








生物技术通报 ›› 2024, Vol. 40 ›› Issue (7): 314-322.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1205
金博阳1( ), 秦仕宇1, 张明达1, 李倩倩1, 文静1, 沈秀丽2, 杜志强1(
), 秦仕宇1, 张明达1, 李倩倩1, 文静1, 沈秀丽2, 杜志强1( )
)
收稿日期:2023-12-24
出版日期:2024-07-26
发布日期:2024-07-30
通讯作者:
杜志强,男,博士,教授,研究方向:无脊椎动物先天免疫;E-mail: nmdzq1981@163.com作者简介:金博阳,男,研究方向:无脊椎动物先天免疫;E-mail: m19556819988@163.com
基金资助:
JIN Bo-yang1( ), QIN Shi-yu1, ZHANG Ming-da1, LI Qian-qian1, WEN Jing1, SHEN Xiu-li2, DU Zhi-qiang1(
), QIN Shi-yu1, ZHANG Ming-da1, LI Qian-qian1, WEN Jing1, SHEN Xiu-li2, DU Zhi-qiang1( )
)
Received:2023-12-24
Published:2024-07-26
Online:2024-07-30
摘要:
【目的】 旨在研究prx 6基因在小龙虾先天免疫中的调控作用机制,探索小龙虾prx 6基因在金黄色葡萄球菌(Staphylococcus aureus)感染后发挥免疫调控作用的可能分子机制。【方法】 选取prx 6基因作为研究对象,提取正常小龙虾各组织的总RNA,利用RT-qPCR分析目的基因在小龙虾各组织中的分布,并检测S. aureus免疫刺激后,prx 6基因在小龙虾相关组织中的表达模式。通过RNA干扰技术(RNAi)敲低prx 6基因表达量,在感染S. aureus后,测定小龙虾肝胰腺中Pc-crustin 3、Pc-crustin 4、Pc-ALF 9和Pc-lectin 1免疫效应基因的相对表达量。同时,进一步对小龙虾血淋巴中的细菌载量进行统计分析。【结果】 通过对prx 6基因在小龙虾各组织的表达水平分析,观察到该基因在肝胰腺的表达量显著高于其他组织。在S. aureus感染后,小龙虾肝胰腺、血细胞和肠组织中prx 6基因的表达量显著上调,在鳃组织仅有早期表达量有所增加。小龙虾存活率的检测结果表明RNAi下调prx 6基因表达量之后,其生存率显著低于dsGFP+S. aureus对照组。为了进一步探索死亡率升高的原因,研究了抗菌肽基因在抗细菌防御中的表达水平。RNAi实验结果显示,在prx 6基因表达量降低的情况下,小龙虾肝胰腺Pc-crustin 3、Pc-crustin 4、Pc-ALF 9以及Pc-lectin 1基因表达水平都出现了显著下降。此外,小龙虾血淋巴中的细菌载量检测结果显示,prx 6基因RNAi组的细菌载量显著高于dsGFP+S. aureus对照组。【结论】 小龙虾prx 6基因通过影响抗菌肽基因的表达,以及血淋巴细菌清除能力来参与抗细菌先天免疫应答。
金博阳, 秦仕宇, 张明达, 李倩倩, 文静, 沈秀丽, 杜志强. 小龙虾prx 6基因在对抗金黄色葡萄球菌感染中的分子作用机制研究[J]. 生物技术通报, 2024, 40(7): 314-322.
JIN Bo-yang, QIN Shi-yu, ZHANG Ming-da, LI Qian-qian, WEN Jing, SHEN Xiu-li, DU Zhi-qiang. Research on the Molecular Mechanism of Crayfish prx 6 in the Process of Defending against Staphylococcus aureus Infection[J]. Biotechnology Bulletin, 2024, 40(7): 314-322.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| prx 6-RT-F | CGGATCACTGGAGGGTCA AACA |
| prx 6-RT-R | GCA ATTTTCATCCTCGGCATCA |
| prx 6-iF | GCGTAATACGACTCACTATAGGATGGTTAACTTAGGCGAT |
| prx 6-iR | GCGTAATACGACTCACTATAGGGTCTATGGCTCTAAGAAT |
| GFP-iF | GCGTAATACGACTCACTATAGGCGAGCTGGACGGCGACGTAAAC |
| GFP-iR | GCGTAATACGACTCACTATAGGCTTGAAGTTCACCTTGATGCC |
| 18S RNA-RT-F | TCTTCTTAGAGGGATTAGCGG |
| 18S RNA-RT-R | AAGGGGATTGAACGGGTTA |
| Pc-ALF 9-RT-F | AGTGGCGTCATACAGGAAGGGG |
| Pc-ALF 9-RT-R | CCAAAGGATGGCGAGAAATAGT |
| Pc-crustin 3-RT-F | TACGTCTTGCCCTCGTCTTA |
| Pc-crustin 3-RT-R | CAGCGTCCTCCTCTTTGTAATC |
| Pc-crustin 4-RT-F | CTCTGACTGCCAGGTCTTT |
| Pc-crustin 4-RT-R | TGCGAGCTGTGATGGTTAG |
| Pc-lectin 1-RT-F | GGGGAGGGCTGCTACTACTTG |
| Pc-lectin 1-RT-R | CGTGCCACCCACCCATAAG |
表1 RT-qPCR引物序列
Table 1 RT-qPCR primer sequences
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| prx 6-RT-F | CGGATCACTGGAGGGTCA AACA |
| prx 6-RT-R | GCA ATTTTCATCCTCGGCATCA |
| prx 6-iF | GCGTAATACGACTCACTATAGGATGGTTAACTTAGGCGAT |
| prx 6-iR | GCGTAATACGACTCACTATAGGGTCTATGGCTCTAAGAAT |
| GFP-iF | GCGTAATACGACTCACTATAGGCGAGCTGGACGGCGACGTAAAC |
| GFP-iR | GCGTAATACGACTCACTATAGGCTTGAAGTTCACCTTGATGCC |
| 18S RNA-RT-F | TCTTCTTAGAGGGATTAGCGG |
| 18S RNA-RT-R | AAGGGGATTGAACGGGTTA |
| Pc-ALF 9-RT-F | AGTGGCGTCATACAGGAAGGGG |
| Pc-ALF 9-RT-R | CCAAAGGATGGCGAGAAATAGT |
| Pc-crustin 3-RT-F | TACGTCTTGCCCTCGTCTTA |
| Pc-crustin 3-RT-R | CAGCGTCCTCCTCTTTGTAATC |
| Pc-crustin 4-RT-F | CTCTGACTGCCAGGTCTTT |
| Pc-crustin 4-RT-R | TGCGAGCTGTGATGGTTAG |
| Pc-lectin 1-RT-F | GGGGAGGGCTGCTACTACTTG |
| Pc-lectin 1-RT-R | CGTGCCACCCACCCATAAG |
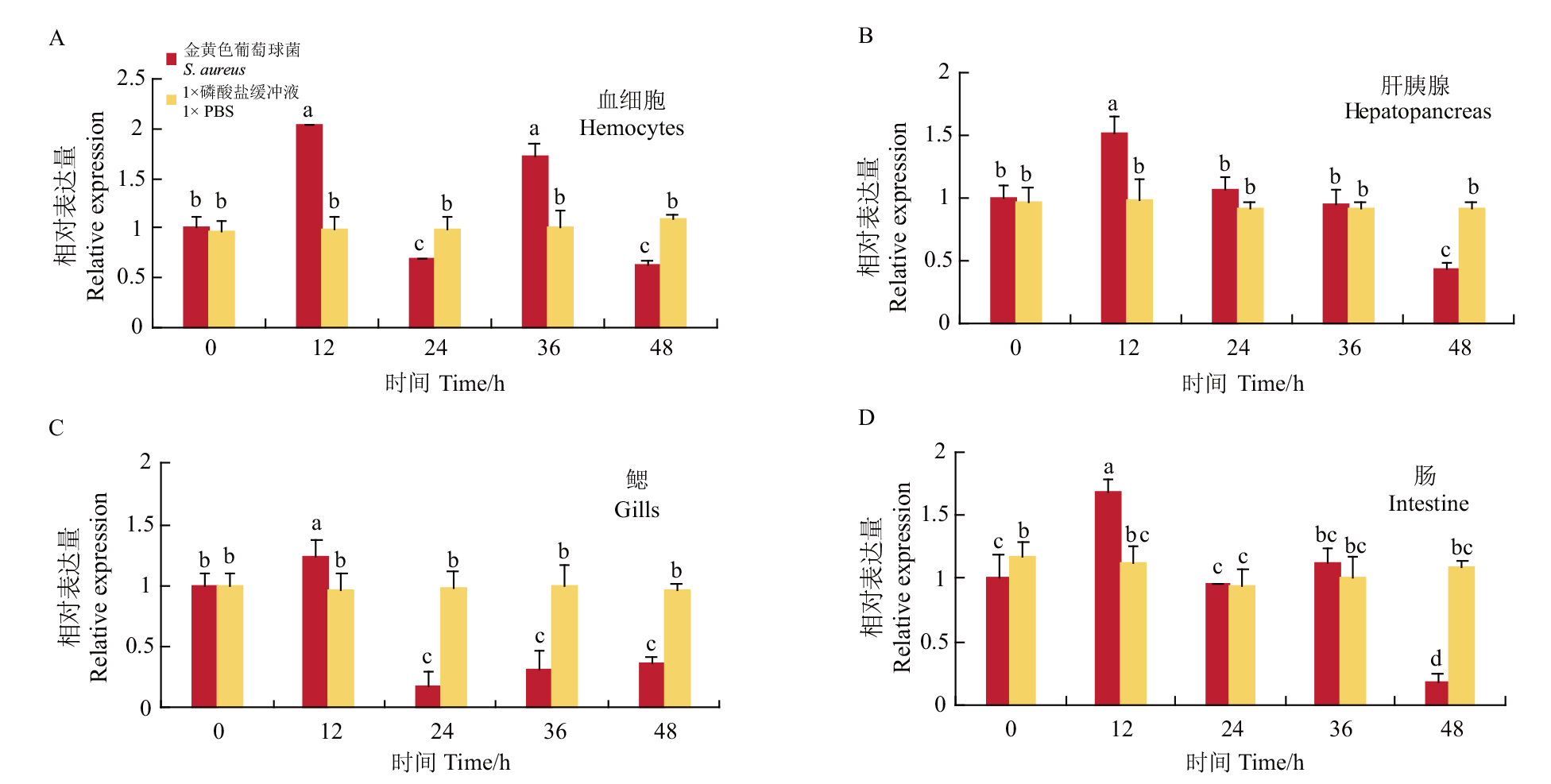
图2 金黄色葡萄球菌刺激后prx 6的表达模式 金黄色葡萄球菌刺激后prx 6在4种组织中的相对表达模式;不同小写字母表示差异显著(P<0.05);相同字母表示差异不显著(P>0.05),下同
Fig. 2 Expression patterns of prx 6 after S. aureus stimulation Relative expression of prx 6 in tissues after S. aureus infection. Different lowercase letters showed significant differences(P<0.05). The same letters indicate insignificant differences(P>0.05). The same below
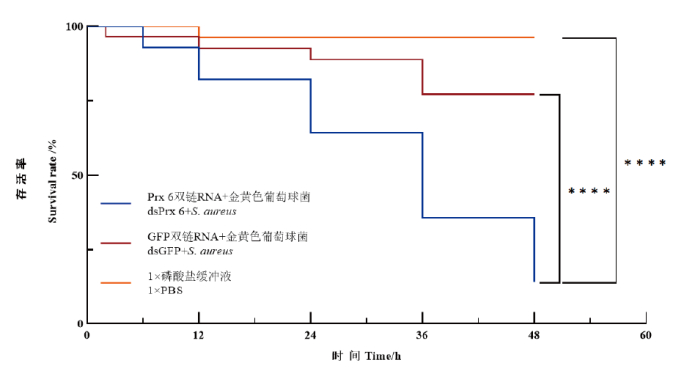
图3 RNAi并且注射金黄色葡萄球菌之后小龙虾存活率统计 ****表示组间差异极显著(P<0.000 1)
Fig. 3 Survival rates of P. clarkii statistics after S. aureus injection in RNAi assay **** indicates highly significant different with control group(P<0.000 1)

图4 RNAi试验中S. aureus刺激后相关抗菌肽基因在肝胰腺中的相对表达量分析结果 18S RNA作为内参对照
Fig. 4 Results for relative expression analysis of Amps genes in crayfish hepatopancreas after S. aureus challenge in RNAi assay 18S RNA is used as inner control
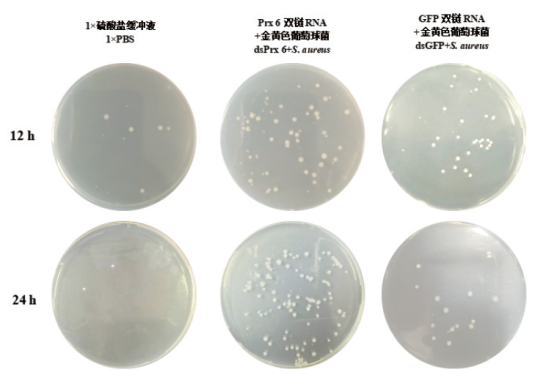
图5 RNAi试验中的小龙虾血淋巴细菌清除结果 分别将1×PBS组、dsPrx 6+S. aureus组和dsGFP+S. aureus组小龙虾的血淋巴涂板在琼脂平板上。检测了12 h和24 h的细菌丰度
Fig. 5 Bacterial clearance experiments results in crayfish hemolymph in RNAi assay The hemolymph of crayfish from 1×PBS, dsPrx 6 + S. aureus and dsGFP + S. aureus groups was coated on the agar plate. Bacterial abundance at 12 and 24 h was detected
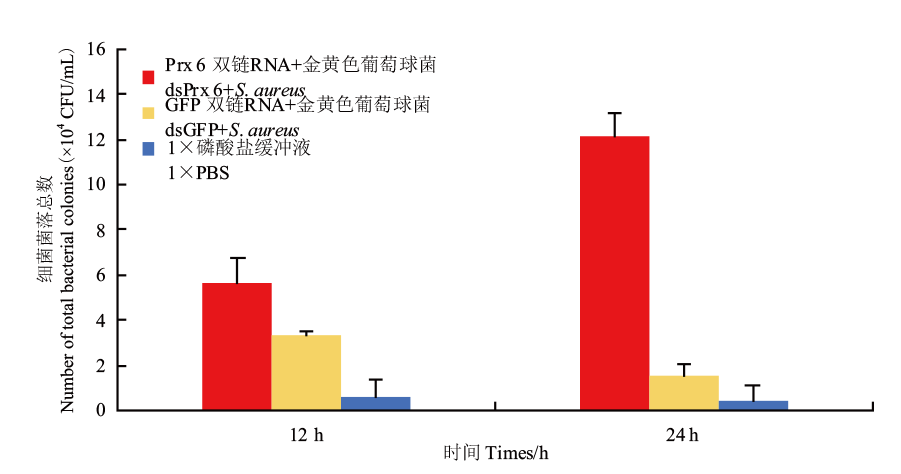
图6 细菌菌落数统计结果 对LB平板中的单个菌落进行计数,使用公式进行计算
Fig. 6 Statistics results of bacterial colonies Individual colonies in the LB plates were counted and cakulated using the formula
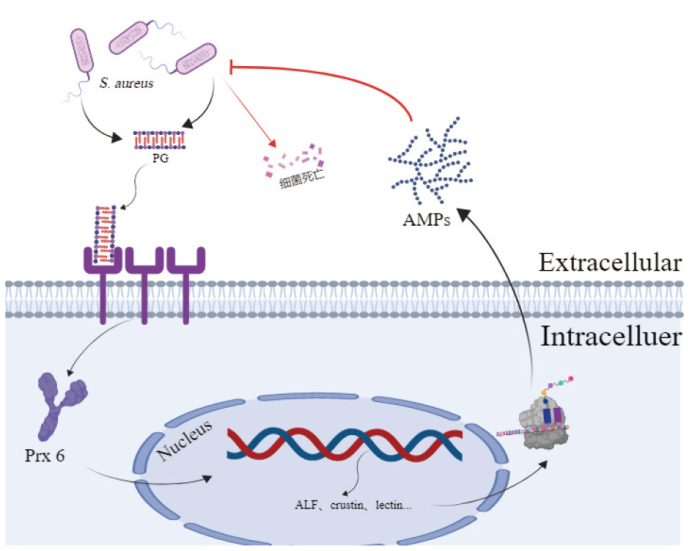
图7 prx 6通过影响抗菌肽基因的表达来抵抗金黄色葡萄球菌侵染的理论推测 小龙虾受到金黄色葡萄球菌刺激后,通过激活体内prx 6,进一步影响抗菌肽基因的转录与表达,分泌出抗菌肽抵抗外来病原菌
Fig. 7 Theoretical speculation for prx 6 affecting the expression of Amps genes to defend against S. aureus infection After S. aureus infection, crayfish can further modulate the transcription and expression of genes related to antimicrobial peptides by activating prx 6, thereby facilitating the secretion of antimicrobial peptides against foreign pathogens
| [1] | 夏越勇, 周国勤, 刘炜. 小龙虾常见病害及防治措施[J]. 水产养殖, 2023, 44(4): 69-72. |
| Xia YY, Zhou GQ, Liu W. Common diseases of crayfish and their control measures[J]. J Aquac, 2023, 44(4): 69-72. | |
| [2] | Qin ZD, Sarath Babu V, Lin HZ, et al. The immune function of prophenoloxidase from red swamp crayfish(Procambarus clarkii)in response to bacterial infection[J]. Fish Shellfish Immunol, 2019, 92: 83-90. |
| [3] | Park S, Ronholm J. Staphylococcus aureus in agriculture: lessons in evolution from a multispecies pathogen[J]. Clin Microbiol Rev, 2021, 34(2): e00182-e00120. |
| [4] | Forshaw TE, Reisz JA, Nelson KJ, et al. Specificity of human sulfiredoxin for reductant and peroxiredoxin oligomeric state[J]. Antioxidants, 2021, 10(6): 946. |
| [5] |
Wood ZA, Schröder E, Robin Harris J, et al. Structure, mechanism and regulation of peroxiredoxins[J]. Trends Biochem Sci, 2003, 28(1): 32-40.
doi: 10.1016/s0968-0004(02)00003-8 pmid: 12517450 |
| [6] |
Troussicot L, Burmann BM, Molin M. Structural determinants of multimerization and dissociation in 2-Cys peroxiredoxin chaperone function[J]. Structure, 2021, 29(7): 640-654.
doi: 10.1016/j.str.2021.04.007 pmid: 33945778 |
| [7] | Qiao K, Wang CC, Huang LQ, et al. Molecular characterization of a new tetrodotoxin-binding protein, peroxiredoxin-1, from Takifugu bimaculatus[J]. Int J Mol Sci, 2022, 23(6): 3071. |
| [8] | Liang XY, Li YM, Chu PF, et al. Grass carp prx 3 elevates host antioxidant activity and induces autophagy to inhibit grass carp reovirus(GCRV)replication[J]. Antioxidants, 2022, 11(10): 1952. |
| [9] | 李辉, 冯伟, 于俊杰, 等. 甲壳动物过氧化物还原酶基因的研究进展[J]. 浙江大学学报: 农业与生命科学版, 2021, 47(3): 284-294. |
| Li H, Feng W, Yu JJ, et al. Research progress of peroxiredoxin gene in crustaceans[J]. J Zhejiang Univ Agric Life Sci, 2021, 47(3): 284-294. | |
| [10] | Lang L, Wolf AC, Riedel M, et al. Substrate promiscuity and hyperoxidation susceptibility as potential driving forces for the co-evolution of Prx5-type and Prx6-type 1-cys peroxiredoxin mechanisms[J]. ACS Catal, 2023, 13(6): 3627-3643. |
| [11] | Zhang RR, Wang Y, Xu C, et al. Characterization of peroxiredoxin from Neocaridina denticulata sinensis and its antioxidant and DNA protection activity analysis[J]. Fish Shellfish Immunol, 2022, 127: 211-218. |
| [12] | Ren XC, Liu XP, Liu QH. Litopenaeus vannamei peroxiredoxin 2-like is involved in WSSV infection by interaction with wsv089 and VP26[J]. Dev Comp Immunol, 2022, 126: 104243. |
| [13] | Wanvimonsuk S, Somboonwiwat K. Peroxiredoxin-4 supplementation modulates the immune response, shapes the intestinal microbiome, and enhances AHPND resistance in Penaeus vannamei[J]. Fish Shellfish Immunol, 2023, 139: 108915. |
| [14] |
Yang YZ, Zhao Y, Yang L, et al. Characterization of 2-Cys peroxiredoxin 3 and 4 in common carp and the immune response against bacterial infection[J]. Comp Biochem Physiol B Biochem Mol Biol, 2018, 217: 60-69.
doi: S1096-4959(17)30201-4 pmid: 29277606 |
| [15] | Liu K, Liu JX, Zhang ZN, et al. Molecular characterization of three peroxiredoxin genes in Portunus pelagicus expressed in response to Vibrio alginolyticus challenge[J]. Aquac Rep, 2022, 27: 101391. |
| [16] | Xia XC, Yu RX, Li MB, et al. Molecular cloning and characterization of two genes encoding peroxiredoxins from freshwater bivalve Anodonta woodiana: Antioxidative effect and immune defense[J]. Fish Shellfish Immunol, 2018, 82: 476-491. |
| [17] | Abbas MN, Kausar S, Cui HJ. The biological role of peroxiredoxins in innate immune responses of aquatic invertebrates[J]. Fish Shellfish Immunol, 2019, 89: 91-97. |
| [18] | Shen HS, Hu YC, Ma YC, et al. In-depth transcriptome analysis of the red swamp crayfish Procambarus clarkii[J]. PLoS One, 2014, 9(10): e110548. |
| [19] | Mu CK, Zhao JM, Wang LL, et al. Molecular cloning and characterization of peroxiredoxin 6 from Chinese mitten crab Eriocheir sinensis[J]. Fish Shellfish Immunol, 2009, 26(6): 821-827. |
| [20] |
Rőszer T. The invertebrate midintestinal gland(“hepatopancreas”)is an evolutionary forerunner in the integration of immunity and metabolism[J]. Cell Tissue Res, 2014, 358(3): 685-695.
doi: 10.1007/s00441-014-1985-7 pmid: 25174684 |
| [21] | Dai LS, Abbas MN, Kausar S, et al. Transcriptome analysis of hepatopancraes of Procambarus clarkii challenged with polyriboinosinic polyribocytidylic acid(poly I: C)[J]. Fish Shellfish Immunol, 2017, 71: 144-150. |
| [22] | Cheng DW, Zhang HK, Liu HX, et al. Identification and molecular characterization of peroxiredoxin 6 from noble scallop Chlamys nobilis revealing its potent immune response and antioxidant property[J]. Fish Shellfish Immunol, 2020, 100: 368-377. |
| [23] | 谢亚凯. 两种过氧化物还原酶在日本囊对虾先天免疫中的功能研究[D]. 济南: 山东大学, 2016. |
| Xie YK. The function analysis of two peroxiredoxin subfamilies in innate immunity of kuruma shrimp marsupenaeus japonicus[D]. Jinan: Shandong University, 2016. | |
| [24] | Ran XQ, Gao L, Yan M, et al. Peroxiredoxin 4 interacts with domeless and participates in antibacterial immune response through the JAK/STAT pathway[J]. Front Immunol, 2022, 13: 907183. |
| [25] | Odnokoz O, Earland N, Badinloo M, et al. Peroxiredoxins play an important role in the regulation of immunity and aging in Drosophila[J]. Antioxidants, 2023, 12(8): 1616. |
| [26] |
Egessa R. Antimicrobial peptides from freshwater invertebrate species: potential for future applications[J]. Mol Biol Rep, 2022, 49(10): 9797-9811.
doi: 10.1007/s11033-022-07483-1 pmid: 35716292 |
| [27] | Guryanova SV, Balandin SV, Belogurova-Ovchinnikova OY, et al. Marine invertebrate antimicrobial peptides and their potential as novel peptide antibiotics[J]. Mar Drugs, 2023, 21(10): 503. |
| [28] | Safronova VN, Bolosov IA, Kruglikov RN, et al. Novel β-hairpin peptide from marine Polychaeta with a high efficacy against gram-negative pathogens[J]. Mar Drugs, 2022, 20(8): 517. |
| [29] | Ding D, Sun XJ, Yan M, et al. The ECSIT mediated Toll3-dorsal-ALFs pathway inhibits bacterial amplification in kuruma shrimp[J]. Front Immunol, 2022, 13: 807326. |
| [30] | Ni MQ, Zhang Y, Zheng JB, et al. HSP40 mediated TLR-Dorsal-AMPs pathway in Portunus trituberculatus[J]. Fish Shellfish Immunol, 2023, 133: 108536. |
| [31] | Mi R, Li XJ, Sun YX, et al. Effects of microbial community and disease resistance against Vibrio splendidus of Yesso scallop(Patinopecten yessoensis)fed supplementary diets of tussah immunoreactive substances and antimicrobial peptides[J]. Fish Shellfish Immunol, 2022, 121: 446-455. |
| [32] | Huang L, Liu Y, Zhang XX, et al. Peroxiredoxin 1 of Procambarus clarkii govern immune responses during pathogen infection[J]. Fish Shellfish Immunol, 2023, 138: 108828. |
| [33] | Rattanadilog Na Phuket T, Charoensapsri W, Amparyup P, et al. Antibacterial activity and immunomodulatory role of a proline-rich antimicrobial peptide SpPR-AMP1 against Vibrio campbellii infection in shrimp Litopenaeus vannamei[J]. Fish Shellfish Immunol, 2023, 132:108479 |
| [34] | Wanvimonsuk S, Jaree P, Kawai T, et al. Prx4 acts as DAMP in shrimp, enhancing bacterial resistance via the toll pathway and prophenoloxidase activation[J]. iScience, 2022, 26(1): 105793. |
| [35] | Huang Y, Jiang Y, Wang MM, et al. Mannose-binding C-type lectin from Procambarus clarkii exhibited antimicrobial activity to mediate crayfish innate immunity[J]. Aquac Rep, 2023, 32: 101707. |
| [1] | 陈晓萌, 张雪静, 张欢, 张宝江, 苏艳. 重组牛乳源金黄色葡萄球菌GapC蛋白优势B细胞抗原表位的预测和筛选[J]. 生物技术通报, 2023, 39(5): 306-313. |
| [2] | 叶红, 王玉昆. 植物PRR免疫受体功能研究进展[J]. 生物技术通报, 2023, 39(12): 1-15. |
| [3] | 张龙喜, 吕琳, 张欢欢, 周金成, 车午男, 董辉. RNAi技术在寄生蜂中的应用研究进展[J]. 生物技术通报, 2023, 39(12): 99-108. |
| [4] | 程深伟, 张克强, 梁军锋, 刘福元, 郜兴亮, 杜连柱. 畜禽养殖粪污中典型致病菌的三重微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2022, 38(9): 271-280. |
| [5] | 王子琰, 王建, 张伦, 桂水清, 卢雪梅. 家蝇抗菌肽MDC对鼠伤寒沙门氏菌的抑菌稳定性研究[J]. 生物技术通报, 2022, 38(3): 149-156. |
| [6] | 刘晓玫, 王东鑫, 张春, 魏双施. AAV介导的RNAi对SARS-CoV-2 S基因表达的抑制作用[J]. 生物技术通报, 2022, 38(3): 188-193. |
| [7] | 郭宇飞, 闫荣媚, 张小茹, 曹威, 刘浩. 代谢工程改造黑曲霉生产葡萄糖二酸[J]. 生物技术通报, 2022, 38(11): 227-237. |
| [8] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [9] | 李凯晴, 李莹, 王艺磊, 邹鹏飞. 受体相互作用蛋白的功能及在硬骨鱼类中的研究进展[J]. 生物技术通报, 2021, 37(5): 197-211. |
| [10] | 潘银来, 邱春辉, 王艺磊, 张子平. RNA药物的发展及其在水产上的应用[J]. 生物技术通报, 2021, 37(2): 203-215. |
| [11] | 邓普荣, 刘勇波. RNAi与转Bt基因技术协同抗虫研究进展[J]. 生物技术通报, 2021, 37(10): 216-224. |
| [12] | 徐雪亮, 王奋山, 刘子荣, 范琳娟, 季香云, 蒋杰贤, 姚英娟. RNA干扰技术在昆虫学领域研究进展[J]. 生物技术通报, 2021, 37(1): 255-261. |
| [13] | 蒋成辉, 曾巧英, 王萌, 潘阳阳, 刘旭明, 尚天甜. CRISPR/Cas9构建srtA基因敲除的金黄色葡萄球菌[J]. 生物技术通报, 2020, 36(9): 253-265. |
| [14] | 杨文文, 倪嘉瑶, 胡蕊洁, 王华忠. 一个RNAi载体上反向重复片段的测序策略[J]. 生物技术通报, 2020, 36(5): 205-210. |
| [15] | 宋华丽, 孙效迎, 孔祥会, 李莉, 裴超. RNA干扰技术在水产动物抗病毒和抗寄生虫研究中的应用研究进展[J]. 生物技术通报, 2020, 36(2): 193-205. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||