








生物技术通报 ›› 2024, Vol. 40 ›› Issue (5): 94-102.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0967
孙擘1,2( ), 王蕊1, 霍永学1, 葛建镕1, 匡猛2(
), 王蕊1, 霍永学1, 葛建镕1, 匡猛2( ), 王凤格1(
), 王凤格1( )
)
收稿日期:2023-10-18
出版日期:2024-05-26
发布日期:2024-03-28
通讯作者:
王凤格,女,博士,研究员,研究方向:玉米分子鉴定;E-mail: gege0106@163.com;作者简介:孙擘,硕士研究生,研究方向:共性技术开发及应用;E-mail: sunbo990818@163.com。王蕊为本文共同第一作者
基金资助:
SUN Bo1,2( ), WANG Rui1, HUO Yong-xue1, GE Jian-rong1, KUANG Meng2(
), WANG Rui1, HUO Yong-xue1, GE Jian-rong1, KUANG Meng2( ), WANG Feng-ge1(
), WANG Feng-ge1( )
)
Received:2023-10-18
Published:2024-05-26
Online:2024-03-28
摘要:
【目的】荧光毛细管电泳由于其检测通量高、分辨率高等优点,被广泛应用于个体鉴定、品种鉴定、物种鉴定等多种应用场景中。尾巴序列为荧光毛细管电泳平台的广泛应用提供了高效的解决方案。为解决已有尾巴序列无法满足多物种使用需求,本研究基于编码转译技术开发高效的通用尾巴序列(universal tailed-sequence, UTS)设计工具。基于此工具设计通用尾巴序列,构建适合多作物的通用型PCR体系和程序,提高荧光电泳通量和灵活性。【方法】基于编码转译技术开发高效的通用尾巴序列设计工具,并对3 755个常用汉字进行编码转译,并设置序列GC含量、发卡结构及同源引物二聚体退火温度等筛选条件得到符合条件的UTS。使用BLAST工具对通用尾巴序列设计工具生成的UTS在多种生物基因组上进行同源性评估,并在玉米、番茄、辣椒、西瓜等作物上进行实验评估,构建物种通用型实验体系及程序。【结果】通过设计工具编码转译并筛选共得到7 436 833个高质量的候选UTS,占所有字组的52.74%。挑选6个UTS在20个作物基因组上的BLAST结果显示其与M13相比具有更好的特异性。通过对通用引物扩增程序进行优化,使通用引物在多个物种上的扩增成功率达到或超过95%,并具有较强的稳定性。【结论】利用编码转译技术开发通用尾巴序列,并为其搭配物种通用型扩增体系和程序,提供一种通量高、成本低的荧光电泳通用检测方法。
孙擘, 王蕊, 霍永学, 葛建镕, 匡猛, 王凤格. 适于多物种的通用尾巴序列设计及通用体系的建立[J]. 生物技术通报, 2024, 40(5): 94-102.
SUN Bo, WANG Rui, HUO Yong-xue, GE Jian-rong, KUANG Meng, WANG Feng-ge. Design of Universal Tailed-sequence and Establishment of the Universal System Suitable for Multiple Species[J]. Biotechnology Bulletin, 2024, 40(5): 94-102.
| 反应组分 Reaction component | 荧光引物扩增体系 Fluorescent primer amplification system | TP-M13-SSR扩增体系 TP-M13-SSR amplification system | UTS通用扩增体系 UTS universal amplification system |
|---|---|---|---|
| ddH2O | / | / | / |
| 10×Buffer | 1× | 1× | 1× |
| MgCl2 | 2.5 mmol/L | 2.5 mmol/L | 2.5 mmol/L |
| dNTPs | 0.15 mmol/L each | 0.15 mmol/L each | 0.15 mmol/L each |
| Taq 酶 Taq enzyme | 1.0 U | 1.0 U | 1.0 U |
| 上游引物 Forward primer | 0.25 μmol/L | 0.015 μmol/L | 0.03 μmol/L |
| 下游引物 Reverse primer | 0.25 μmol/L | 0.1 μmol/L | 0.1 μmol/L |
| 通用尾巴序列 Universal tailed-sequence | / | 0.1 μmol/L | 0.1 μmol/L |
| 模板DNA Template DNA | 5 ng/μL | 5 ng/μL | 5 ng/μL |
表1 荧光引物与通用引物序列PCR扩增体系
Table 1 PCR amplification system of fluorescent primers and universal primer sequences
| 反应组分 Reaction component | 荧光引物扩增体系 Fluorescent primer amplification system | TP-M13-SSR扩增体系 TP-M13-SSR amplification system | UTS通用扩增体系 UTS universal amplification system |
|---|---|---|---|
| ddH2O | / | / | / |
| 10×Buffer | 1× | 1× | 1× |
| MgCl2 | 2.5 mmol/L | 2.5 mmol/L | 2.5 mmol/L |
| dNTPs | 0.15 mmol/L each | 0.15 mmol/L each | 0.15 mmol/L each |
| Taq 酶 Taq enzyme | 1.0 U | 1.0 U | 1.0 U |
| 上游引物 Forward primer | 0.25 μmol/L | 0.015 μmol/L | 0.03 μmol/L |
| 下游引物 Reverse primer | 0.25 μmol/L | 0.1 μmol/L | 0.1 μmol/L |
| 通用尾巴序列 Universal tailed-sequence | / | 0.1 μmol/L | 0.1 μmol/L |
| 模板DNA Template DNA | 5 ng/μL | 5 ng/μL | 5 ng/μL |
| 通用尾巴序列类别 UTS category | 扩增程序 Amplification program |
|---|---|
| UTS通用扩增程序 UTS universal amplification program | 95℃,5 min; 95℃,40 s,55℃,35 s,72℃,45 s,35 cycle; 72℃,10 min;4℃ store |
| TP-M13-SSR扩增程序 TP-M13-SSR amplification program | 95℃,5 min; 95℃,40 s,Primer annealing temperature,35 s,72℃,45 s,8 cycle; 95℃,40 s,M13 Tailed-sequence annealing temperature,35 s,72℃,45 s,30 cycle; 72℃,10 min;4℃ |
表2 UTS与M13的PCR扩增程序
Table 2 PCR amplification program for UTS and M13
| 通用尾巴序列类别 UTS category | 扩增程序 Amplification program |
|---|---|
| UTS通用扩增程序 UTS universal amplification program | 95℃,5 min; 95℃,40 s,55℃,35 s,72℃,45 s,35 cycle; 72℃,10 min;4℃ store |
| TP-M13-SSR扩增程序 TP-M13-SSR amplification program | 95℃,5 min; 95℃,40 s,Primer annealing temperature,35 s,72℃,45 s,8 cycle; 95℃,40 s,M13 Tailed-sequence annealing temperature,35 s,72℃,45 s,30 cycle; 72℃,10 min;4℃ |
| UTS编号UTS code | 汉字字组 Chinese character | 编码后序列 Sequence after encoding(5'-3') | 序列GC含量 GC content of sequence/% | 发卡结构Tm Hairpin Tm value/℃ | 同源二聚体Tm值 Self-dimer Tm value/℃ |
|---|---|---|---|---|---|
| UTS01 | 孙擘(Sun Bo) | GACCAAGCACACGACAACTGACAT | 50.0 | / | -29.7 |
| UTS02 | 电泳(Dian Yong) | GACGACCTAGCCGACAAGTGAGTG | 58.3 | / | 9.3 |
| UTS03 | 荧光(Ying Guang) | GAATATGCAACGGACCATCCATAC | 45.8 | 35.9 | -25.8 |
| UTS04 | 基因(Ji Yin) | GACCACGGAGAAGACCACAGAATT | 50.0 | / | -19.6 |
| UTS05 | 分子(Fen Zi) | GACCATATATCAGACCAAGCACTT | 41.7 | / | -33.0 |
| UTS06 | 玉米(Yu Mi) | GACGATGAATACGACGAGTCAGTG | 50.0 | 34.7 | -0.4 |
表3 通用引物设计工具设计引物评估情况
Table 3 Evaluation of primers designed by UTS design tool
| UTS编号UTS code | 汉字字组 Chinese character | 编码后序列 Sequence after encoding(5'-3') | 序列GC含量 GC content of sequence/% | 发卡结构Tm Hairpin Tm value/℃ | 同源二聚体Tm值 Self-dimer Tm value/℃ |
|---|---|---|---|---|---|
| UTS01 | 孙擘(Sun Bo) | GACCAAGCACACGACAACTGACAT | 50.0 | / | -29.7 |
| UTS02 | 电泳(Dian Yong) | GACGACCTAGCCGACAAGTGAGTG | 58.3 | / | 9.3 |
| UTS03 | 荧光(Ying Guang) | GAATATGCAACGGACCATCCATAC | 45.8 | 35.9 | -25.8 |
| UTS04 | 基因(Ji Yin) | GACCACGGAGAAGACCACAGAATT | 50.0 | / | -19.6 |
| UTS05 | 分子(Fen Zi) | GACCATATATCAGACCAAGCACTT | 41.7 | / | -33.0 |
| UTS06 | 玉米(Yu Mi) | GACGATGAATACGACGAGTCAGTG | 50.0 | 34.7 | -0.4 |
| 物种名称Species name | 通用引物名称Universal primer name | ||||||
|---|---|---|---|---|---|---|---|
| M13 | UTS01 | UTS02 | UTS03 | UTS04 | UTS05 | UTS06 | |
| 玉米Zea mays L. | 0.004 | 8.2 | 2.1 | 0.13 | 0.52 | 0.52 | 0.13 |
| 水稻Oryza sativa L. | 0.026 | 19 | 4.7 | 4.7 | 4.7 | 4.7 | 1.2 |
| 小麦Triticum aestivum L. | 0.006 | 0.061 | 15 | 15 | 3.8 | 0.96 | 3.8 |
| 大豆Glycine max (L.)Merr. | 0.013 | 1.8 | 29 | 29 | 7.2 | 0.45 | 7.2 |
| 大麦Hordeum vulgare L. | 0.004 | 0.45 | 7 | 7 | 1.8 | 1.8 | 1.8 |
| 高粱Sorghum bicolor (L.)Moench | 3.7 | 8.7 | 34 | 2.2 | 2.2 | 8.7 | 2.2 |
| 甘薯Dioscorea esculenta (Lour.)Burkill | 1.7 | 10 | 2.6 | 0.67 | 0.67 | 2.6 | 0.17 |
| 马铃薯Solanum tuberosum L. | 0.022 | 3.2 | 50 | 3.2 | 0.21 | 0.81 | 50 |
| 棉花Gossypium hirsutum L. | 0.39 | 3.1 | 3.1 | 12 | 3.1 | 0.2 | 3.1 |
| 向日葵Helianthus annuus L. | 0.75 | 1.5 | 1.5 | 6 | 6 | 1.5 | 0.38 |
| 甘蔗Saccharum officinarum L. | 0.5 | 3.6 | 0.91 | 3.6 | 0.23 | 0.23 | 0.23 |
| 番茄Solanum lycopersicum L. | 0.024 | 21 | 5.2 | 1.3 | 5.2 | 1.3 | 21 |
| 黄瓜Cucumis sativus L. | 4.9 | 0.56 | 2.2 | 8.8 | 0.56 | 0.56 | 0.56 |
| 辣椒Capsicum annuum L. | 1.8 | 16 | 4.1 | 4.1 | 0.27 | 1 | 0.27 |
| 西瓜Citrullus lanatus subsp. Vuaris | 7.4 | 13 | 0.21 | 0.21 | 3.2 | 0.81 | 0.81 |
| 甜瓜Cucumis melo L. | 2 | 5.3 | 5.3 | 5.3 | 5.3 | 5.3 | 5.3 |
| 梨Pyrus L. | 5.9 | 0.065 | 16 | 0.065 | 0.065 | 1 | 4 |
| 枣Ziziphus jujuba(L.)Lam. | 6.7 | 0.22 | 3.4 | 3.4 | 0.22 | 0.86 | 13 |
| 菜豆Phaseolus vulgaris L. | 0.26 | 0.47 | 7.3 | 1.8 | 1.8 | 0.47 | 0.12 |
| 苹果Malus pumila Mill. | 1.6 | 0.15 | 2.3 | 0.58 | 2.3 | 0.58 | 0.15 |
表4 M13通用引物和6条UTS序列在20个作物基因组上的BLAST分析结果
Table 4 BLAST assessments for M13 universal primers and six UTS sequences across the genomes of 20 crops
| 物种名称Species name | 通用引物名称Universal primer name | ||||||
|---|---|---|---|---|---|---|---|
| M13 | UTS01 | UTS02 | UTS03 | UTS04 | UTS05 | UTS06 | |
| 玉米Zea mays L. | 0.004 | 8.2 | 2.1 | 0.13 | 0.52 | 0.52 | 0.13 |
| 水稻Oryza sativa L. | 0.026 | 19 | 4.7 | 4.7 | 4.7 | 4.7 | 1.2 |
| 小麦Triticum aestivum L. | 0.006 | 0.061 | 15 | 15 | 3.8 | 0.96 | 3.8 |
| 大豆Glycine max (L.)Merr. | 0.013 | 1.8 | 29 | 29 | 7.2 | 0.45 | 7.2 |
| 大麦Hordeum vulgare L. | 0.004 | 0.45 | 7 | 7 | 1.8 | 1.8 | 1.8 |
| 高粱Sorghum bicolor (L.)Moench | 3.7 | 8.7 | 34 | 2.2 | 2.2 | 8.7 | 2.2 |
| 甘薯Dioscorea esculenta (Lour.)Burkill | 1.7 | 10 | 2.6 | 0.67 | 0.67 | 2.6 | 0.17 |
| 马铃薯Solanum tuberosum L. | 0.022 | 3.2 | 50 | 3.2 | 0.21 | 0.81 | 50 |
| 棉花Gossypium hirsutum L. | 0.39 | 3.1 | 3.1 | 12 | 3.1 | 0.2 | 3.1 |
| 向日葵Helianthus annuus L. | 0.75 | 1.5 | 1.5 | 6 | 6 | 1.5 | 0.38 |
| 甘蔗Saccharum officinarum L. | 0.5 | 3.6 | 0.91 | 3.6 | 0.23 | 0.23 | 0.23 |
| 番茄Solanum lycopersicum L. | 0.024 | 21 | 5.2 | 1.3 | 5.2 | 1.3 | 21 |
| 黄瓜Cucumis sativus L. | 4.9 | 0.56 | 2.2 | 8.8 | 0.56 | 0.56 | 0.56 |
| 辣椒Capsicum annuum L. | 1.8 | 16 | 4.1 | 4.1 | 0.27 | 1 | 0.27 |
| 西瓜Citrullus lanatus subsp. Vuaris | 7.4 | 13 | 0.21 | 0.21 | 3.2 | 0.81 | 0.81 |
| 甜瓜Cucumis melo L. | 2 | 5.3 | 5.3 | 5.3 | 5.3 | 5.3 | 5.3 |
| 梨Pyrus L. | 5.9 | 0.065 | 16 | 0.065 | 0.065 | 1 | 4 |
| 枣Ziziphus jujuba(L.)Lam. | 6.7 | 0.22 | 3.4 | 3.4 | 0.22 | 0.86 | 13 |
| 菜豆Phaseolus vulgaris L. | 0.26 | 0.47 | 7.3 | 1.8 | 1.8 | 0.47 | 0.12 |
| 苹果Malus pumila Mill. | 1.6 | 0.15 | 2.3 | 0.58 | 2.3 | 0.58 | 0.15 |
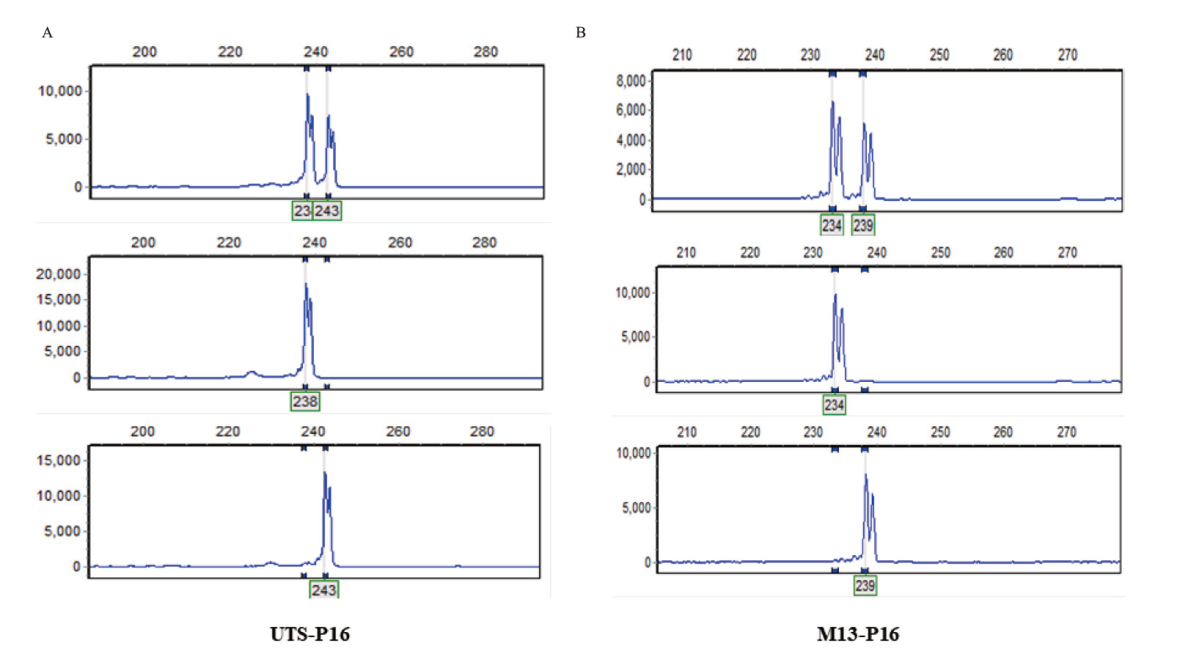
图1 UTS与M13尾巴序列反应程序和反应体系的扩增峰型比较 A:UTS01- P16在三份玉米样品上的扩增结果;B:M13-P16在三份玉米种质资源上的扩增结果
Fig. 1 Comparison of amplified peaks between UTS and M13 tailed-sequence reaction procedures and reaction system A: Amplification results of UTS01-P16 on three corn samples. B: Amplification results of M13-P16 on three corn samples
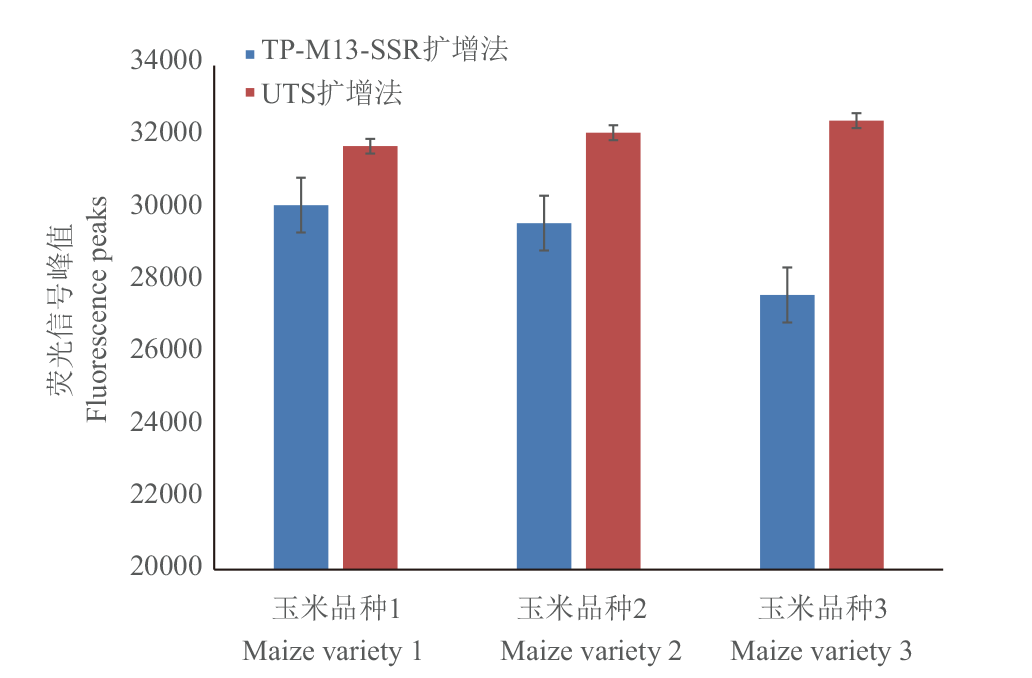
图2 UTS通用引物与M13接头反应程序和反应体系的扩增峰值比较 纵坐标为扩增峰荧光峰值,误差线表示标准偏差
Fig. 2 Comparison of amplification peaks between UTS universal primers and M13 reaction procedures and reaction systems Error line in the figure refers to the standard deviation
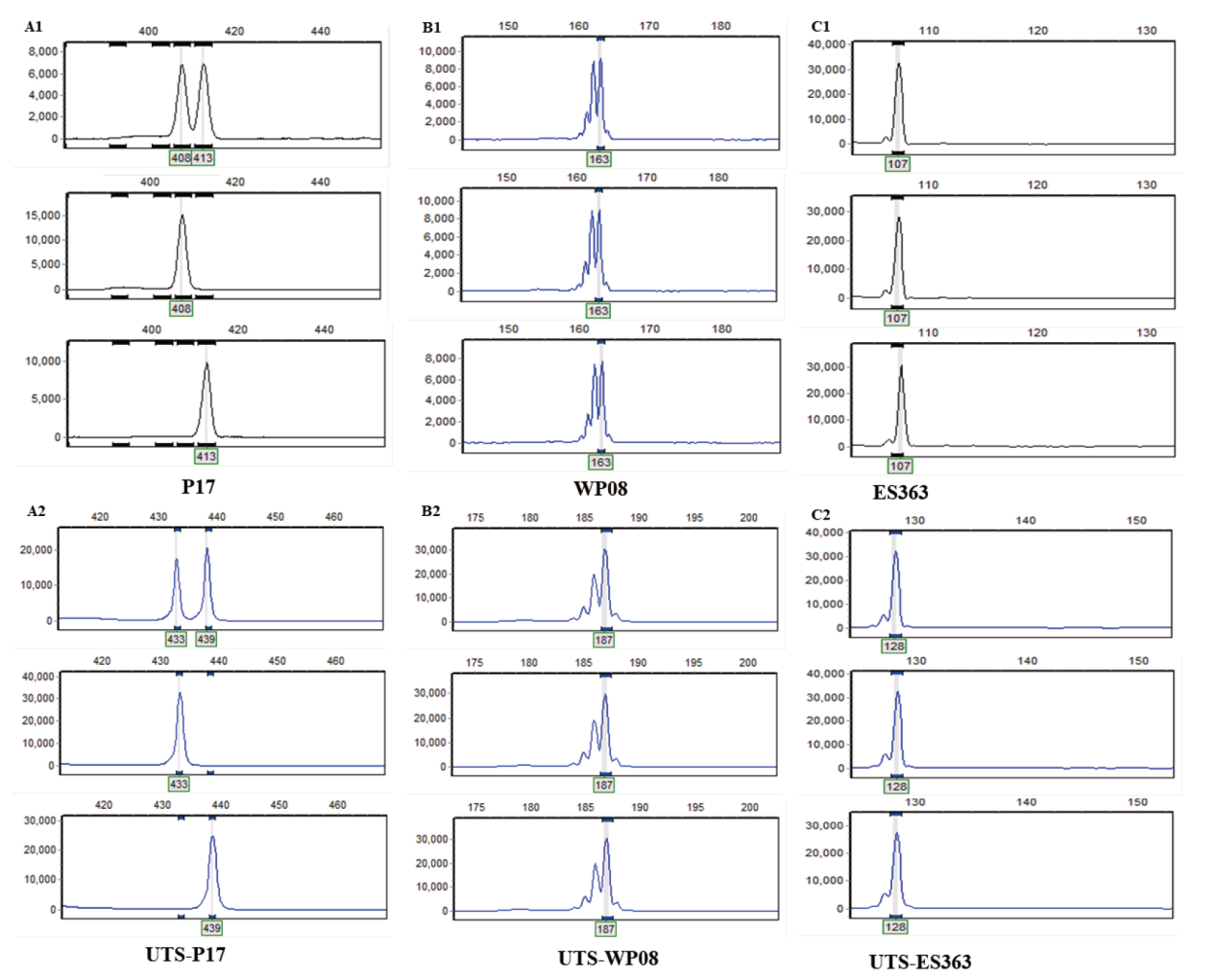
图3 不同作物荧光引物和通用尾巴序列的荧光毛细管电泳图 A1:UTS01- P17在三份玉米样品上的扩增结果;A2:P17荧光引物在三份玉米种质资源上的扩增结果;B1:UTS01- WP08在三份西瓜种质资源上的扩增结果;B2:WP08荧光引物在三份西瓜种质资源上的扩增结果;C1:UTS01- ES36308在三份辣椒种质资源上的扩增结果;C2:ES363荧光引物在三份西瓜种质资源上的扩增结果
Fig. 3 Fluorescent capillary electropherograms of different crops using fluorescent primers and UTS A1: Amplified results of UTS01-P17 on three corn samples. A2: Amplified results of P17 fluorescent primer on three corn germplasm resources. B1: Amplified results of UTS01-WP08 on three watermelon germplasm resources. B2: Amplified results of WP08 fluorescent primer on three watermelon germplasm resources. C1: Amplified results of UTS01-ES36308 on three pepper germplasm resources. C2: Amplified results of ES363 fluorescent primer on three watermelon germplasm resources
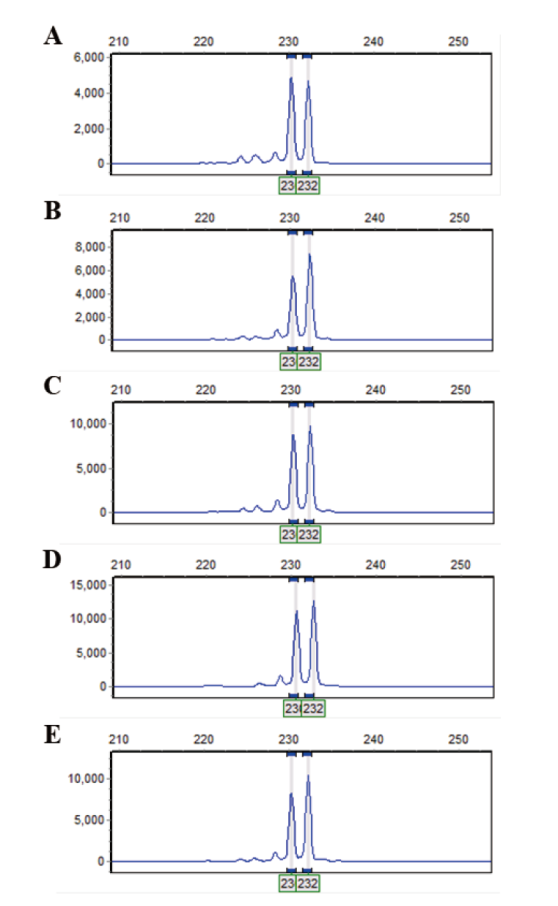
图4 UTS02-UTS06连接番茄SOL_SSR145引物的扩增结果 A:UTS02- SOL_SSR145的扩增结果;B:UTS03- SOL_SSR145的扩增结果;C:UTS04- SOL_SSR145的扩增结果;D:UTS05- SOL_SSR145的扩增结果;E:UTS06- SOL_SSR145的扩增结果
Fig. 4 Amplified results of UTS02-UTS06 ligated to tomato SOL_SSR145 primer A: Amplified results of UTS02- SOL_SSR145. B: Amplified results of UTS03- SOL_SSR145. C: Amplified results of UTS04- SOL_SSR145. D: Amplified results of UTS05- SOL_SSR145. E: Amplified results of UTS06- SOL_SSR145
| 物种名称 Species | 测试引物总数Total number of primers tested | M13扩增成功率M13 amplified success rate/% | 单UTS扩增成功率Single UTS amplified success rate/% | 两个UTS扩增成功率Two UTS amplified success rate/% |
|---|---|---|---|---|
| 玉米Z. mays | 40 | 72.5 | 85.0 | 95.0 |
| 西瓜C. lanatus | 28 | 96.4 | 100.0 | 100.0 |
| 番茄S. lycopersicum | 24 | 87.5 | 91.7 | 100.0 |
| 辣椒C. annuum | 22 | 86.4 | 86.4 | 95.5 |
表5 通用尾巴序列与M13接头的多作物扩增效果
Table 5 UTS- and M13-amplified effects on various crops
| 物种名称 Species | 测试引物总数Total number of primers tested | M13扩增成功率M13 amplified success rate/% | 单UTS扩增成功率Single UTS amplified success rate/% | 两个UTS扩增成功率Two UTS amplified success rate/% |
|---|---|---|---|---|
| 玉米Z. mays | 40 | 72.5 | 85.0 | 95.0 |
| 西瓜C. lanatus | 28 | 96.4 | 100.0 | 100.0 |
| 番茄S. lycopersicum | 24 | 87.5 | 91.7 | 100.0 |
| 辣椒C. annuum | 22 | 86.4 | 86.4 | 95.5 |
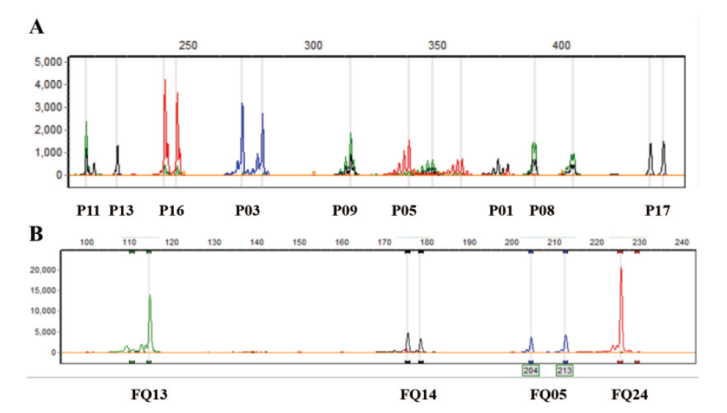
图5 UTS多重检测体系 A:UTS在玉米上的多重检测体系;B:UTS在番茄上的多重检测体系
Fig. 5 Multiple detection systems for UTS A: Multiple detection system for UTS on maize. B: Multiple detection system for UTS on tomatoes
| [1] | 谭智广. 低功耗聚合酶链式反应温度控制系统的研制[D]. 大连: 大连理工大学, 2023. |
| Tan GZ. Development of low power consumption temperature control system of PCR[D]. Dalian: Dalian University of Technology, 2023. | |
| [2] | Zhu HL, Zhang HQ, Xu Y, et al. PCR past, present and future[J]. BioTechniques, 2020, 69(4): 317-325. |
| [3] | 郑戈文. 农作物种子检测中常用电泳方法的比较分析[J]. 中国种业, 2018(11): 35-37. |
| Zheng GW. Comparative analysis of electrophoresis methods commonly used in crop seed detection[J]. China Seed Ind, 2018(11): 35-37. | |
| [4] | 王爱娜, 王灏, 李殿荣, 等. 琼脂糖与聚丙烯酰胺凝胶电泳在SSR鉴定杂交油菜种子纯度中的比较[J]. 西北农业学报, 2012, 21(8): 101-106. |
| Wang AN, Wang H, Li DR, et al. Comparison of agarose and polyacrylamide gel electrophoresis for SSR in purity identification of qinyou 33 hybrid rape seed[J]. Acta Agric Boreali Occidentalis Sin, 2012, 21(8): 101-106. | |
| [5] | 王竹林, 杨睿, 刘联正, 等. 聚丙烯酰胺凝胶电泳银染的影响因素[J]. 实验室研究与探索, 2011, 30(9): 15-17, 113. |
| Wang ZL, Yang R, Liu LZ, et al. Factors of silver staining of polyacrylamide gel electrophoresis[J]. Res Explor Lab, 2011, 30(9): 15-17, 113. | |
| [6] |
冯琬淇, 张福顺, 刘乃新. 毛细管与聚丙烯酰胺凝胶电泳检测甜菜SSR位点的比较研究[J]. 中国农学通报, 2022, 38(36): 34-41.
doi: 10.11924/j.issn.1000-6850.casb2022-0412 |
|
Feng WQ, Zhang FS, Liu NX. SSR polymorphism in sugar beet: detection by polyacrylamide gel electrophoresis and capillary electrophoresis[J]. Chin Agric Sci Bull, 2022, 38(36): 34-41.
doi: 10.11924/j.issn.1000-6850.casb2022-0412 |
|
| [7] | 刘文彬, 许理文, 王凤格, 等. 基于两种荧光毛细管电泳平台筛选评估玉米新型SSR引物[J]. 玉米科学, 2017, 25(2): 24-30. |
| Liu WB, Xu LW, Wang FG, et al. Evaluating and screening new maize SSR primer based on two kinds of fluorescent capillary eelectrophoresis platform[J]. J Maize Sci, 2017, 25(2): 24-30. | |
| [8] |
管俊娇, 杨晓洪, 张鹏, 等. 基于荧光检测技术的青稞品种鉴定方法的建立[J]. 生物技术通报, 2021, 37(11): 293-302.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-1494 |
| Guan JJ, Yang XH, Zhang P, et al. Establishment of a method identifying the varieties of hulless barley based on fluorescence detection technology[J]. Biotechnol Bull, 2021, 37(11): 293-302. | |
| [9] | 方梅, 胡波, 侯媛媛, 等. 毛细管电泳在基因研究中的应用[J]. 生物技术通报, 2006(2): 60-66. |
| Fang M, Hu B, Hou YY, et al. The application of capillary electrophoresis to gene analysis[J]. Biotechnol Bull, 2006(2): 60-66. | |
| [10] | 李会勇, 王天宇, 黎裕, 等. TP-M13自动荧光检测法在高粱SSR基因型鉴定中的应用[J]. 植物遗传资源学报, 2005, 6(1): 68-70. |
| Li HY, Wang TY, Li Y, et al. Application of the TP-M13 automated fluorescent-lablled system of SSR genotyping in sorghum[J]. J Plant Genet Resour, 2005, 6(1): 68-70. | |
| [11] |
Schuelke M. An economic method for the fluorescent labeling of PCR fragments[J]. Nat Biotechnol, 2000, 18(2): 233-234.
doi: 10.1038/72708 pmid: 10657137 |
| [12] | 匡猛, 王凤格, 赵久然, 等. 玉米SSR引物尾巴序列的设计方法[J]. 农业生物技术学报, 2007, 15(6): 1072-1073. |
| Kuang M, Wang FG, Zhao JR, et al. A method of designing tail sequence for maize SSR primers[J]. J Agric Biotechnol, 2007, 15(6): 1072-1073. | |
| [13] |
曾斌, 马鑫鑫, 余镇藩, 等. 新疆野扁桃的TP-M13-SSR荧光标记引物的筛选[J]. 中国农学通报, 2019, 35(1): 45-49.
doi: 10.11924/j.issn.1000-6850.casb18070067 |
| Zeng B, Ma XX, Yu ZF, et al. Wild almond(Prunus tenella batsch.) in Xinjiang: screening of TP-M13-SSR primers in capillary fluorescent electrophoresis[J]. Chin Agric Sci Bull, 2019, 35(1): 45-49. | |
| [14] | 赵紫薇, 任洁, 刘亚维, 等. 一种适用于玉米胚乳DNA提取的新方法[J]. 分子植物育种, 2023: 1-10. |
| Zhao ZW, Ren J, Liu YW, et al. A New Method for DNA Extraction from Maize Endosperm[J]. Molecular Plant Breeding, 2023: 1-10. | |
| [15] | 国家市场监督管理总局, 国家标准化管理委员会. 主要农作物品种真实性和纯度SSR分子标记检测玉米: GB/T 39914—2021[S]. 北京: 中国标准出版社, 2021. |
| State Administration of Market Supervision and administration, Standardization Administration of the People's Republic of China. Variety genuineness and purity testing of main crops with SSR markers—Maize: GB/T 39914—2021[S]. Beijing: Standards Press of China, 2021. | |
| [16] | 中华人民共和国农业部. 番茄品种鉴定技术规程 Indel分子标记法: NY/T 2471—2013[S]. 北京: 中国农业出版社, 2014. |
| Ministry of Agriculture of the People's Republic of China. Identification of tomato varieties—Indel marker method: NY/T 2471—2013[S]. Beijing: China Agriculture Press, 2014. | |
| [17] | 中华人民共和国农业部. 西瓜品种鉴定技术规程 SSR分子标记法: NY/T 2472—2013[S]. 北京: 中国农业出版社, 2014. |
| Ministry of Agriculture of the People's Republic of China. Identification of watermelon varieties—SSR marker method: NY/T 2472—2013[S]. Beijing: China Agriculture Press, 2014. | |
| [18] | 中华人民共和国农业部. 辣椒品种鉴定技术规程 SSR分子标记法: NY/T 2475—2013[S]. 北京: 中国农业出版社, 2014. |
| Ministry of Agriculture of the People's Republic of China. Identification of pepper varieties—SSR marker method: NY/T 2475—2013[S]. Beijing: China Agriculture Press, 2014. | |
| [19] | 刘志斋, 王天宇, 黎裕. TP-M13-SSR技术及其在玉米遗传多样性研究中的应用[J]. 玉米科学, 2007, 15(6): 10-15, 31. |
| Liu ZZ, Wang TY, Li Y. TP-M13-SSR technique and its applications in the analysis of genetic diversity in maize[J]. J Maize Sci, 2007, 15(6): 10-15, 31. | |
| [20] | 李小娟, 刘文雅, 何彦峰, 等. 葫芦藓叶绿体基因组密码子使用模式分析[J]. 分子植物育种, 2023: 1-15. |
| Li XJ, Liu WY, He YF, et al. Analysis of codon usage patterns in chloroplast genomes of funaria hygrometrica[J]. Molecular Plant Breeding, 2023: 1-15. | |
| [21] |
仇学文, 李丹, 甘玉迪, 等. 豇豆叶绿体基因组密码子使用偏好性分析[J]. 核农学报, 2023, 37(6): 1118-1129.
doi: 10.11869/j.issn.1000-8551.2023.06.1118 |
|
Qiu XW, Li D, Gan YD, et al. Analysis of codon usage bias in the cowpea chloroplast genome[J]. J Nucl Agric Sci, 2023, 37(6): 1118-1129.
doi: 10.11869/j.issn.1000-8551.2023.06.1118 |
|
| [22] | 雷佳欣, 张丽娟, 高丹丹, 等. 文冠果基因组密码子偏好性分析[J]. 西北林学院学报, 2023, 38(4): 104-110. |
| Lei JX, Zhang LJ, Gao DD, et al. Codon usage bias of Xanthoceras sorbifolium genome[J]. J Northwest For Univ, 2023, 38(4): 104-110. |
| [1] | 钟匀, 林春, 刘正杰, 董陈文华, 毛自朝, 李兴玉. 芦笋皂苷合成相关糖基转移酶基因克隆及原核表达分析[J]. 生物技术通报, 2024, 40(4): 255-263. |
| [2] | 杨淇, 魏子迪, 宋娟, 童堃, 杨柳, 王佳涵, 刘海燕, 栾维江, 马轩. 水稻组蛋白H1三突变体的创建和转录组学分析[J]. 生物技术通报, 2024, 40(4): 85-96. |
| [3] | 郑巧, 林华, 徐浩, 安微, 薛昌华, 张婧, 韩国全. SARS-CoV-2 Omicron 变异株多重微滴式数字 PCR 定量方法的建立及应用[J]. 生物技术通报, 2024, 40(2): 80-89. |
| [4] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [5] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [6] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [7] | 宋海娜, 吴心桐, 杨鲁豫, 耿喜宁, 张华敏, 宋小龙. 葱鳞葡萄孢菌诱导下韭菜RT-qPCR内参基因的筛选和验证[J]. 生物技术通报, 2023, 39(3): 101-115. |
| [8] | 李天顺, 李宸葳, 王佳, 朱龙佼, 许文涛. 功能核酸筛选过程中次级文库的有效制备[J]. 生物技术通报, 2023, 39(3): 116-122. |
| [9] | 余世洲, 曹领改, 王世泽, 刘勇, 边文杰, 任学良. 烟草种质基因分型核心SNP标记的开发[J]. 生物技术通报, 2023, 39(3): 89-100. |
| [10] | 穆德添, 万凌云, 章瑶, 韦树根, 陆英, 付金娥, 田艺, 潘丽梅, 唐其. 钩藤管家基因筛选及生物碱合成相关基因的表达分析[J]. 生物技术通报, 2023, 39(2): 126-138. |
| [11] | 滕梦鑫, 徐亚, 何静, 汪奇, 乔飞, 李敬阳, 李新国. 香蕉MaMC6的克隆及原核表达分析[J]. 生物技术通报, 2023, 39(12): 179-186. |
| [12] | 李会杰, 董莲华, 陈桂芳, 刘思渊, 杨佳怡, 杨靖亚. 食品中椰毒假单胞菌微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2023, 39(1): 127-136. |
| [13] | 胡雪莹, 张越, 郭雅杰, 仇天雷, 高敏, 孙兴滨, 王旭明. 不同施肥处理农田土壤中噬菌体与细菌携带抗生素抗性基因的比较[J]. 生物技术通报, 2022, 38(9): 116-126. |
| [14] | 程深伟, 张克强, 梁军锋, 刘福元, 郜兴亮, 杜连柱. 畜禽养殖粪污中典型致病菌的三重微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2022, 38(9): 271-280. |
| [15] | 曹英芳, 赵新, 刘双, 李瑞环, 刘娜, 徐石勇, 高芳瑞, 马卉, 兰青阔, 檀建新, 王永. 抗除草剂大豆GE-J12实时荧光定量PCR检测方法的建立[J]. 生物技术通报, 2022, 38(7): 146-152. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||