








生物技术通报 ›› 2023, Vol. 39 ›› Issue (8): 137-147.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0138
赵思佳1( ), 王晓璐2, 孙纪录1, 田健2(
), 王晓璐2, 孙纪录1, 田健2( ), 张杰2(
), 张杰2( )
)
收稿日期:2023-02-19
出版日期:2023-08-26
发布日期:2023-09-05
通讯作者:
田健,男,博士,研究员,研究方向:蛋白分子设计;E-mail: tianjian@caas.cn;作者简介:赵思佳,女,硕士研究生,研究方向:食品微生物;E-mail: sijia_zhao@163.com
基金资助:
ZHAO Si-jia1( ), WANG Xiao-lu2, SUN Ji-lu1, TIAN Jian2(
), WANG Xiao-lu2, SUN Ji-lu1, TIAN Jian2( ), ZHANG Jie2(
), ZHANG Jie2( )
)
Received:2023-02-19
Published:2023-08-26
Online:2023-09-05
摘要:
本研究旨在以毕赤酵母为底盘细胞构建赤藓糖醇生产菌株。通过调控糖酵解途径中磷酸果糖激酶基因pfk的表达,敲除副产物阿拉伯糖醇和核糖醇生产相关基因,过表达不同来源的4-磷酸赤藓糖磷酸化酶、赤藓糖还原酶和糖醇磷酸酶,构建毕赤酵母赤藓糖醇生产菌株,对过表达戊糖磷酸途径关键酶转酮酶(TKL)、磷酸核酮糖差向异构酶(RPE)及赤藓糖还原酶对赤藓糖醇产量的影响也进行了探究。结果表明,过表达酿酒酵母来源的糖醇磷酸酶基因pyp1及大肠杆菌来源的4-磷酸赤藓糖磷酸化酶基因yidA的工程菌株C8具有赤藓糖醇生产能力,摇瓶发酵产量为30 mg/L;进一步过表达tkl和rpe后,菌株C10摇瓶发酵产量提高约40倍,达到1.2 g/L,高密度发酵产量为10.6 g/L;赤藓糖还原酶的过量表达并没有提升赤藓糖醇的产量,反而提高了副产物的产量。本研究首次在毕赤酵母中成功构建了赤藓糖醇合成通路,为改造毕赤酵母高效生产赤藓糖醇及其他高价值化合物奠定基础。
赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147.
ZHAO Si-jia, WANG Xiao-lu, SUN Ji-lu, TIAN Jian, ZHANG Jie. Modification of Pichia pastoris for Erythritol Production by Metabolic Engineering[J]. Biotechnology Bulletin, 2023, 39(8): 137-147.

图2 毕赤酵母菌株C0和C2的表型验证 A:菌株C0和C2的生长曲线;B:阿拉伯糖醇和核糖醇的生产
Fig. 2 Phenotype verification of P. pastoris C0 and C2 strains A: Growth curves of C0 and C2 strains; B: productions of arabitol and ribitol
| 基因Gene name | 功能Function | 来源Source | 蛋白分子量Protein molecular weight/kD |
|---|---|---|---|
| pyp1 | 糖醇磷酸酶基因Sugar alcohol phosphatase gene | S. cerevisiae | 30.7 |
| yidA | 去磷酸化酶基因Dphosphorylase gene | E. coli MG1655 | 32.9 |
| err1 | 赤藓糖还原酶基因Erythrose reductase gene | T. reesei | 39.5 |
| er(NADH) | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica | 39.4 |
| er(NADPH) | 赤藓糖还原酶基因Erythrose reductase gene | C. magnoliae JH110 | 34.6 |
| er1 | 赤藓糖还原酶基因Erythrose reductase gene | M. megachiliensis | 39.6 |
| er3 | 赤藓糖还原酶基因Erythrose reductase gene | M. megachiliensis | 39.8 |
| ER10 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica CLIB122 | 39.1 |
| ER25 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica CLIB122 | 38.2 |
| JX885 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica | 31.4 |
| rpe1 | 差向异构酶基因Epimerase gene | S. cerevisiae | 29.2 |
表1 本研究中涉及的外源基因
Table 1 Exogenous genes involved in this study
| 基因Gene name | 功能Function | 来源Source | 蛋白分子量Protein molecular weight/kD |
|---|---|---|---|
| pyp1 | 糖醇磷酸酶基因Sugar alcohol phosphatase gene | S. cerevisiae | 30.7 |
| yidA | 去磷酸化酶基因Dphosphorylase gene | E. coli MG1655 | 32.9 |
| err1 | 赤藓糖还原酶基因Erythrose reductase gene | T. reesei | 39.5 |
| er(NADH) | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica | 39.4 |
| er(NADPH) | 赤藓糖还原酶基因Erythrose reductase gene | C. magnoliae JH110 | 34.6 |
| er1 | 赤藓糖还原酶基因Erythrose reductase gene | M. megachiliensis | 39.6 |
| er3 | 赤藓糖还原酶基因Erythrose reductase gene | M. megachiliensis | 39.8 |
| ER10 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica CLIB122 | 39.1 |
| ER25 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica CLIB122 | 38.2 |
| JX885 | 赤藓糖还原酶基因Erythrose reductase gene | Y. lipolytica | 31.4 |
| rpe1 | 差向异构酶基因Epimerase gene | S. cerevisiae | 29.2 |
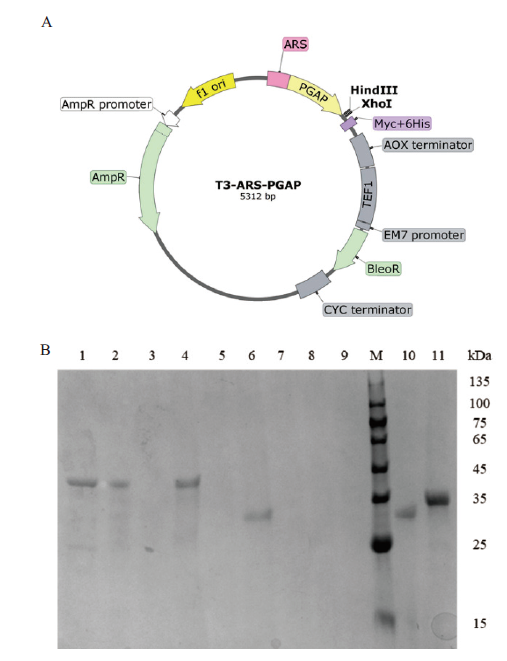
图4 外源基因在毕赤酵母中表达 A:T-ARS-PGAP质粒图谱;B:SDS-PAGE检测外源基因表达
Fig. 4 Expressions of exogenous genes in P. pastoris A: T-ARS-PGAP plasmid map; B: SDS-PAGE detection of the expression of exogenous genes. 1-11: err1, er(NADH), er(NADPH), er, er3, pyp1, ER10, ER25, JX885, rpe1, yidA
| 化合物名称Compound name | 最高浓度Maximum concentration/(g·L-1) |
|---|---|
| 赤藓糖醇 Erythritol | 0 |
| 阿拉伯糖醇 Arabitol | 1.4 |
| 核糖醇 Ribitol | 5.6 |
表2 突变株C5高密度发酵产物
Table 2 Products of high-density fermentation of mutant C5
| 化合物名称Compound name | 最高浓度Maximum concentration/(g·L-1) |
|---|---|
| 赤藓糖醇 Erythritol | 0 |
| 阿拉伯糖醇 Arabitol | 1.4 |
| 核糖醇 Ribitol | 5.6 |
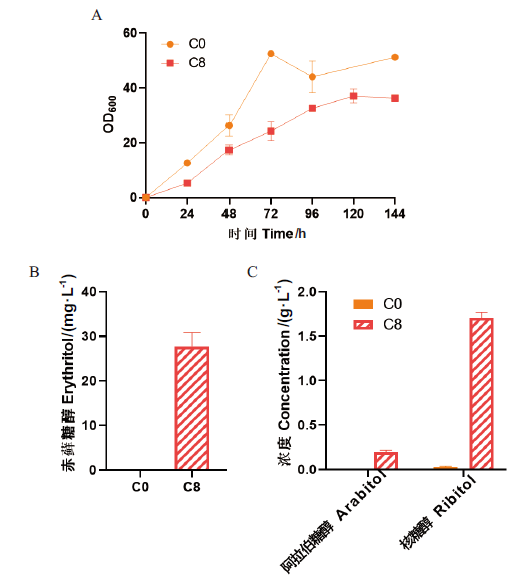
图5 毕赤酵母菌株C0和C8的表型验证 A:生长曲线;B:赤藓糖醇产量;C:阿拉伯糖醇和核糖醇产量
Fig. 5 Fermentation profiles of P. pastoris C0 and C8 strains A: Growth curve. B: Production of erythritol. C: Production of arabitol and ribitol
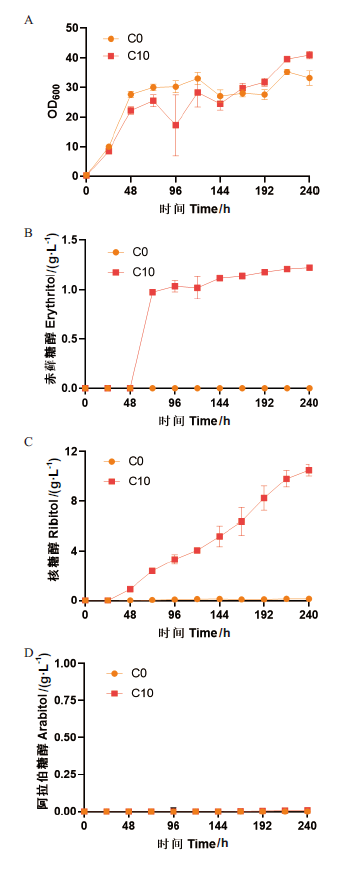
图6 毕赤酵母菌株C0和C10的表型验证 A:生长曲线;B:赤藓糖醇产量;C:核糖醇产量;D:阿拉伯糖醇产量
Fig. 6 Phenotype verification of P. pastoris C0 and C10 strains A: Growth curve. B: Production of erythritol. C: Production of ribitol. D: Production of arabitol
| 化合物名称 Compound name | 最高浓度Maximum concentration/(g·L-1) | |
|---|---|---|
| C11 | C12 | |
| 赤藓糖醇 Erythritol | 1.2 | 1.2 |
| 核糖醇 Ribitol | 1.6 | 4.0 |
| 阿拉伯糖醇 Arabitol | 4.9 | 5.1 |
表3 突变株C11和C12摇瓶发酵产物
Table 3 Products of shake-flask fermentation of mutant C11 and C12
| 化合物名称 Compound name | 最高浓度Maximum concentration/(g·L-1) | |
|---|---|---|
| C11 | C12 | |
| 赤藓糖醇 Erythritol | 1.2 | 1.2 |
| 核糖醇 Ribitol | 1.6 | 4.0 |
| 阿拉伯糖醇 Arabitol | 4.9 | 5.1 |
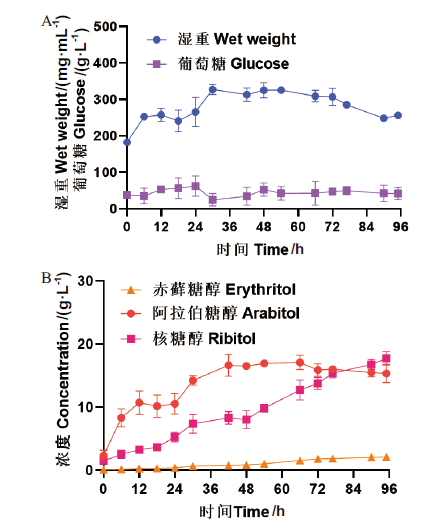
图8 毕赤酵母菌株C11高密度发酵 A:湿重和葡萄糖含量;B:赤藓糖醇、核糖醇和阿拉伯糖醇产量
Fig. 8 High-cell-density fermentation with strain P. pastoris C11 A: Wet weight and glucose concentration. B: Production of erythritol, ribitol and arabitol
| [1] |
Hijosa-Valsero M, Garita-Cambronero J, Paniagua-García AI, et al. By-products of sugar factories and wineries as feedstocks for erythritol generation[J]. Food Bioprod Process, 2021, 126: 345-355.
doi: 10.1016/j.fbp.2021.02.001 URL |
| [2] | 中国食品科学技术学会. 赤藓糖醇的科学共识[J]. 中国食品学报, 2022, 22(12): 405-412. |
| Chinese Institute of Food Science and Technology. Scientific consensus on erythritol[J]. J Chin Inst Food Sci Technol, 2022, 22(12): 405-412. | |
| [3] |
Martău GA, Coman V, Vodnar DC. Recent advances in the biotechnological production of erythritol and mannitol[J]. Crit Rev Biotechnol, 2020, 40(5): 608-622.
doi: 10.1080/07388551.2020.1751057 pmid: 32299245 |
| [4] | 邱学良. 产赤藓糖醇亚罗解脂酵母的耐热机制分析及组合策略改造[D]. 无锡: 江南大学, 2020. |
| Qiu XL. Thermotolerance mechanism analysis and combination strategy transformation of erythritol producing Yarrowia lipolytica[D]. Wuxi: Jiangnan University, 2020. | |
| [5] |
den Hartog GJM, Boots AW, Adam-Perrot A, et al. Erythritol is a sweet antioxidant[J]. Nutrition, 2010, 26(4): 449-458.
doi: 10.1016/j.nut.2009.05.004 pmid: 19632091 |
| [6] | 张妍, 张丽, 李宝磊, 等. 赤藓糖醇的国内外研究进展[J]. 饮料工业, 2022, 25(5): 76-79. |
| Zhang Y, Zhang L, Li BL, et al. Research progress of erythritol at home and abroad[J]. Beverage Ind, 2022, 25(5): 76-79. | |
| [7] | 中华人民共和国工业和信息化部. 口腔清洁护理用品牙膏中赤藓糖醇含量的测定高效液相色谱法: QB/T 5408—2019[S]. 北京: 中国轻工业出版社, 2019. |
| Ministry of Industry and Information of the People's Republic of China. Oral hygiene care products determination of erythritol content in toothpaste high performance liquid chromatography: QB/T 5408—2019[S]. Beijing: China Light Industry Press, 2019. | |
| [8] |
Liang PX, Cao MF, Li J, et al. Expanding sugar alcohol industry: Microbial production of sugar alcohols and associated chemocatalytic derivatives[J]. Biotechnol Adv, 2023, 64: 108105.
doi: 10.1016/j.biotechadv.2023.108105 URL |
| [9] | 隋松森, 王松江, 郭传庄, 等. 微生物发酵法产赤藓糖醇的研究进展[J]. 中国食品添加剂, 2021, 32(6): 125-131. |
| Sui SS, Wang SJ, Guo CZ, et al. Research progress in erythritol production by microbial fermentation[J]. China Food Addit, 2021, 32(6): 125-131. | |
| [10] | Stapley JA, Genders DJ, Atherton DM, et al. Methods for the electrolytic production of erythrose or erythritol: United States: US7955489[P]. 2011-06-07. |
| [11] |
Richter H, Vlad D, Unden G. Significance of pantothenate for glucose fermentation by Oenococcus oeni and for suppression of the erythritol and acetate production[J]. Arch Microbiol, 2001, 175(1): 26-31.
pmid: 11271417 |
| [12] |
Gänzle MG, Vermeulen N, Vogel RF. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough[J]. Food Microbiol, 2007, 24(2): 128-138.
pmid: 17008155 |
| [13] |
Veiga-da-Cunha M, Santos H, Van Schaftingen E. Pathway and regulation of erythritol formation in Leuconostoc oenos[J]. J Bacteriol, 1993, 175(13): 3941-3948.
pmid: 8391532 |
| [14] |
Khatape AB, Dastager SG, Rangaswamy V. An overview of erythritol production by yeast strains[J]. FEMS Microbiol Lett, 2022, 369(1): fnac107.
doi: 10.1093/femsle/fnac107 URL |
| [15] |
Rakicka-Pustułka M, Mirończuk AM, Celińska E, et al. Scale-up of the erythritol production technology - Process simulation and techno-economic analysis[J]. J Clean Prod, 2020, 257: 120533.
doi: 10.1016/j.jclepro.2020.120533 URL |
| [16] |
Deshpande MS, Kulkarni PP, Kumbhar PS, et al. Erythritol production from sugar based feedstocks by Moniliella pollinis using lysate of recycled cells as nutrients source[J]. Process Biochem, 2022, 112: 45-52.
doi: 10.1016/j.procbio.2021.11.020 URL |
| [17] |
Yang SL, Pan XW, Wang Q, et al. Enhancing erythritol production from crude glycerol in a wild-type Yarrowia lipolytica by metabolic engineering[J]. Front Microbiol, 2022, 13: 1054243.
doi: 10.3389/fmicb.2022.1054243 URL |
| [18] |
Li LZ, Yang TY, Guo WQ, et al. Construction of an efficient mutant strain of Trichosporonoides oedocephalis with HOG1 gene deletion for production of erythritol[J]. J Microbiol Biotechnol, 2016, 26(4): 700-709.
doi: 10.4014/jmb.1510.10049 URL |
| [19] |
Gómez-Ramírez IV, Corrales-García LL, Possani LD, et al. Expression in Pichia pastoris of human antibody fragments that neutralize venoms of Mexican scorpions[J]. Toxicon, 2023, 223: 107012.
doi: 10.1016/j.toxicon.2022.107012 URL |
| [20] |
Guo F, Dai ZX, Peng WF, et al. Metabolic engineering of Pichia pastoris for malic acid production from methanol[J]. Biotechnol Bioeng, 2021, 118(1): 357-371.
doi: 10.1002/bit.v118.1 URL |
| [21] | Cai P, Wu XY, Deng J, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris[J]. Proc Natl Acad Sci USA, 2022, 119(29): e2201711119. |
| [22] |
Zhang QQ, Wang XL, Luo HY, et al. Metabolic engineering of Pichia pastoris for myo-inositol production by dynamic regulation of central metabolism[J]. Microb Cell Fact, 2022, 21(1): 112.
doi: 10.1186/s12934-022-01837-x |
| [23] |
Abdel-Mawgoud AM, Markham KA, Palmer CM, et al. Metabolic engineering in the host Yarrowia lipolytica[J]. Metab Eng, 2018, 50: 192-208.
doi: S1096-7176(18)30273-8 pmid: 30056205 |
| [24] |
Kobayashi Y, Yoshida J, Iwata H, et al. Gene expression and function involved in polyol biosynthesis of Trichosporonoides megachiliensis under hyper-osmotic stress[J]. J Biosci Bioeng, 2013, 115(6): 645-650.
doi: 10.1016/j.jbiosc.2012.12.004 pmid: 23294575 |
| [25] |
Lee DH, Lee YJ, Ryu YW, et al. Molecular cloning and biochemical characterization of a novel erythrose reductase from Candida magnoliae JH110[J]. Microb Cell Fact, 2010, 9: 43.
doi: 10.1186/1475-2859-9-43 |
| [26] |
Carly F, Vandermies M, Telek S, et al. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering[J]. Metab Eng, 2017, 42: 19-24.
doi: 10.1016/j.ymben.2017.05.002 URL |
| [27] |
Jovanović B, Mach RL, Mach-Aigner AR. Characterization of erythrose reductases from filamentous fungi[J]. AMB Express, 2013, 3(1): 43.
doi: 10.1186/2191-0855-3-43 pmid: 23924507 |
| [28] |
Janek T, Dobrowolski A, Biegalska A, et al. Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis[J]. Microb Cell Fact, 2017, 16(1): 118.
doi: 10.1186/s12934-017-0733-6 URL |
| [29] |
Shenoy A, Yalamanchili S, Davis AR, et al. Expression and display of glycoengineered antibodies and antibody fragments with an engineered yeast strain[J]. Antibodies, 2021, 10(4): 38.
doi: 10.3390/antib10040038 URL |
| [30] |
De Brabander P, Uitterhaegen E, Delmulle T, et al. Challenges and progress towards industrial recombinant protein production in yeasts: a review[J]. Biotechnol Adv, 2023, 64: 108121.
doi: 10.1016/j.biotechadv.2023.108121 URL |
| [31] |
Ávila-Fernández Á, Montiel S, Rodríguez-Alegría ME, et al. Simultaneous enzyme production, Levan-type FOS synthesis and sugar by-products elimination using a recombinant Pichia pastoris strain expressing a levansucrase-endolevanase fusion enzyme[J]. Microb Cell Fact, 2023, 22(1): 18.
doi: 10.1186/s12934-022-02009-7 pmid: 36703199 |
| [32] |
Inokuma K, Miyamoto S, Morinaga K, et al. Direct production of 4-hydroxybenzoic acid from cellulose using cellulase-displaying Pichia pastoris[J]. Biotechnol Bioeng, 2023, 120(4): 1097-1107.
doi: 10.1002/bit.v120.4 URL |
| [33] |
Chen SL, Liu TS, Zhang WG, et al. Cofactor engineering for efficient production of α-farnesene by rational modification of NADPH and ATP regeneration pathway in Pichia pastoris[J]. Int J Mol Sci, 2023, 24(2): 1767.
doi: 10.3390/ijms24021767 URL |
| [34] |
Hijosa-Valsero M, Paniagua-García AI, Díez-Antolínez R. Cell immobilization for erythritol production[J]. J Fungi, 2022, 8(12): 1286.
doi: 10.3390/jof8121286 URL |
| [1] | 吴巧茵, 施友志, 李林林, 彭政, 谭再钰, 刘利平, 张娟, 潘勇. 类胡萝卜素降解菌株的原位筛选及其在雪茄提质增香中的应用[J]. 生物技术通报, 2023, 39(9): 192-201. |
| [2] | 苗永美, 苗翠苹, 于庆才. 枯草芽孢杆菌BBs-27发酵液性质及脂肽对黄色镰刀菌的抑菌作用[J]. 生物技术通报, 2023, 39(9): 255-267. |
| [3] | 薛宁, 王瑾, 李世新, 刘叶, 程海娇, 张玥, 毛雨丰, 王猛. 多基因同步调控结合高通量筛选构建高产L-苯丙氨酸的谷氨酸棒杆菌工程菌株[J]. 生物技术通报, 2023, 39(9): 268-280. |
| [4] | 徐发迪, 徐康, 孙东明, 李萌蕾, 赵建志, 鲍晓明. 基于杨木(Populus sp.)的二代燃料乙醇技术研究进展[J]. 生物技术通报, 2023, 39(9): 27-39. |
| [5] | 张岳一, 兰社益, 裴海闰, 封棣. 多菌种联用发酵燕麦麸皮工艺优化及发用功效评价[J]. 生物技术通报, 2023, 39(9): 58-70. |
| [6] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [7] | 李雨真, 梅天秀, 李治文, 王淇, 李俊, 邹岳, 赵心清. 红酵母基因组和代谢工程改造研究进展[J]. 生物技术通报, 2023, 39(7): 67-79. |
| [8] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [9] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [10] | 李昕悦, 周明海, 樊亚超, 廖莎, 张风丽, 刘晨光, 孙悦, 张霖, 赵心清. 基于转运蛋白工程提升微生物菌株耐受性和生物制造效率的研究进展[J]. 生物技术通报, 2023, 39(11): 123-136. |
| [11] | 车永梅, 刘广超, 郭艳苹, 叶青, 赵方贵, 刘新. 一种耐盐复合菌剂的制备和促生作用研究[J]. 生物技术通报, 2023, 39(11): 217-225. |
| [12] | 孙言秋, 谢采芸, 汤岳琴. 耐高温酿酒酵母的构建与高温耐受机制解析[J]. 生物技术通报, 2023, 39(11): 226-237. |
| [13] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [14] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [15] | 任海伟, 孙一帆, 任雨薇, 郭晓鹏, 潘立超, 张丙云, 李金平. 基于文献计量的青贮添加剂研究进展[J]. 生物技术通报, 2022, 38(8): 261-274. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||