧, 李斌, 赵熠辉, 杨俊彪, 闫家铭, 靳雨璠, 陈稷, 黄进. 镉胁迫下水稻OsPT1的表达及功能分析[J]. 生物技术通报, 2023, 39(1): 166-174. |
| [14] | 陈光, 李佳, 杜瑞英, 王旭. 水稻盐敏感突变体ss2的鉴定与基因功能分析[J]. 生物技术通报, 2022, 38(9): 158-166. |
| [15] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||