








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 86-99.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0663
收稿日期:2023-07-12
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
姜艳军,男,博士,教授,研究方向:生物催化与转化、环境友好的化工过程;E-mail: yanjunjiang@hebut.edu.cn作者简介:李亮,女,博士,副教授,研究方向:生物工程;E-mail: liangli@hebut.edu.cn
基金资助:
LI Liang( ), XU Shan-shan, JIANG Yan-jun(
), XU Shan-shan, JIANG Yan-jun( )
)
Received:2023-07-12
Published:2024-01-26
Online:2024-02-06
摘要:
麦角硫因(ergothioneine, ERG)作为一种稀有的天然含硫组氨酸衍生物,已被证明具有强大的抗氧化性和诸多生物学功能。因此,ERG受到越来越多研究人员和产品开发人员的关注。目前,ERG已被广泛应用于食品、化妆品和医疗等行业。研究表明只有少数细菌和真菌可体内合成ERG,植物、动物和人类自身均不能直接合成ERG,只能从其他来源获取。ERG可通过生物提取法、化学合成法以及生物合成法获得,但由于传统生产方式(生物提取法和化学合成法)存在产量低、生产效率差和生产成本较高等问题,限制了该产品的规模化生产和应用。因此,亟需开发一种高效、经济且安全、可靠的ERG生产方式以满足市场的需求。近年来合成生物学快速发展,利用基因工程、蛋白质工程和代谢工程等技术提高生物合成法生产ERG的能力已逐渐成为研究热点。本文将论述ERG的生物学活性和功能,介绍ERG生物合成途径和ERG在食品、化妆品和医疗等行业的应用前景,比较ERG主要的生产方式,总结并梳理近年来采取各种工程策略通过生物合成法生产ERG的研究进展;并就如何利用基因工程、蛋白质工程和代谢工程提高ERG产量提出几点工程策略,以期为生物合成法高产ERG提供理论参考和研究思路。
李亮, 徐姗姗, 姜艳军. 生物合成法生产麦角硫因的研究进展[J]. 生物技术通报, 2024, 40(1): 86-99.
LI Liang, XU Shan-shan, JIANG Yan-jun. Research Progress in the Production of Ergothioneine by Biosynthesis[J]. Biotechnology Bulletin, 2024, 40(1): 86-99.
| 生产方式 Production method | 分类 Classification | 优点 Advantages | 缺点 Disadvantages | 参考文献 Reference |
|---|---|---|---|---|
| 生物提取法 Bioextraction | 回流提取法 Reflux extraction | 针对性强,收率较高 High pertinence, and high yield | 相对耗时Relatively time-consuming | [ |
| 酶解提取法 Enzymatic extraction | 提取速度快,条件温和 Fast extraction speed, and moderate conditions | 酶活范围较窄,提取条件苛刻 Narrow enzyme activity range, hash extraction condition | [ | |
| 超声微波联合法 Ultrasonic and microwave extraction | 减少萃取溶剂和能耗,提取效率高 Lower extraction solvent and energy consumption, and high extraction efficiency | 产量低 Low yield | [ | |
| 化学合成法 Chemical synthesis | 路线(1)和(2)Route(1)and(2) | —— | 路线冗长复杂,反应温度较高,资源浪费,收率低 Long and complex route, high reaction temperature, waste of resource, and low yield | [ |
| 路线(3)Route(3) | 收率高于路线(1)和(2)Yields higher than that in route(1)and(2) | 原料昂贵;中间体纯化使用两次反向层析柱,导致成本增加;毫克级别High feedstock cost; intermediate purification uses two reverse chromatography columns, which increases cost; milligram level | [ | |
| 路线(4)Route(4) | “一锅法”制备,路线短;无中间纯化过程 Prepared by one-pot method, short route; no intermediate purification | 原料昂贵且来源少 Expensive and few feedstock | [ | |
| 路线(5)Route(5) | 操作简单,原料低廉易得,步骤简短,条件相对温和可控,产量较高 Simple operation, cheap and readily available feedstock, short route, relatively moderate and controllable conditions, and high yield | 使用具有危害性的化学试剂,增加废液和废物处理成本 Use hazardous chemical reagents, increase waste liquid and waste disposal costs | [ | |
| 生物合成法 Biosynthesis | 微生物液体发酵Microbial liquid fermentation | 可食用菌发酵,天然属性,安全性高 Edible mushroom fermentation, natural properties and high safety | 发酵周期长,产率低 Long fermentation period, and low yield | [ |
| 生物转化 Biotransformation | 直接以前体氨基酸作为底物,原料成本低;工艺简单;产品浓度较高 Direct precursor amino acids as substrates, low feedstock cost; simple technology; higher product concentration | 产率低;表达复合酶体外催化,经济适用性差 Low yield, in vitro catalysis of expression complex enzymes, and low economic practicality | [ | |
| ERG工程菌株的发酵Fermentation of ERG engineered strains | 以常见菌株作为底盘菌,方便获得;减少外源添加,原料成本低;无有害试剂,环境友好;发酵周期短;操作简单 Common strains are chassis bacteria, which are readily available; reduce exogenous additions, low feedstock cost; no hazardous chemical reagents, environment-friendly; short fermentation period; simple operation | - | [ |
表1 麦角硫因生产方式对比
Table 1 Comparison of ergothioneine production methods
| 生产方式 Production method | 分类 Classification | 优点 Advantages | 缺点 Disadvantages | 参考文献 Reference |
|---|---|---|---|---|
| 生物提取法 Bioextraction | 回流提取法 Reflux extraction | 针对性强,收率较高 High pertinence, and high yield | 相对耗时Relatively time-consuming | [ |
| 酶解提取法 Enzymatic extraction | 提取速度快,条件温和 Fast extraction speed, and moderate conditions | 酶活范围较窄,提取条件苛刻 Narrow enzyme activity range, hash extraction condition | [ | |
| 超声微波联合法 Ultrasonic and microwave extraction | 减少萃取溶剂和能耗,提取效率高 Lower extraction solvent and energy consumption, and high extraction efficiency | 产量低 Low yield | [ | |
| 化学合成法 Chemical synthesis | 路线(1)和(2)Route(1)and(2) | —— | 路线冗长复杂,反应温度较高,资源浪费,收率低 Long and complex route, high reaction temperature, waste of resource, and low yield | [ |
| 路线(3)Route(3) | 收率高于路线(1)和(2)Yields higher than that in route(1)and(2) | 原料昂贵;中间体纯化使用两次反向层析柱,导致成本增加;毫克级别High feedstock cost; intermediate purification uses two reverse chromatography columns, which increases cost; milligram level | [ | |
| 路线(4)Route(4) | “一锅法”制备,路线短;无中间纯化过程 Prepared by one-pot method, short route; no intermediate purification | 原料昂贵且来源少 Expensive and few feedstock | [ | |
| 路线(5)Route(5) | 操作简单,原料低廉易得,步骤简短,条件相对温和可控,产量较高 Simple operation, cheap and readily available feedstock, short route, relatively moderate and controllable conditions, and high yield | 使用具有危害性的化学试剂,增加废液和废物处理成本 Use hazardous chemical reagents, increase waste liquid and waste disposal costs | [ | |
| 生物合成法 Biosynthesis | 微生物液体发酵Microbial liquid fermentation | 可食用菌发酵,天然属性,安全性高 Edible mushroom fermentation, natural properties and high safety | 发酵周期长,产率低 Long fermentation period, and low yield | [ |
| 生物转化 Biotransformation | 直接以前体氨基酸作为底物,原料成本低;工艺简单;产品浓度较高 Direct precursor amino acids as substrates, low feedstock cost; simple technology; higher product concentration | 产率低;表达复合酶体外催化,经济适用性差 Low yield, in vitro catalysis of expression complex enzymes, and low economic practicality | [ | |
| ERG工程菌株的发酵Fermentation of ERG engineered strains | 以常见菌株作为底盘菌,方便获得;减少外源添加,原料成本低;无有害试剂,环境友好;发酵周期短;操作简单 Common strains are chassis bacteria, which are readily available; reduce exogenous additions, low feedstock cost; no hazardous chemical reagents, environment-friendly; short fermentation period; simple operation | - | [ |
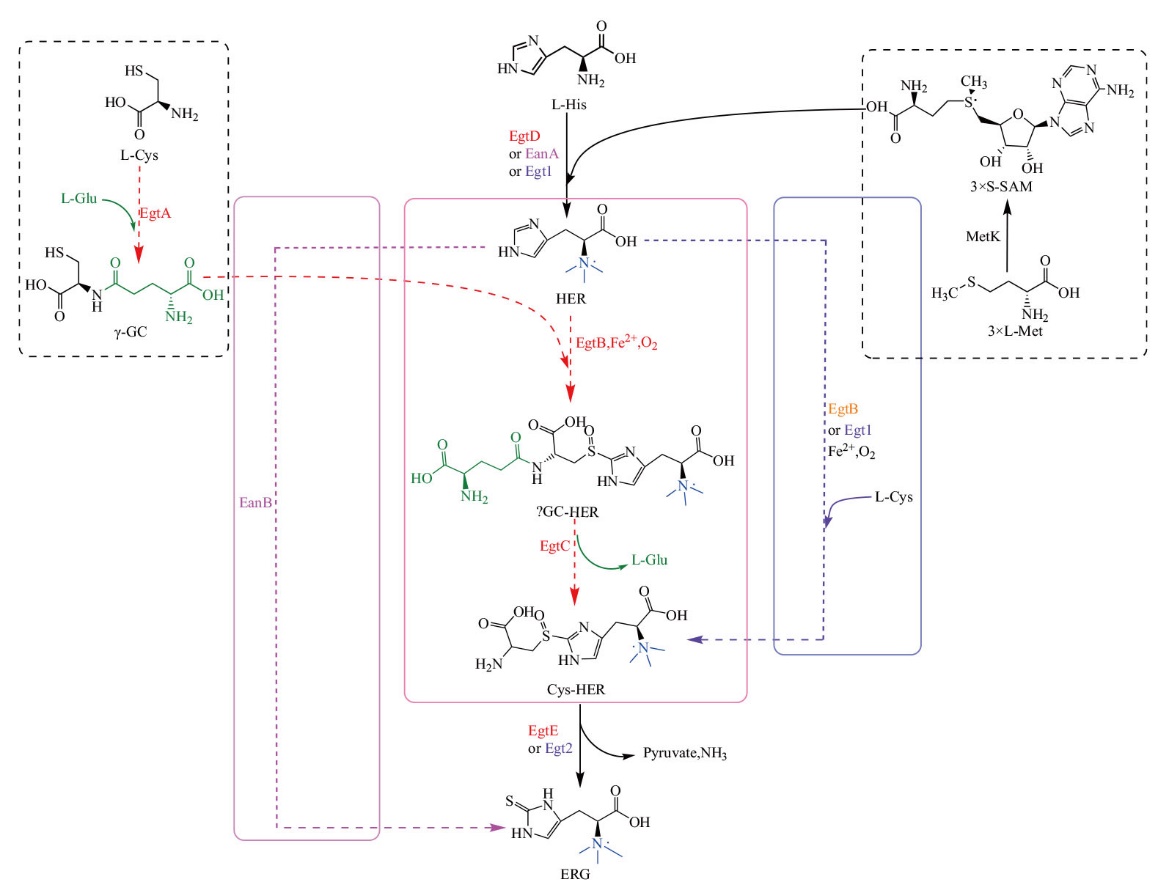
图3 麦角硫因的生物合成途径 SAM:S-腺苷蛋氨酸;γ-GC:γ-谷氨酰-半胱氨酸;HER:组氨酸甜菜碱;γGC-HER:γ-谷氨酰-组氨酸甜菜碱亚砜;Cys-HER:组氨酸甜菜碱半胱氨酸亚砜;MetK:S-腺苷蛋氨酸合成酶;黑色代表两条或两条以上的公共生物合成途径;红色代表ERG在M. smegmatis中的生物合成途径(EgtA:γ-谷氨酰半胱氨酸连接酶;EgtB:单核非血红素依赖性氧化酶;EgtC:酰胺转移酶;EgtD:SAM依赖性组氨酸甲基转移酶;EgtE:PLP依赖性C-S裂解酶);紫色代表ERG在N. crassa中的生物合成途径[Egt1:双功能酶(SAM依赖性组氨酸甲基转移酶和单核非血红素依赖性氧化酶);Egt2:PLP依赖性C-S裂解酶];橙色代表ERG在甲基杆菌等细菌中的生物合成途径(EgtB:类似真菌Egt1);粉色代表ERG在厌氧菌中的生物合成途径(EanA:甲基转移酶;EanB:硫转移酶);绿色代表L-Glu在M. smegmatis生物合成途径中的参与
Fig. 3 Biosynthetic pathway of ergothioneine SAM: S-adenosylmethionine; γ-GC:γ-glutamyl-cysteine; HER: hercynine; γGC-HER: hercynyl-γ-glutamyl-cysteine sulfoxide; Cys-HER: hercynyl-cysteine sulfoxide; MetK: S-adenosylmethionine synthetase. Black represents two or more public biosynthetic pathways. Red represents biosynthetic pathways of ERG in M. smegmatis(EgtA: γ-glutamyl cysteine synthase; EgtB: mononuclear non-heme iron enzyme; EgtC: amidotransferase; EgtD: SAM-dependent histidine methyltransferase; EgtE: PLP-mediated C-S lyase). Purple represents biosynthetic pathways of ERG in N. crassa[Egt1: Bifunctional enzymes(SAM-dependent histidine methyltransferase and mononuclear non-heme iron enzyme); Egt2: PLP-mediated C-S lyase]. Orange represents biosynthetic pathways of ERG in Methylobacterium(EgtB: Similar to fungi Egt1). Pink represents biosynthetic pathways of ERG in anaerobic bacteria(EanA: Methyltransferase; EanB: rhodanese-like sulfur transferase). Green represents the participation of L-Glu in M. smegmatis biosynthetic pathway
| 菌株 Strain | 关键策略 Key strategy | 发酵时间 Fermentation period/h | 产量 Yield | 生产效率Production efficiency/(mg·L-1·h-1) | 参考文献 Reference |
|---|---|---|---|---|---|
| 大肠杆菌E. coli BW25113 | 过表达egtBCDEMs和gshA基因 | 72 | 24 mg/L | 0.3 | [ |
| 大肠杆菌E. coli MG1655 | 优化表达egtABCDE基因 | 60 | 437.6 mg/L | 7.3 | [ |
| 大肠杆菌E. coli BW25113 | 过表达egtDEMs 和egtBMp基因;表达cysE*、serA* 和ydeD 基因;敲除metJ 基因 | 192 | 657 mg/L | 3.4 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达egtBCDEMs、egt1Sp 和egtA基因;过表达thrA 和serA T410STOP 基因 | 108 | 710.53 mg/L | 6.6 | [ |
| 大肠杆菌E. coli BL21(DE3) | 过表达egtBCDEMs、egt1Sp、egtA、thrA 和serAT410STOP 基因 | 108 | 1.1 g/L | 10.2 | [ |
| 大肠杆菌E. coli BW25113 | 过表达egtABCDEMs 基因;表达gshA、cysE*、serA* 和ydeD基因;敲除metJ 基因 | 216 | 1.31 g/L | 6.1 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达 egtBCDEMs 和egt1Sp 基因;过表达egtAMs、thrA 和serA T410STOP基因 | 108 | 2.01 g/L | 18.6 | [ |
| 大肠杆菌E. coli MG1655 | 表达egtBCDEMs、egtB*Ms、egt2Nc 和hisG* 基因;双拷贝表达gshA;过表达hisDBCHAFI基因 | 52 | 2.9 g/L | 55.8 | [ |
| 大肠杆菌E. coli BW25113 | 过表达tregt1和tregt2 基因 | 143 | 4.34 g/L | 30.4 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达egtEMs 基因;半理性设计和随机突变EgtD 和TNcEgt1 | 96 | 5.4 g/L | 56.3 | [ |
| 酿酒酵母Saccharomyces cerevisiae | 过表达Poegt1、Peegt1 和Ptegt1 基因 | — | 2.5 mg/L | — | [ |
| 酿酒酵母 S. cerevisiae | 过表达egt1Gf 和egt2Gf 基因 | 168 | 20.61 mg/L | 0.1 | [ |
| 酿酒酵母 S. cerevisiae | 共表达双拷贝 egt1Nc 和egt2Cp 基因 | 84 | 598 mg/L | 7.1 | [ |
| 圆红冬孢酵母 Rhodotorula toruloides | 表达egt1Nc基因 | 96 | 1.5 g/L | 15.6 | [ |
| 解脂耶氏酵母 Yarrowia lipolytica | 共表达双拷贝egt1Nc 和egt2Cp 基因 | 220 | 1.63 g/L | 7.4 | [ |
| 酿酒酵母 S. cerevisiae | 共表达双拷贝egt1Nc和egt2Cp 基因;过表达 MET14;敲除spe2 基因 | 160 | 2.4 g/L | 15 | [ |
| 粟酒裂殖酵母Schizosaccharomyces pombe | 经过多轮紫外线和氯化锂突变处理 | 148 | 12.5 g/L | 84.5 | [ |
| 谷氨酸棒状杆菌 C. glutamicum | 过表达egtDEMs 和egtBMp基因 | 336 | 100 mg/L | 0.3 | [ |
| 谷氨酸棒状杆菌 C. glutamicum | 表达egt1Sp 和egt2Sp基因;过表达cysE、cysK和cysR 基因;加强硫同化和磷酸戊糖途径;敲除sdaA基因 | 36 | 264 mg/L | 7.3 | [ |
| 甲基杆菌属Methylobacterium aquaticum | 过表达egtBDMs 基因;敲除hutH 基因 | 168 | 7.0 mg/g 干重 Dry weight | — | [ |
| 米曲霉 Aspergillus oryzae | 过表达egt1Nc 和egt2Nc基因 | — | 231 mg/kg 培养基 Medium | — | [ |
| 蛹虫草 Cordyceps militaris | 过表达EgtDMs、CmE1B和CmEgt2 | — | 2.5 g/kg 干重 Dry weight | — | [ |
| 枯草芽孢杆菌 Bacillus subtilis | 优化表达egtABCDE 基因 | 60 | 568.4 mg/L | 9.5 | [ |
| 新金色分枝杆菌 Mycolicibacterium neoaurum | 过表达egtABCDEMn、hisG、hisC 和allB1 基因;敲除假定裂解酶基因 Mn_3042;过表达metK 和ahcY 基因 | 216 | 1.56 g/L | 7.2 | [ |
表2 麦角硫因基因工程菌株的发酵水平
Table 2 Fermentation levels of genetically engineered strains for ergothioneine
| 菌株 Strain | 关键策略 Key strategy | 发酵时间 Fermentation period/h | 产量 Yield | 生产效率Production efficiency/(mg·L-1·h-1) | 参考文献 Reference |
|---|---|---|---|---|---|
| 大肠杆菌E. coli BW25113 | 过表达egtBCDEMs和gshA基因 | 72 | 24 mg/L | 0.3 | [ |
| 大肠杆菌E. coli MG1655 | 优化表达egtABCDE基因 | 60 | 437.6 mg/L | 7.3 | [ |
| 大肠杆菌E. coli BW25113 | 过表达egtDEMs 和egtBMp基因;表达cysE*、serA* 和ydeD 基因;敲除metJ 基因 | 192 | 657 mg/L | 3.4 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达egtBCDEMs、egt1Sp 和egtA基因;过表达thrA 和serA T410STOP 基因 | 108 | 710.53 mg/L | 6.6 | [ |
| 大肠杆菌E. coli BL21(DE3) | 过表达egtBCDEMs、egt1Sp、egtA、thrA 和serAT410STOP 基因 | 108 | 1.1 g/L | 10.2 | [ |
| 大肠杆菌E. coli BW25113 | 过表达egtABCDEMs 基因;表达gshA、cysE*、serA* 和ydeD基因;敲除metJ 基因 | 216 | 1.31 g/L | 6.1 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达 egtBCDEMs 和egt1Sp 基因;过表达egtAMs、thrA 和serA T410STOP基因 | 108 | 2.01 g/L | 18.6 | [ |
| 大肠杆菌E. coli MG1655 | 表达egtBCDEMs、egtB*Ms、egt2Nc 和hisG* 基因;双拷贝表达gshA;过表达hisDBCHAFI基因 | 52 | 2.9 g/L | 55.8 | [ |
| 大肠杆菌E. coli BW25113 | 过表达tregt1和tregt2 基因 | 143 | 4.34 g/L | 30.4 | [ |
| 大肠杆菌E. coli BL21(DE3) | 表达egtEMs 基因;半理性设计和随机突变EgtD 和TNcEgt1 | 96 | 5.4 g/L | 56.3 | [ |
| 酿酒酵母Saccharomyces cerevisiae | 过表达Poegt1、Peegt1 和Ptegt1 基因 | — | 2.5 mg/L | — | [ |
| 酿酒酵母 S. cerevisiae | 过表达egt1Gf 和egt2Gf 基因 | 168 | 20.61 mg/L | 0.1 | [ |
| 酿酒酵母 S. cerevisiae | 共表达双拷贝 egt1Nc 和egt2Cp 基因 | 84 | 598 mg/L | 7.1 | [ |
| 圆红冬孢酵母 Rhodotorula toruloides | 表达egt1Nc基因 | 96 | 1.5 g/L | 15.6 | [ |
| 解脂耶氏酵母 Yarrowia lipolytica | 共表达双拷贝egt1Nc 和egt2Cp 基因 | 220 | 1.63 g/L | 7.4 | [ |
| 酿酒酵母 S. cerevisiae | 共表达双拷贝egt1Nc和egt2Cp 基因;过表达 MET14;敲除spe2 基因 | 160 | 2.4 g/L | 15 | [ |
| 粟酒裂殖酵母Schizosaccharomyces pombe | 经过多轮紫外线和氯化锂突变处理 | 148 | 12.5 g/L | 84.5 | [ |
| 谷氨酸棒状杆菌 C. glutamicum | 过表达egtDEMs 和egtBMp基因 | 336 | 100 mg/L | 0.3 | [ |
| 谷氨酸棒状杆菌 C. glutamicum | 表达egt1Sp 和egt2Sp基因;过表达cysE、cysK和cysR 基因;加强硫同化和磷酸戊糖途径;敲除sdaA基因 | 36 | 264 mg/L | 7.3 | [ |
| 甲基杆菌属Methylobacterium aquaticum | 过表达egtBDMs 基因;敲除hutH 基因 | 168 | 7.0 mg/g 干重 Dry weight | — | [ |
| 米曲霉 Aspergillus oryzae | 过表达egt1Nc 和egt2Nc基因 | — | 231 mg/kg 培养基 Medium | — | [ |
| 蛹虫草 Cordyceps militaris | 过表达EgtDMs、CmE1B和CmEgt2 | — | 2.5 g/kg 干重 Dry weight | — | [ |
| 枯草芽孢杆菌 Bacillus subtilis | 优化表达egtABCDE 基因 | 60 | 568.4 mg/L | 9.5 | [ |
| 新金色分枝杆菌 Mycolicibacterium neoaurum | 过表达egtABCDEMn、hisG、hisC 和allB1 基因;敲除假定裂解酶基因 Mn_3042;过表达metK 和ahcY 基因 | 216 | 1.56 g/L | 7.2 | [ |
| [1] | Tanret C. The new base drawn from rye ergot, ergothioneine[J]. Compt Rend, 1909,(149): 222-224. |
| [2] |
潘虹余, 郭丽琼, 林俊芳. 麦角硫因机体内的分布与代谢和其在疾病中的作用研究进展[J]. 食品科学, 2019, 40(23): 334-340.
doi: 10.7506/spkx1002-6630-20181016-158 |
| Pan HY, Guo LQ, Lin JF. Recent advances in understanding the in vivo distribution and metabolism of ergothioneine and its roles in disease prevention[J]. Food Sci, 2019, 40(23): 334-340. | |
| [3] | 林陈水, 付水星, 黎小军, 等. 一种稀有的天然氨基酸-麦角硫因[J]. 氨基酸和生物资源, 2006, 28(1): 63-67. |
| Lin CS, Fu SX, Li XJ, et al. Ergothioneine-a rare natural amino acid[J]. Amino Acids Biotic Resour, 2006, 28(1): 63-67. | |
| [4] |
Kalaras MD, Richie JP, Calcagnotto A, et al. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione[J]. Food Chem, 2017, 233: 429-433.
doi: S0308-8146(17)30691-X pmid: 28530594 |
| [5] | 木开代斯·买合木提, 陈建, 焦春伟, 等. L-麦角硫因生物合成与应用研究进展[J]. 天然产物研究与开发, 2022, 34(4): 713-721. |
| Mukaidaisi M, Chen J, Jiao CW, et al. Progress in biosynthesis and application of L-ergothioneine[J]. Nat Prod Res Dev, 2022, 34(4): 713-721. | |
| [6] |
Kitsanayanyong L, Ohshima T. Ergothioneine: a potential antioxidative and antimelanosis agent for food quality preservation[J]. FEBS Lett, 2022, 596(10): 1330-1347.
doi: 10.1002/feb2.v596.10 URL |
| [7] | 何鑫怡, 周子艺, 陈媛媛, 等. 麦角硫因生物活性及其在食品工业中的应用[J]. 食品与发酵工业, 2023, 49(10): 285-292. |
| He XY, Zhou ZY, Chen YY, et al. Bioactivity of ergothioneine and its application in food industry: a review[J]. Food Ferment Ind, 2023, 49(10): 285-292. | |
| [8] | 张晓娜, 徐鹤然, 化璟琳, 等. 麦角硫因生物学功能及在化妆品功效原料中的应用[J]. 当代化工研究, 2021(16): 154-158. |
| Zhang XN, Xu HR, Hua JL, et al. The biological function and application of ergothioneine in cosmetic efficacy raw materials[J]. Mod Chem Res, 2021(16): 154-158. | |
| [9] |
Liu HM, Tang W, Wang XY, et al. Safe and effective antioxidant: the biological mechanism and potential pathways of ergothioneine in the skin[J]. Molecules, 2023, 28(4): 1648.
doi: 10.3390/molecules28041648 URL |
| [10] |
Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease[J]. Biochim Biophys Acta, 2012, 1822(5): 784-793.
doi: 10.1016/j.bbadis.2011.09.017 pmid: 22001064 |
| [11] | 高青莹, 徐建雄. 麦角硫因的抗氧化特性及其干预氧化应激相关疾病的研究进展[J]. 天然产物研究与开发, 2023, 35(6):1081-1087. |
| Gao QY, Xu JX. Antioxidative properties of ergothioneine and research progress of its intervention in oxidative stress-related diseases[J]. Nat Prod Res Dev, 2023, 35(6):1081-1087. | |
| [12] |
Fu TT, Shen L. Ergothioneine as a natural antioxidant against oxidative stress-related diseases[J]. Front Pharmacol, 2022, 13: 850813.
doi: 10.3389/fphar.2022.850813 URL |
| [13] | 冯路路, 鄂恒超, 张艳梅, 等. 食用菌中麦角硫因提取分离和检测方法研究进展[J]. 食用菌学报, 2021, 28(1): 115-123. |
| Feng LL, E HC, Zhang YM, et al. Research progress on extraction, separation and detection of ergothionine in edible fungi[J]. Acta Edulis Fungi, 2021, 28(1): 115-123. | |
| [14] | 马晓雪, 陈旭东, 吴志文, 等. 天然抗氧化剂麦角硫因的合成工艺研究[J]. 合成化学, 2022, 30(9): 743-748. |
| Ma XX, Chen XD, Wu ZW, et al. Process research of natural antioxidant ergothioneine[J]. Chin J Synth Chem, 2022, 30(9): 743-748. | |
| [15] | 刘琦, 毛雨丰, 廖小平, 等. 麦角硫因生物合成研究的新进展[J]. 生物工程学报, 2022, 38(4): 1408-1420. |
| Liu Q, Mao YF, Liao XP, et al. Recent progress in ergothioneine biosynthesis: a review[J]. Chin J Biotechnol, 2022, 38(4): 1408-1420. | |
| [16] | 黄明汝, 潘俊锋, 刘建. 复合酶及其在制备麦角硫因中的应用: CN112301013B[P]. 2022-11-08. |
| Huang MR, Pan JF, Liu J. Compound enzyme and application thereof in preparation of ergothioneine: CN112301013B[P]. 2022-11-08. | |
| [17] |
Osawa R, Kamide T, Satoh Y, et al. Heterologous and high production of ergothioneine in Escherichia coli[J]. J Agric Food Chem, 2018, 66(5): 1191-1196.
doi: 10.1021/acs.jafc.7b04924 URL |
| [18] | EFSA Panel on Dietetic Products, Nutrition and Allergies(NDA), Turck D, et al. Statement on the safety of synthetic l-ergothioneine as a novel food - supplementary dietary exposure and safety assessment for infants and young children, pregnant and breastfeeding women[J]. EFSA J, 2017, 15(11): e05060. |
| [19] | 周波. 麦角硫因的营养学研究概述[J]. 沈阳医学院学报, 2021, 23(3): 193-197. |
| Zhou B. An outline review of the nutritional research on ergothioneine[J]. J Shenyang Med Coll, 2021, 23(3): 193-197. | |
| [20] |
Kohl E, Steinbauer J, Landthaler M, et al. Skin ageing[J]. J Eur Acad Dermatol Venereol, 2011, 25(8): 873-884.
doi: 10.1111/j.1468-3083.2010.03963.x pmid: 21261751 |
| [21] |
Gründemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter[J]. Proc Natl Acad Sci USA, 2005, 102(14): 5256-5261.
doi: 10.1073/pnas.0408624102 pmid: 15795384 |
| [22] |
Liao WC, Wu WH, Tsai PC, et al. Kinetics of ergothioneine inhibition of mushroom tyrosinase[J]. Appl Biochem Biotechnol, 2012, 166(2): 259-267.
doi: 10.1007/s12010-011-9421-x pmid: 22068690 |
| [23] |
Obayashi K, Kurihara K, Okano Y, et al. L-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-alpha and MMP-1 expression in UV-irradiated human dermal fibroblasts[J]. J Cosmet Sci, 2005, 56(1): 17-27.
pmid: 15744438 |
| [24] |
Shukla Y, Kulshrestha OP, Khuteta KP. Ergothioneine content in normal and senile human cataractous lenses[J]. Indian J Med Res, 1981, 73: 472-473.
pmid: 7275246 |
| [25] |
Paul BD. Ergothioneine: a stress vitamin with antiaging, vascular, and neuroprotective roles?[J]. Antioxid Redox Signal, 2022, 36(16/17/18): 1306-1317.
doi: 10.1089/ars.2021.0043 URL |
| [26] |
Kerley RN, McCarthy C, Kell DB, et al. The potential therapeutic effects of ergothioneine in pre-eclampsia[J]. Free Radic Biol Med, 2018, 117: 145-157.
doi: 10.1016/j.freeradbiomed.2017.12.030 URL |
| [27] |
Cheah IK, Halliwell B. Could ergothioneine aid in the treatment of coronavirus patients?[J]. Antioxidants, 2020, 9(7): 595.
doi: 10.3390/antiox9070595 URL |
| [28] |
Han YW, Tang XY, Zhang YT, et al. The current status of biotechnological production and the application of a novel antioxidant ergothioneine[J]. Crit Rev Biotechnol, 2021, 41(4): 580-593.
doi: 10.1080/07388551.2020.1869692 pmid: 33550854 |
| [29] |
Hu W, Song H, Her AS, et al. Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway[J]. Org Lett, 2014, 16(20): 5382-5385.
doi: 10.1021/ol502596z URL |
| [30] | 康振, 陈坚, 堵国成, 等. 一种发酵合成麦角硫因的工程菌株及其构建方法: CN110358719B[P]. 2021-05-04. |
| Kang Z, Chen J, Du GC, et al. Engineering strain for fermented synthesis of ergothioneine and construction method thereof: CN110358719B[P]. 2021-05-04. | |
| [31] |
Kamide T, Takusagawa S, Tanaka N, et al. High production of ergothioneine in Escherichia coli using the sulfoxide synthase from Methylobacterium strains[J]. J Agric Food Chem, 2020, 68(23): 6390-6394.
doi: 10.1021/acs.jafc.0c01846 URL |
| [32] | 王丽, 王阳, 李江华, 等. 产麦角硫因大肠杆菌工程菌株的构建与优化[J]. 生物工程学报, 2022, 38(2): 796-806. |
| Wang L, Wang Y, Li JH, et al. Construction and optimization of ergothioneine-producing Escherichia coli[J]. Chin J Biotechnol, 2022, 38(2): 796-806. | |
| [33] | 康振, 王阳, 王丽, 等. 高效合成麦角硫因的工程菌的构建方法与应用: CN113234652B[P]. 2022-09-27. |
| Kang Z, Wang Y, Wang L, et al. Construction method of engineering bacteria for efficiently synthesizing ergothioneine and application thereof: CN113234652B[P]. 2022-09-27. | |
| [34] |
Tanaka N, Kawano Y, Satoh Y, et al. Gram-scale fermentative production of ergothioneine driven by overproduction of cysteine in Escherichia coli[J]. Sci Rep, 2019, 9(1): 1895.
doi: 10.1038/s41598-018-38382-w |
| [35] | 陈佳敏, 王阳, 堵国成, 等. 优化前体供给与细胞膜通透性强化大肠杆菌合成麦角硫因[J]. 食品与生物技术学报, 2022, 41(8): 43-52. |
| Chen JM, Wang Y, Du GC, et al. Enhancement of ergothioneine synthesis in Escherichia coli via optimization of precursor supply and cell membrane permeability[J]. J Food Sci Biotechnol, 2022, 41(8): 43-52. | |
| [36] | 马倩, 田道光, 谢希贤, 等. 一种生产麦角硫因的基因工程菌株及其应用: CN112251392B[P]. 2022-09-09. |
| Ma Q, Tian DG, Xie XX, et al. Genetically engineered strain for producing ergothioneine and application: CN112251392B[P]. 2022-09-09. | |
| [37] |
Chen ZH, He YZ, Wu XY, et al. Toward more efficient ergothioneine production using the fungal ergothioneine biosynthetic pathway[J]. Microb Cell Fact, 2022, 21(1): 76.
doi: 10.1186/s12934-022-01807-3 pmid: 35525939 |
| [38] |
Zhang LW, Tang JW, Feng MQ, et al. Engineering methyltransferase and sulfoxide synthase for high-yield production of ergothioneine[J]. J Agric Food Chem, 2023, 71(1): 671-679.
doi: 10.1021/acs.jafc.2c07859 URL |
| [39] | 潘涛, 林金德, 余颖豪, 等. 酿酒酵母表达侧耳源单基因生物合成麦角硫因[J]. 食品科学, 2022, 43(10): 214-219. |
| Pan T, Lin JD, Yu YH, et al. Expression in Saccharomyces cerevisiae of single ergothioneine synthase genes from Pleurotus[J]. Food Sci, 2022, 43(10): 214-219. | |
| [40] |
Yu YH, Pan HY, Guo LQ, et al. Successful biosynthesis of natural antioxidant ergothioneine in Saccharomyces cerevisiae required only two genes from Grifola frondosa[J]. Microb Cell Fact, 2020, 19(1): 164.
doi: 10.1186/s12934-020-01421-1 |
| [41] |
van der Hoek SA, Darbani B, Zugaj KE, et al. Engineering the yeast Saccharomyces cerevisiae for the production of L-(+)-ergothioneine[J]. Front Bioeng Biotechnol, 2019, 7: 262.
doi: 10.3389/fbioe.2019.00262 URL |
| [42] | 范文超, 高书良, 王金刚, 等. 一种构建麦角硫因生产菌的方法: CN111534535B[P]. 2022-02-22. |
| Fan WC, Gao SL, Wang JG, et al. Method for constructing ergothionine producing strain: CN111534535B[P]. 2022-02-22. | |
| [43] |
van der Hoek SA, Rusnák M, Jacobsen IH, et al. Engineering ergothioneine production in Yarrowia lipolytica[J]. FEBS Lett, 2022, 596(10): 1356-1364.
doi: 10.1002/feb2.v596.10 URL |
| [44] |
van der Hoek SA, Rusnák M, Wang GK, et al. Engineering precursor supply for the high-level production of ergothioneine in Saccharomyces cerevisiae[J]. Metab Eng, 2022, 70: 129-142.
doi: 10.1016/j.ymben.2022.01.012 URL |
| [45] | Zhou LQ, Xiang T, Yang MX, et al. Yeast strain and use thereof and preparation method of ergothioneine: US20230220428[P]. 2023-07-13. |
| [46] |
Hirasawa T, Shimoyamada Y, Tachikawa Y, et al. Ergothioneine production by Corynebacterium glutamicum harboring heterologous biosynthesis pathways[J]. J Biosci Bioeng, 2023, 135(1): 25-33.
doi: 10.1016/j.jbiosc.2022.10.002 URL |
| [47] |
Kim M, Jeong DW, Oh JW, et al. Efficient synthesis of food-derived antioxidant L-ergothioneine by engineered Corynebacterium glutamicum[J]. J Agric Food Chem, 2022, 70(5): 1516-1524.
doi: 10.1021/acs.jafc.1c07541 URL |
| [48] |
Fujitani Y, Alamgir KM, Tani A. Ergothioneine production using Methylobacterium species, yeast, and fungi[J]. J Biosci Bioeng, 2018, 126(6): 715-722.
doi: S1389-1723(18)30295-0 pmid: 29910189 |
| [49] |
Takusagawa S, Satoh Y, Ohtsu I, et al. Ergothioneine production with Aspergillus oryzae[J]. Biosci Biotechnol Biochem, 2019, 83(1): 181-184.
doi: 10.1080/09168451.2018.1527210 URL |
| [50] |
Chen BX, Xue LN, Wei T, et al. Enhancement of ergothioneine production by discovering and regulating its metabolic pathway in Cordyceps militaris[J]. Microb Cell Fact, 2022, 21(1): 169.
doi: 10.1186/s12934-022-01891-5 |
| [51] |
Xiong LB, Xie ZY, Ke J, et al. Engineering Mycolicibacterium neoaurum for the production of antioxidant ergothioneine[J]. Food Bioeng, 2022, 1(1): 26-36.
doi: 10.1002/fbe2.v1.1 URL |
| [52] |
Wu HY, Tian DG, Fan XG, et al. Highly efficient production of L-histidine from glucose by metabolically engineered Escherichia coli[J]. ACS Synth Biol, 2020, 9(7): 1813-1822.
doi: 10.1021/acssynbio.0c00163 URL |
| [53] | Liu H, Fang GC, Wu H, et al. L-cysteine production in Escherichia coli based on rational metabolic engineering and modular strategy[J]. Biotechnol J, 2018, 13(5): e1700695. |
| [54] |
Li H, Wang BS, Li YR, et al. Metabolic engineering of Escherichia coli W3110 for the production of L-methionine[J]. J Ind Microbiol Biotechnol, 2017, 44(1): 75-88.
doi: 10.1007/s10295-016-1870-3 URL |
| [55] |
Pluskal T, Ueno M, Yanagida M. Genetic and metabolomic dissection of the ergothioneine and selenoneine biosynthetic pathway in the fission yeast, S. pombe, and construction of an overproduction system[J]. PLoS One, 2014, 9(5): e97774.
doi: 10.1371/journal.pone.0097774 URL |
| [56] |
Alamgir KM, Masuda S, Fujitani Y, et al. Production of ergothioneine by Methylobacterium species[J]. Front Microbiol, 2015, 6: 1185.
doi: 10.3389/fmicb.2015.01185 pmid: 26579093 |
| [57] |
Wu YK, Liu YF, Lv XQ, et al. Applications of CRISPR in a microbial cell factory: from genome reconstruction to metabolic network reprogramming[J]. ACS Synth Biol, 2020, 9(9): 2228-2238.
doi: 10.1021/acssynbio.0c00349 pmid: 32794766 |
| [58] |
Ye CC, Yang YT, Chen X, et al. Metabolic engineering of Escherichia coli BW25113 for the production of 5-Aminolevulinic Acid based on CRISPR/Cas9 mediated gene knockout and metabolic pathway modification[J]. J Biol Eng, 2022, 16(1): 26.
doi: 10.1186/s13036-022-00307-7 |
| [59] |
Sao Emani C, Williams MJ, Wiid IJ, et al. Ergothioneine is a secreted antioxidant in Mycobacterium smegmatis[J]. Antimicrob Agents Chemother, 2013, 57(7): 3202-3207.
doi: 10.1128/AAC.02572-12 URL |
| [1] | 何思成, 张紫瑗, 韩雨晴, 苗琳, 张翠英, 于爱群. 解脂耶氏酵母细胞工厂生产多不饱和脂肪酸的研究进展[J]. 生物技术通报, 2024, 40(1): 72-85. |
| [2] | 吴巧茵, 施友志, 李林林, 彭政, 谭再钰, 刘利平, 张娟, 潘勇. 类胡萝卜素降解菌株的原位筛选及其在雪茄提质增香中的应用[J]. 生物技术通报, 2023, 39(9): 192-201. |
| [3] | 苗永美, 苗翠苹, 于庆才. 枯草芽孢杆菌BBs-27发酵液性质及脂肽对黄色镰刀菌的抑菌作用[J]. 生物技术通报, 2023, 39(9): 255-267. |
| [4] | 薛宁, 王瑾, 李世新, 刘叶, 程海娇, 张玥, 毛雨丰, 王猛. 多基因同步调控结合高通量筛选构建高产L-苯丙氨酸的谷氨酸棒杆菌工程菌株[J]. 生物技术通报, 2023, 39(9): 268-280. |
| [5] | 徐发迪, 徐康, 孙东明, 李萌蕾, 赵建志, 鲍晓明. 基于杨木(Populus sp.)的二代燃料乙醇技术研究进展[J]. 生物技术通报, 2023, 39(9): 27-39. |
| [6] | 张岳一, 兰社益, 裴海闰, 封棣. 多菌种联用发酵燕麦麸皮工艺优化及发用功效评价[J]. 生物技术通报, 2023, 39(9): 58-70. |
| [7] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [8] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [9] | 叶云芳, 田清尹, 施婷婷, 王亮, 岳远征, 杨秀莲, 王良桂. 植物中β-紫罗兰酮生物合成及调控研究进展[J]. 生物技术通报, 2023, 39(8): 91-105. |
| [10] | 王玲, 卓燊, 付学森, 刘紫璇, 刘笑蓉, 王志辉, 周日宝, 刘湘丹. 莲生物碱生物合成途径及相关基因研究进展[J]. 生物技术通报, 2023, 39(7): 56-66. |
| [11] | 李雨真, 梅天秀, 李治文, 王淇, 李俊, 邹岳, 赵心清. 红酵母基因组和代谢工程改造研究进展[J]. 生物技术通报, 2023, 39(7): 67-79. |
| [12] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [13] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [14] | 王慕镪, 陈琦, 马薇, 李春秀, 欧阳鹏飞, 许建和. 机器学习方法在酶定向进化中的应用进展[J]. 生物技术通报, 2023, 39(4): 38-48. |
| [15] | 李海宁, 张红兵, 耿革霞, 李冉, 贾振华. 非天然氨基酸的应用及生物合成策略[J]. 生物技术通报, 2023, 39(12): 43-55. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||