








生物技术通报 ›› 2024, Vol. 40 ›› Issue (8): 63-73.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0205
武帅1( ), 辛燕妮1, 买春海1, 穆晓娅1, 王敏1, 岳爱琴1, 赵晋忠3, 吴慎杰2, 杜维俊1(
), 辛燕妮1, 买春海1, 穆晓娅1, 王敏1, 岳爱琴1, 赵晋忠3, 吴慎杰2, 杜维俊1( ), 王利祥1(
), 王利祥1( )
)
收稿日期:2024-03-04
出版日期:2024-08-26
发布日期:2024-09-05
通讯作者:
杜维俊,女,博士,研究方向:作物遗传育种;E-mail: duweijun68@126.com;作者简介:武帅,男,硕士,研究方向:大豆遗传育种;E-mail: 1925531945@qq.com
基金资助:
WU Shuai1( ), XIN Yan-ni1, MAI Chun-hai1, MU Xiao-ya1, WANG Min1, YUE Ai-qin1, ZHAO Jin-zhong3, WU Shen-jie2, DU Wei-jun1(
), XIN Yan-ni1, MAI Chun-hai1, MU Xiao-ya1, WANG Min1, YUE Ai-qin1, ZHAO Jin-zhong3, WU Shen-jie2, DU Wei-jun1( ), WANG Li-xiang1(
), WANG Li-xiang1( )
)
Received:2024-03-04
Published:2024-08-26
Online:2024-09-05
摘要:
【目的】谷氨酰胺合成酶(glutamine synthetase, GS)是氮代谢“GS-GOGAT循环”中的关键酶,研究GS在大豆中的家族成员以及对外界胁迫的响应情况。【方法】利用生物信息学方法,在大豆中全面鉴定GS基因,明确大豆GmGSs基因的位置与结构、蛋白的理化性质以及组织表达模式等,并对非生物胁迫的应答响应进行研究。【结果】从大豆中共鉴定出8个GS基因,位于8条染色体上,对应编码的氨基酸序列长度为356-432 aa。在10个保守基序中,8个GmGS都包含9个保守基序,GmGS7和GmGS8比其他成员多一个motif10保守基序。启动子顺式作用元件分析表明,GmGSs的启动子中包含丰富的光响应元件、激素响应元件和逆境胁迫响应元件。转录组数据分析表明GmGSs基因在所有组织中均有表达,其中GmGS3和GmGS4在各组织中表达量较高。荧光定量qPCR结果表明:在不同浓度的氯化铵处理后,大豆GS家族中GmGS4、GmGS5和GmGS7对低浓度铵盐处理后期响应最显著。且高盐胁迫处理后,GmGS5在根、茎、叶组织中表达量下降;GmGS7在根、茎组织中表达量上升。【结论】GS在大豆中共有8个成员,其中GmGS7在氯化铵处理和盐胁迫中均参与响应。
武帅, 辛燕妮, 买春海, 穆晓娅, 王敏, 岳爱琴, 赵晋忠, 吴慎杰, 杜维俊, 王利祥. 大豆GS基因家族全基因组鉴定及胁迫响应分析[J]. 生物技术通报, 2024, 40(8): 63-73.
WU Shuai, XIN Yan-ni, MAI Chun-hai, MU Xiao-ya, WANG Min, YUE Ai-qin, ZHAO Jin-zhong, WU Shen-jie, DU Wei-jun, WANG Li-xiang. Genome-wide Identification and Stress Response Analysis of Soybean GS Gene Family[J]. Biotechnology Bulletin, 2024, 40(8): 63-73.
| 基因ID Gene ID | 正向引物 Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| GmCYP2 | CGGGACCAGTGTGCTTCTTCA | CCCCTCCACTACAAAGGCTCG |
| GmGS1 | TAACACCAAACAGAGTCATTCACC | TGAGGTTGATGAGATCCGAAA |
| GmGS2 | GACTAATTTCCGGGGTTTCG | GGAGACCTTTTTCTTCCTCACAG |
| GmGS3 | GCTTTTCTTAGTAGGATTTGGTCTC | TAACAATCGGAAAACGAGGGA |
| GmGS4 | GTGGAAGCCATGAGCAAAACT | CGAGGGAAAGGAATAGAAAACA |
| GmGS5 | TTGGAAACCATAAGCAGCCTC | GCCAAGCATTGAAGTGTGAGA |
| GmGS6 | GCAACGTCAAAACAATCACATG | AACAACAGGCGAGGTAGTCACA |
| GmGS7 | GCTGGTGTTGTGCTCTCTCT | TCTTCCCACACGGATTGAGC |
| GmGS8 | ACCCAAAGGTCCAAGCAG | CCAGGATAGCCACCAACG |
表1 引物序列
Table 1 Primer sequence
| 基因ID Gene ID | 正向引物 Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| GmCYP2 | CGGGACCAGTGTGCTTCTTCA | CCCCTCCACTACAAAGGCTCG |
| GmGS1 | TAACACCAAACAGAGTCATTCACC | TGAGGTTGATGAGATCCGAAA |
| GmGS2 | GACTAATTTCCGGGGTTTCG | GGAGACCTTTTTCTTCCTCACAG |
| GmGS3 | GCTTTTCTTAGTAGGATTTGGTCTC | TAACAATCGGAAAACGAGGGA |
| GmGS4 | GTGGAAGCCATGAGCAAAACT | CGAGGGAAAGGAATAGAAAACA |
| GmGS5 | TTGGAAACCATAAGCAGCCTC | GCCAAGCATTGAAGTGTGAGA |
| GmGS6 | GCAACGTCAAAACAATCACATG | AACAACAGGCGAGGTAGTCACA |
| GmGS7 | GCTGGTGTTGTGCTCTCTCT | TCTTCCCACACGGATTGAGC |
| GmGS8 | ACCCAAAGGTCCAAGCAG | CCAGGATAGCCACCAACG |
| 基因名称 Gene name | 基因座位ID Gene locus ID | 转录本ID Transcript ID | 氨基酸数量Number of amino acids/aa | 理论等电点Theoretical pI | 分子量Molecular weight/kD | 不稳定系Instability index | 亲水平均数 Grand average of hydropathicity | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| GmGS1 | Glyma.09g173200 | KRH39032 | 356 | 5.22 | 39212.02 | 40.29 | -0.467 | Cytoplasmic |
| GmGS2 | Glyma.07g104500 | KRH48675 | 356 | 5.32 | 39148 | 40.49 | -0.442 | Cytoplasmic |
| GmGS3 | Glyma.11g215500 | KRH30930 | 356 | 5.48 | 38990.93 | 37.96 | -0.374 | Cytoplasmic |
| GmGS4 | Glyma.18g041100 | KRG97948 | 356 | 5.48 | 39140.98 | 39.47 | -0.425 | Cytoplasmic |
| GmGS5 | Glyma.14g213300 | KRH17324 | 356 | 6.12 | 39208.29 | 38.63 | -0.444 | Cytoplasmic |
| GmGS6 | Glyma.02g244000 | KRH72976 | 356 | 6.03 | 39326.47 | 39.75 | -0.428 | Cytoplasmic |
| GmGS7 | Glyma.13g210800 | KRH48676 | 432 | 6.42 | 47653.72 | 42.55 | -0.466 | Chloroplast |
| GmGS8 | Glyma.15g102000 | KRH30931 | 432 | 6.73 | 47691.83 | 43.16 | -0.458 | Chloroplast |
表2 大豆GS基因理化性质
Table 2 Physical and chemical properties analysis of GS genes in G. max
| 基因名称 Gene name | 基因座位ID Gene locus ID | 转录本ID Transcript ID | 氨基酸数量Number of amino acids/aa | 理论等电点Theoretical pI | 分子量Molecular weight/kD | 不稳定系Instability index | 亲水平均数 Grand average of hydropathicity | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| GmGS1 | Glyma.09g173200 | KRH39032 | 356 | 5.22 | 39212.02 | 40.29 | -0.467 | Cytoplasmic |
| GmGS2 | Glyma.07g104500 | KRH48675 | 356 | 5.32 | 39148 | 40.49 | -0.442 | Cytoplasmic |
| GmGS3 | Glyma.11g215500 | KRH30930 | 356 | 5.48 | 38990.93 | 37.96 | -0.374 | Cytoplasmic |
| GmGS4 | Glyma.18g041100 | KRG97948 | 356 | 5.48 | 39140.98 | 39.47 | -0.425 | Cytoplasmic |
| GmGS5 | Glyma.14g213300 | KRH17324 | 356 | 6.12 | 39208.29 | 38.63 | -0.444 | Cytoplasmic |
| GmGS6 | Glyma.02g244000 | KRH72976 | 356 | 6.03 | 39326.47 | 39.75 | -0.428 | Cytoplasmic |
| GmGS7 | Glyma.13g210800 | KRH48676 | 432 | 6.42 | 47653.72 | 42.55 | -0.466 | Chloroplast |
| GmGS8 | Glyma.15g102000 | KRH30931 | 432 | 6.73 | 47691.83 | 43.16 | -0.458 | Chloroplast |
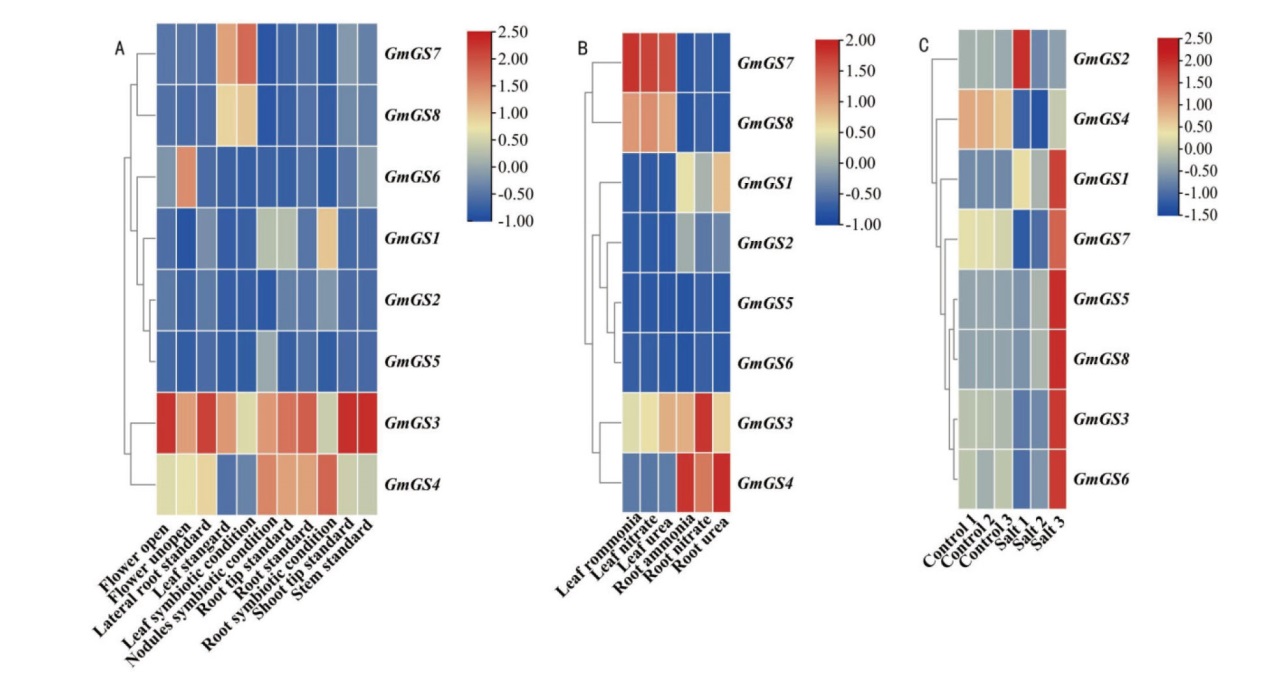
图6 大豆GS基因家族在不同组织(A)、不同氮处理(B)以及盐处理后(C)表达模式分析
Fig. 6 Expression patterns of G. max GS gene family in different tissues(A), nitrogen treatment(B), and salt treatment(C)
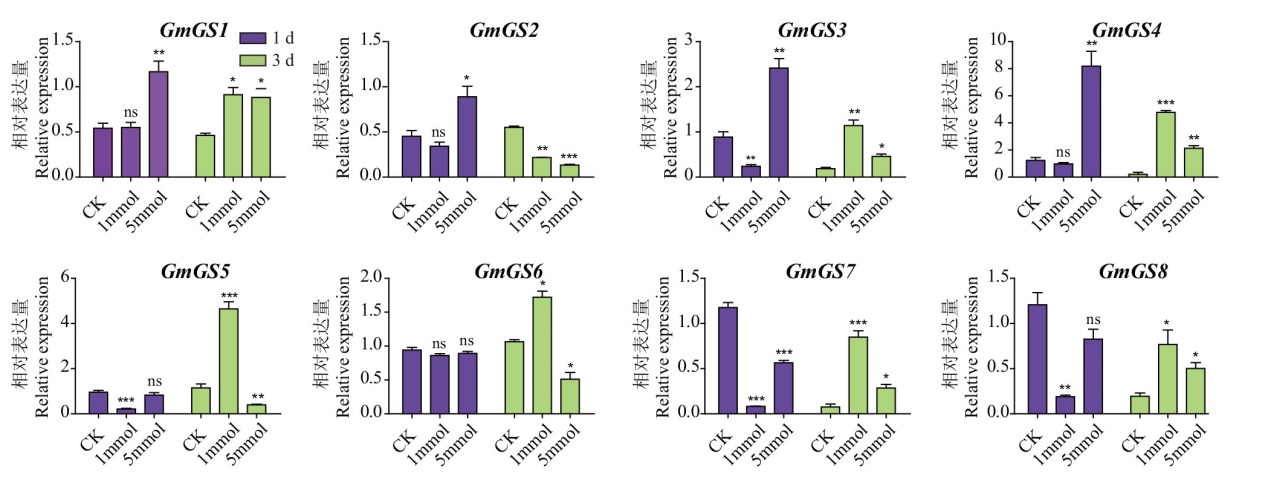
图7 大豆GS基因家族在铵盐处理后表达模式分析 1 d、3 d 分别表示处理后的1 d和3 d,CK、1 mmol、5 mmol分别表示氯化铵处理的浓度0 mmol/L、1 mmol/L、5 mmol/L。*、**、*** 分别表示与对照相比10%、5%、1%显著性水平,ns代表无显著性差异,下同
Fig. 7 Expression patterns of G. max GS family genes after ammonium salt treatment 1 d and 3 d indicate 1 and 3 days after treatment. CK, 1 mmol, and 5 mmol are the concentrations of ammonium chloride treatment, representing 0, 1 mmol/L, and 5 mmol/L, respectively. *, **, *** refers to 10%, 5% and 1% significance levels compared to control, and ns to no significant difference. The same below
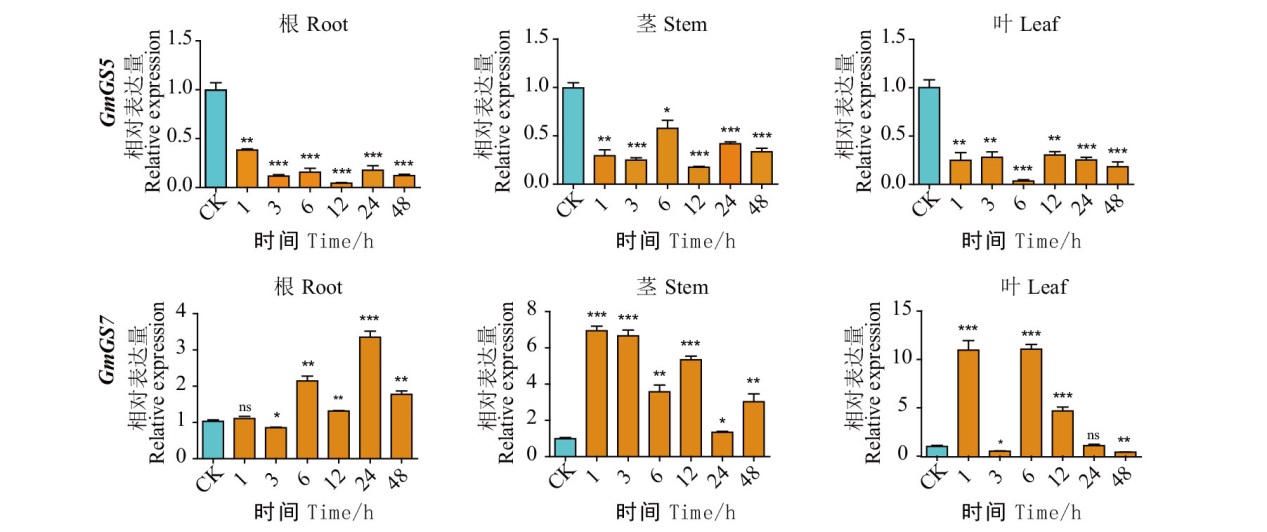
图8 大豆GS基因家族在盐处理后表达模式分析 CK表示未处理对照,1、3、6、12、24、48表示150 mmol/L NaCI 处理后的时间,分别从左到右取样了GmGS5和GmGS7的根、茎、叶组织部位
Fig. 8 Expression patterns of G. max GS family genes after salt treatment CK indicates the untreated control, 1, 3, 6, 12, 24, 48 indicate the time after 150 mmol/L NaCI treatment, and the root, stem, and leaf tissue sites of GmGS5 and GmGS7 were sampled from left to right, respectively
| [1] | Zhang Y, Shen SH, Liu YJ. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure[J]. Polym Degrad Stab, 2013, 98(2): 514-518. |
| [2] | 徐洪超, 商靖, 刘铭荟, 等. 氮代谢相关酶的研究进展[J]. 安徽农业科学, 2022, 50(4): 17-20. |
| Xu HC, Shang J, Liu MH, et al. Research progress of enzymes related to nitrogen metabolism[J]. J Anhui Agric Sci, 2022, 50(4): 17-20. | |
| [3] |
Bao AL, Zhao ZQ, Ding GD, et al. The stable level of glutamine synthetase 2 plays an important role in rice growth and in carbon-nitrogen metabolic balance[J]. Int J Mol Sci, 2015, 16(6): 12713-12736.
doi: 10.3390/ijms160612713 pmid: 26053400 |
| [4] | Lee KT, Liao HS, Hsieh MH. Glutamine metabolism, sensing and signaling in plants[J]. Plant Cell Physiol, 2023, 64(12): 1466-1481. |
| [5] | 陈胜勇, 李观康, 汪云, 等. 谷氨酰胺合成酶的研究进展[J]. 中国农学通报, 2010, 26(22): 45-49. |
|
Chen SY, Li GK, Wang Y, et al. The research progress of glutamine synthetase[J]. Chin Agric Sci Bull, 2010, 26(22): 45-49.
doi: 10.11924/j.issn.1000-6850.2010-1942 |
|
| [6] | 韩娜, 葛荣朝, 赵宝存, 等. 植物谷氨酰胺合成酶研究进展[J]. 河北师范大学学报, 2004, 28(4): 407-410, 423. |
| Han N, Ge RC, Zhao BC, et al. Research development of the glutamine synthetase in plants[J]. J Hebei Norm Univ Nat Sci, 2004, 28(4): 407-410, 423. | |
| [7] | 王小纯, 张同勋, 李高飞, 等. 小麦谷氨酰胺合成酶基因克隆与其表达特性分析[J]. 河南农业大学学报, 2012, 46(5): 487-492. |
| Wang XC, Zhang TX, Li GF, et al. Cloning of glutamine synthetases in wheat and analysis of their expression characteristics[J]. J Henan Agric Univ, 2012, 46(5): 487-492. | |
| [8] |
Morey KJ, Ortega JL, Sengupta-Gopalan C. Cytosolic glutamine synthetase in soybean is encoded by a multigene family, and the members are regulated in an organ-specific and developmental manner[J]. Plant Physiol, 2002, 128(1): 182-193.
pmid: 11788764 |
| [9] |
王晓波, 滕婉, 何雪, 等. 大豆谷氨酰胺合成酶基因的分类及根瘤特异表达GmGS1β2基因功能的初步分析[J]. 作物学报, 2013, 39(12): 2145-2153.
doi: 10.3724/SP.J.1006.2013.02145 |
| Wang XB, Teng W, He X, et al. Classification of glutamine synthetase gene and preliminary functional analysis of the nodule-predominantly expressed gene GmGS1β2 in soybean[J]. Acta Agron Sin, 2013, 39(12): 2145-2153. | |
| [10] | 王嘉文, 吴刚, 徐云敏. 谷氨酰胺合成酶在植物氮同化及再利用中的研究进展[J]. 分子植物育种, 2019, 17(4): 1373-1377. |
| Wang JW, Wu G, Xu YM. Research progress of glutamine synthetase in plant nitrogen assimilation and recycling[J]. Mol Plant Breed, 2019, 17(4): 1373-1377. | |
| [11] | 肖急祥, 曾长英, 彭明. 低氮条件下木薯谷氨酰胺合成酶(GS)酶活及GS家族基因表达分析[J]. 分子植物育种, 2016, 14(1): 21-25. |
| Xiao JX, Zeng CY, Peng M. Enzyme activity and expression analysis of glutamine synthetase GS gene in cassava under low-nitrogen stress[J]. Mol Plant Breed, 2016, 14(1): 21-25. | |
| [12] |
Sakakibara H, Shimizu H, Hase T, et al. Molecular identification and characterization of cytosolic isoforms of glutamine synthetase in maize roots[J]. J Biol Chem, 1996, 271(47): 29561-29568.
doi: 10.1074/jbc.271.47.29561 pmid: 8939884 |
| [13] | Caputo C, Criado MV, Roberts IN, et al. Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants[J]. Plant Physiol Biochem, 2009, 47(5): 335-342. |
| [14] | 张晓娇. 小麦GS基因家族的鉴定及非生物胁迫响应分析[D]. 郑州: 河南农业大学, 2022. |
| Zhang XJ. Identification and abiotic stress analysis of GS gene family in wheat(Triticum aestivum L.)[D]. Zhengzhou: Henan Agricultural University, 2022. | |
| [15] | 石慧, 王思明. 大豆在中国的历史变迁及其动因探究[J]. 农业考古, 2019(3): 32-39. |
| Shi H, Wang SM. Research on historical development and motivations of soybeans in China[J]. Agric Archaeol, 2019(3): 32-39. | |
| [16] | 张昊, 王文涛. 大豆产业国际竞争力提升的长效机制研究[J]. 湖南农业科学, 2022(6): 81-86. |
| Zhang H, Wang WT. Study on the long-term mechanism of improving the international competitiveness of soybean industry[J]. Hunan Agric Sci, 2022(6): 81-86. | |
| [17] |
石广成, 杨万明, 杜维俊, 等. 大豆耐盐种质的筛选及其耐盐生理特性分析[J]. 生物技术通报, 2022, 38(4): 174-183.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0843 |
| Shi GC, Yang WM, Du WJ, et al. Screening of salt-tolerant soybean germplasm and physiological characteristics analysis of its salt tolerance[J]. Biotechnol Bull, 2022, 38(4): 174-183. | |
| [18] | 王晓丽, 王敏, 岳爱琴, 等. 大豆TGL基因家族全基因组鉴定及高盐胁迫响应研究[J]. 大豆科学, 2022, 41(1): 12-19. |
| Wang XL, Wang M, Yue AQ, et al. Genome-wide identification of soybean TGL gene family and the response to high salt stress[J]. Soybean Sci, 2022, 41(1): 12-19. | |
| [19] |
Gasteiger E, Gattiker A, Hoogland C, et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis[J]. Nucleic Acids Res, 2003, 31(13): 3784-3788.
doi: 10.1093/nar/gkg563 pmid: 12824418 |
| [20] | 陈奕博, 杨万明, 岳爱琴, 等. 大豆LIM转录因子家族鉴定及盐胁迫响应分析[J]. 中国农业大学学报, 2023, 28(4): 26-40. |
| Chen YB, Yang WM, Yue AQ, et al. Identification of soybean LIM transcription factor family and analysis of salt stress response[J]. J China Agric Univ, 2023, 28(4): 26-40. | |
| [21] | Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching[J]. Nucleic Acids Res, 2009, 37(Web Server issue): W202-W208. |
| [22] |
Sun L, Song GS, Guo WJ, et al. Dynamic changes in genome-wide Histone3 Lysine27 trimethylation and gene expression of soybean roots in response to salt stress[J]. Front Plant Sci, 2019, 10: 1031.
doi: 10.3389/fpls.2019.01031 pmid: 31552061 |
| [23] | Bolay P, Muro-Pastor MI, Florencio FJ, et al. The distinctive regulation of cyanobacterial glutamine synthetase[J]. Life, 2018, 8(4): 52. |
| [24] | 姜佳佳. 大豆GmGS1 β2基因及其启动子功能的初步研究[D]. 合肥: 安徽农业大学, 2018. |
| Jiang JJ. Study on function of GmGS1 β2 gene and its promoter in soybean(Glycine max)[D]. Hefei: Anhui Agricultural University, 2018. | |
| [25] |
Castro-Rodríguez V, García-Gutiérrez A, Canales J, et al. The glutamine synthetase gene family in Populus[J]. BMC Plant Biol, 2011, 11: 119.
doi: 10.1186/1471-2229-11-119 pmid: 21867507 |
| [26] | Castro-Rodríguez V, García-Gutiérrez A, Cañas RA, et al. Redundancy and metabolic function of the glutamine synthetase gene family in poplar[J]. BMC Plant Biol, 2015, 15(1): 20. |
| [27] |
Yan Y, Zhang ZH, Sun HW, et al. Nitrate confers rice adaptation to high ammonium by suppressing its uptake but promoting its assimilation[J]. Mol Plant, 2023, 16(12): 1871-1874.
doi: 10.1016/j.molp.2023.11.008 pmid: 37994015 |
| [28] | 李常健, 林清华, 张楚富. 高等植物谷氨酰胺合成酶研究进展[J]. 生物学杂志, 2001, 18(4): 1-3. |
| Li CJ, Lin QH, Zhang CF. Progress of the studies on glutamine synthetase in higher plants[J]. Joural Biol, 2001, 18(4): 1-3. | |
| [29] |
Guan M, Møller IS, Schjoerring JK. Two cytosolic glutamine synthetase isoforms play specific roles for seed germination and seed yield structure in Arabidopsis[J]. J Exp Bot, 2015, 66(1): 203-212.
doi: 10.1093/jxb/eru411 pmid: 25316065 |
| [30] | Konishi N, Ishiyama K, Beier MP, et al. Contributions of two cytosolic glutamine synthetase isozymes to ammonium assimilation in Arabidopsis roots[J]. J Exp Bot, 2017, 68(3): 613-625. |
| [31] | 张春丹, 郑中尧, 刘聪, 等. 拟南芥耐铵突变体的筛选及其AtGLN基因表达分析[J]. 分子植物育种, 2022, 20(18): 6057-6066. |
| Zhang CD, Zheng ZY, Liu C, et al. The screening of high ammonium tolerance mutants of Arabidopsis thaliana and the AtGLN expression analysis[J]. Mol Plant Breed, 2022, 20(18): 6057-6066. | |
| [32] |
Hoshida H, Tanaka Y, Hibino T, et al. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase[J]. Plant Mol Biol, 2000, 43(1): 103-111.
doi: 10.1023/a:1006408712416 pmid: 10949377 |
| [33] | Debouba M, Gouia H, Suzuki A, et al. NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings[J]. J Plant Physiol, 2006, 163(12): 1247-1258. |
| [1] | 高萌萌, 赵天宇, 焦馨悦, 林春晶, 关哲允, 丁孝羊, 孙妍妍, 张春宝. 大豆细胞质雄性不育系及其恢复系的比较转录组分析[J]. 生物技术通报, 2024, 40(7): 137-149. |
| [2] | 王芳, 于璐, 齐泽铮, 周长军, 于吉东. 大豆镰刀菌根腐病拮抗菌的筛选及生防效果[J]. 生物技术通报, 2024, 40(7): 216-225. |
| [3] | 白志元, 徐菲, 杨午, 王明贵, 杨玉花, 张海平, 张瑞军. 大豆细胞质雄性不育弱恢复型杂种F1育性转变的转录组分析[J]. 生物技术通报, 2024, 40(6): 134-142. |
| [4] | 刘蓉, 田闵玉, 李光泽, 谭成方, 阮颖, 刘春林. 甘蓝型油菜REVEILLE家族鉴定及诱导表达分析[J]. 生物技术通报, 2024, 40(6): 161-171. |
| [5] | 李嘉欣, 李鸿燕, 刘丽娥, 张恬, 周武. 沙棘NRAMP基因家族鉴定及铅胁迫下表达分析[J]. 生物技术通报, 2024, 40(5): 191-202. |
| [6] | 李景艳, 周家婧, 袁媛, 苏晓艺, 乔文慧, 薛岩磊, 李国婧, 王瑞刚. 拟南芥AtiPGAM2基因参与非生物胁迫的响应[J]. 生物技术通报, 2024, 40(5): 215-224. |
| [7] | 娄银, 高浩竣, 王茜, 牛景萍, 王敏, 杜维俊, 岳爱琴. 大豆GmHMGS基因的鉴定及表达模式分析[J]. 生物技术通报, 2024, 40(4): 110-121. |
| [8] | 李慧, 文钰芳, 王悦, 纪超, 石国优, 罗英, 周勇, 李志敏, 吴晓玉, 杨有新, 刘建萍. 盐胁迫下辣椒CaPIF4的表达特性与功能分析[J]. 生物技术通报, 2024, 40(4): 148-158. |
| [9] | 高玉坤, 张建东, 杨溥原, 陈东明, 王志博, 田颐瑾, Zakey Eldinn.E.A.Khlid, 崔江慧, 常金华. 高粱根际土壤细菌群落对盐胁迫的响应[J]. 生物技术通报, 2024, 40(4): 203-216. |
| [10] | 高志伟, 魏明, 于祖隆, 伍国强, 魏俊龙. 耐盐植物促生菌W-1鉴定及其对红豆草耐盐性的影响[J]. 生物技术通报, 2024, 40(4): 217-227. |
| [11] | 钟匀, 林春, 刘正杰, 董陈文华, 毛自朝, 李兴玉. 芦笋皂苷合成相关糖基转移酶基因克隆及原核表达分析[J]. 生物技术通报, 2024, 40(4): 255-263. |
| [12] | 沈天虹, 齐孝博, 赵瑞丰, 马欣荣. 微藻盐胁迫响应分子机制研究进展[J]. 生物技术通报, 2024, 40(3): 89-99. |
| [13] | 李昊, 伍国强, 魏明, 韩悦欣. 甜菜BvBADH基因家族全基因组鉴定及其高盐胁迫下的表达分析[J]. 生物技术通报, 2024, 40(2): 233-244. |
| [14] | 徐扬, 张瑞英, 戴良香, 张冠初, 丁红, 张智猛. 盐胁迫下氮素对花生种子萌发和种子际细菌菌群结构的调控[J]. 生物技术通报, 2024, 40(2): 253-265. |
| [15] | 王雨晴, 马子奇, 侯嘉欣, 宗钰琪, 郝晗睿, 刘国元, 魏辉, 连博琳, 陈艳红, 张健. 盐胁迫下植物根系分泌物的成分分析与生态功能研究进展[J]. 生物技术通报, 2024, 40(1): 12-23. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||