








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 141-147.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0611
• 薯类作物生物技术专题(专题主编:徐建飞,尚轶) • 上一篇 下一篇
袁兰1,2( ), 黄娅楠2, 张贝妮1,2, 熊雨萌1, 王洪洋1(
), 黄娅楠2, 张贝妮1,2, 熊雨萌1, 王洪洋1( )
)
收稿日期:2024-07-01
出版日期:2024-09-26
发布日期:2024-10-12
通讯作者:
王洪洋,男,博士,副教授,研究方向:马铃薯抗晚疫病分子遗传育种;E-mail: hongyang8318@ynnu.edu.cn作者简介:袁兰,女,硕士研究生,研究方向:马铃薯晚疫病防控;E-mail: 2508701413@qq.com
基金资助:
YUAN Lan1,2( ), HUANG Ya-nan2, ZHANG Bei-ni1,2, XIONG Yu-meng1, WANG Hong-yang1(
), HUANG Ya-nan2, ZHANG Bei-ni1,2, XIONG Yu-meng1, WANG Hong-yang1( )
)
Received:2024-07-01
Published:2024-09-26
Online:2024-10-12
摘要:
【目的】染色体倍性鉴定是马铃薯种质资源评价的重要内容。建立马铃薯染色体倍性的高通量样品制备方法,可为后续大规模开展马铃薯倍性鉴定工作奠定基础。【方法】以30份马铃薯孤雌生殖诱导后代作为实验材料,比较使用低温钢珠打样法、液氮研磨法和刀片切碎法制备细胞核悬液的效果,并对已知二倍体马铃薯IVP101和四倍体马铃薯HTJ349-3进行倍性鉴定。【结果】低温钢珠打样法制备的细胞核悬液,荧光信号明显,细胞裂解充分,杂质少。同时,由于低温钢珠打法简单易操作,大大缩短了样品制备时间,其鉴定效率比起液氮研磨法提高45-60倍,比刀片切碎法提高105-180倍。利用该方法可以准确检测出马铃薯IVP101和HTJ349-3的染色体倍性。【结论】基于流式细胞仪的低温钢珠打样法,其检测结果同液氮研磨法、刀片切碎法制样方法一样准确,可实现高通量鉴定马铃薯染色体倍性。
袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147.
YUAN Lan, HUANG Ya-nan, ZHANG Bei-ni, XIONG Yu-meng, WANG Hong-yang. High-throughput Sample Preparation Method for the Identification of Potato Ploidy Using Flow Cytometry[J]. Biotechnology Bulletin, 2024, 40(9): 141-147.
| 样品Sample | 浓度Concentration | 分子量 Molecular weight | 添加量 Additive amount |
|---|---|---|---|
| MgCl2·6H2O | 0.045 mol/L | 203.3 | 4.574 g |
| Na3C6H5O7·2H2O | 0.030 mol/L | 294.1 | 4.411 g |
| C7H15NO4S | 0.020 mol/L | 209.27 | 2.093 g |
| Triton X-100 | 0.1%(体积比) | 0.5 mL |
表1 500 mL裂解液的配制
Table 1 Preparation of 500 mL lysis buffer
| 样品Sample | 浓度Concentration | 分子量 Molecular weight | 添加量 Additive amount |
|---|---|---|---|
| MgCl2·6H2O | 0.045 mol/L | 203.3 | 4.574 g |
| Na3C6H5O7·2H2O | 0.030 mol/L | 294.1 | 4.411 g |
| C7H15NO4S | 0.020 mol/L | 209.27 | 2.093 g |
| Triton X-100 | 0.1%(体积比) | 0.5 mL |
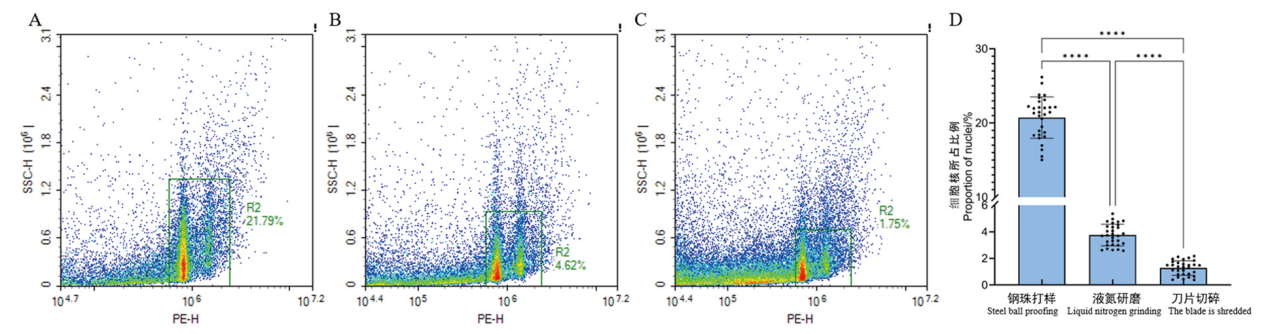
图1 不同制样方法得到的细胞核悬液中细胞核所占的比例 A:低温钢珠打样的密度图;B:液氮研磨法的密度图;C:刀片切碎法的密度图;D:细胞核占比图;采用t检验进行差异显著性分析;****表示P<0.001;n=30
Fig. 1 Proportion of nuclei in nuclear suspension obtained by different sample preparation methods A: Density map of low temperature steel-bullet beating. B: Density map of liquid nitrogen grinding method. C: Density map of blade cutting method. D: Proportion map of nucleus. The significance of the difference was analyzed by t test. **** indicates P<0.001; n =30

图2 不同制样方法得到的细胞核悬液中细胞核DNA含量的直方图 A:低温钢珠打样法;B:液氮研磨法;C:刀片切碎法;D:3种制样法M4峰值;E:3种制样法M6峰值;F:3种制样法M4M6峰值之间比值;采用t检验进行差异显著性分析;ns表示统计学上无显著性差异;n=30
Fig. 2 Histograms of nuclear DNA content in nuclear suspension obtained by different sample preparation methods A: Low temperature steel-bullet beating method. B: Liquid nitrogen grinding method. C: Blade chopping method. D: M4 peak values of three sample preparation methods. E: M6 peak values of three sample preparation methods. F: M4M6 peak value between three sample preparation methods. The significance of the difference was analyzed by t test. ns indicates no statistically significant difference. n=30
| 制样方法 Sample preparation method | 1个样品耗时 Time taken for 1 sample/min | 30个样品耗时Time taken for 30 samples/min |
|---|---|---|
| 低温钢珠打样法Low-temperature steel-bullet beating method | 0.5-1 | 1-2 |
| 液氮研磨法 Liquid nitrogen grinding method | 2-3 | 60-90 |
| 刀片切碎法 Blade shredding method | 6-7 | 180-210 |
表2 三种制样法制备样品时长的比较
Table 2 Comparison of sample preparation time of three sample preparation methods
| 制样方法 Sample preparation method | 1个样品耗时 Time taken for 1 sample/min | 30个样品耗时Time taken for 30 samples/min |
|---|---|---|
| 低温钢珠打样法Low-temperature steel-bullet beating method | 0.5-1 | 1-2 |
| 液氮研磨法 Liquid nitrogen grinding method | 2-3 | 60-90 |
| 刀片切碎法 Blade shredding method | 6-7 | 180-210 |
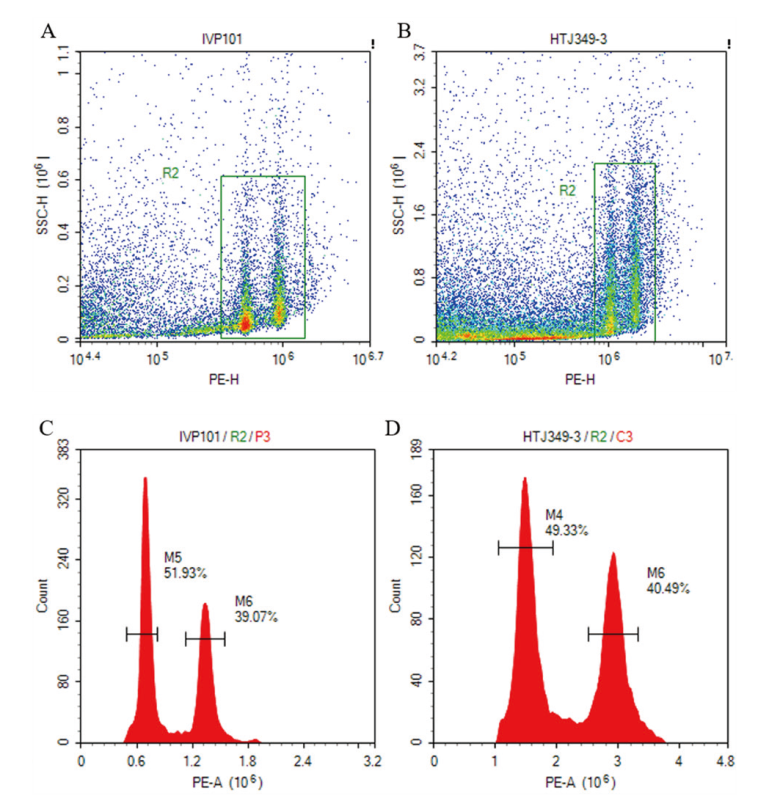
图3 四倍体马铃薯HTJ349-3和二倍体马铃薯IVP101密度图和直方图 A:二倍体IVP101的密度图;B:四倍体HTJ349-3的密度图;C:二倍体IVP101的直方图;D:四倍体HTJ349-3的直方图
Fig. 3 Density and histogram of tetraploid potato HTJ349-3 and diploid potato IVP101 A: Density map of diploid IVP101. B: Density map of tetraploid HTJ349-3. C: Histogram of diploid IVP101. D: Histogram of tetraploid HTJ349-3
| [1] |
Calliope SR, Lobo MO, Sammán NC. Biodiversity of Andean potatoes: morphological, nutritional and functional characterization[J]. Food Chem, 2018, 238: 42-50.
doi: S0308-8146(16)32087-8 pmid: 28867100 |
| [2] | André CM, Oufir M, Hoffmann L, et al. Influence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes(Solanum tuberosum L.)[J]. J Food Compos Anal, 2009, 22(6): 517-524. |
| [3] | Naumann M, Koch M, Thiel H, et al. The importance of nutrient management for potato production part II: plant nutrition and Tuber quality[J]. Potato Res, 2020, 63(1): 121-137. |
| [4] | Singh B, Raigond P, Dutt S, et al. Nutrition in potato and its food products[M]// Vegetables for Nutrition and Entrepreneurship. Singapore: Springer, 2023: 179-201. |
| [5] |
Tian JH, Chen JL, Lv FY, et al. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes[J]. Food Chem, 2016, 197 Pt B: 1264-1270.
doi: 10.1016/j.foodchem.2015.11.049 pmid: 26675866 |
| [6] | Sampaio SL, Petropoulos SA, Alexopoulos A, et al. Potato peels as sources of functional compounds for the food industry: a review[J]. Trends Food Sci Technol, 2020, 103: 118-129. |
| [7] | Zaheer K, Akhtar MH. Potato production, usage, and nutrition—a review[J]. Crit Rev Food Sci Nutr, 2016, 56(5): 711-721. |
| [8] | Wang QB, Zhang W. China's potato industry and potential impacts on the global market[J]. Am J Potato Res, 2004, 81(2): 101-109. |
| [9] | 刘园园, 董建科, 应静文, 等. 利用野生种Solanum boliviense创制马铃薯抗寒种质[J]. 作物学报, 2024, 50(6): 1384-1393. |
| Liu YY, Dong JK, Ying JW, et al. Creating cold-resistant germplasm of potato by using wild species Solanum boliviense[J]. Acta Agron Sin, 2024, 50(6): 1384-1393. | |
| [10] |
徐建飞, 金黎平. 马铃薯遗传育种研究: 现状与展望[J]. 中国农业科学, 2017, 50(6): 990-1015.
doi: 10.3864/j.issn.0578-1752.2017.06.003 |
|
Xu JF, Jin LP. Advances and perspectives in research of potato genetics and breeding[J]. Sci Agric Sin, 2017, 50(6): 990-1015.
doi: 10.3864/j.issn.0578-1752.2017.06.003 |
|
| [11] | 弓娜, 田新民, 周香艳, 等. 流式细胞术在植物学研究中的应用——检测植物核DNA含量和倍性水平[J]. 中国农学通报, 2011, 27(9): 21-27. |
| Gong N, Tian XM, Zhou XY, et al. Applications of flow cytometry in plant research—analysis of nuclear DNA content and ploidy level in plant cells[J]. Chin Agric Sci Bull, 2011, 27(9): 21-27. | |
| [12] |
杨亚杰, 李昱樱, 申状状, 等. 草棉同源多倍体后代筛选及性状鉴定[J]. 生物技术通报, 2022, 38(5): 64-73.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-1288 |
|
Yang YJ, Li YY, Shen ZZ, et al. Selection and character identification for autopolyploid progenies of Gossypium herbaceum[J]. Biotechnol Bull, 2022, 38(5): 64-73.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-1288 |
|
| [13] | 陈瑞阳, 宋文芹, 李秀兰. 植物染色体标本制备的去壁、低渗法及其在细胞遗传学中的意义[J]. 遗传学报, 1982, 9(2):151-159, 173-174. |
| Chen RY, Song WQ, Li XL. Dewalling and hypotonic methods for preparation of plant chromosome specimens and their significance in cytogenetics[J]. Acta Genetica Sinica, 1982, 9(2):151-159, 173-174. | |
| [14] | Smith TW, Kron P, Martin SL. flowPloidy: an R package for genome size and ploidy assessment of flow cytometry data[J]. Appl Plant Sci, 2018, 6(7): e01164. |
| [15] | 赵书涛, 晓东, 王策, 等. 流式细胞仪的原理、应用及最新进展[J]. 现代生物医学进展, 2011, 11(22): 4378-4381. |
| Zhao ST, Xiao D, Wang C, et al. Principles, applications and latest developments of flow cytometer[J]. Prog Mod Biomed, 2011, 11(22): 4378-4381. | |
| [16] |
张文滔, 李嘉铭, 王铫铃, 等. 蝴蝶兰种质资源倍性鉴定及育性相关性研究[J]. 核农学报, 2024, 38(6):1012-1023.
doi: 10.11869/j.issn.1000-8551.2024.06.1012 |
| Zhang WT, Li JM, Wang YL, et al. Identification of ploidy and fertility correlation of phalaenopsis germplasm resources[J]. Journal of Nuclear Agricultural Sciences, 2024, 38(6):1012-1023. | |
| [17] | 侯秋梅, 周艳, 杨朔, 等. 不同香水月季染色体倍性鉴定及其繁育系统分析[J]. 西北植物学报, 2024, 44(4): 654-661. |
| Hou QM, Zhou Y, Yang S, et al. Chromosome ploidy identification and breeding system analysis of rose with different perfumes[J]. Acta Bot Boreali Occidentalia Sin, 2024, 44(4): 654-661. | |
| [18] | 李拓键, 屈燕, 王兵益, 等. 不同倍性云南山茶染色体核型分析[J]. 中南林业科技大学学报, 2023, 43(3): 167-174. |
| Li TJ, Qu Y, Wang BY, et al. Chromosome karyotype analysis of Camellia reticulata with different ploidy[J]. J Cent South Univ For Technol, 2023, 43(3): 167-174. | |
| [19] |
吴方圆, 蔡娅, 郝丙青, 等. 流式细胞仪检测香花油茶、越南油茶基因组大小方法的建立及应用[J]. 热带作物学报, 2023, 44(8):1542-1550.
doi: 10.3969/j.issn.1000-2561.2023.08.003 |
| Wu FY, Cai Y, Hao BQ, et al. Establishment and application of flow cytometry method for detecting genome size of Camellia oleifera and Camellia oleifera Vietnam[J]. Chinese Journal of Tropical Crops, 2023, 44(8):1542-1550. | |
| [20] |
熊文艳, 普冉, 刘云礼, 等. 基于流式细胞术对27种石斛的倍性鉴定和基因组大小分析[J]. 热带作物学报, 2022, 43(11): 2249-2257.
doi: 10.3969/j.issn.1000-2561.2022.11.009 |
| Xiong WY, Pu R, Liu YL, et al. Estimation ploidy and genome size of 27 Dendrobium species by flow cytometry[J]. Chin J Trop Crops, 2022, 43(11): 2249-2257. | |
| [21] | Motsa MM, Bester C, Slabbert MM, et al. Flow cytometry: a quick method to determine ploidy levels in honeybush(Cyclopia spp.)[J]. Genet Resour Crop Evol, 2018, 65(6): 1711-1724. |
| [22] |
邵雪花, 李桂兰, 肖维强, 等. 基于流式细胞仪鉴定番石榴倍性方法的建立及应用[J]. 生物技术通报, 2024, 40(2): 48-54.
doi: 10.13560/j.cnki.biotech.bull.1985.2023-0844 |
| Shao XH, Li GL, Xiao WQ, et al. Establishment and application of a method for identification of guava ploidy based on flow cytometry[J]. Biotechnol Bull, 2024, 40(2): 48-54. | |
| [23] | 陈宜木, 周群, 钟颖颖, 等. 利用流式细胞术鉴定68份三角梅基因组大小与染色体倍性[J]. 亚热带植物科学, 2022, 51(6): 417-423. |
| Chen YM, Zhou Q, Zhong YY, et al. Genome size estimation and ploidy identification of 68 servings Bougainvillea by flow cytometry[J]. Subtrop Plant Sci, 2022, 51(6): 417-423. | |
| [24] |
李春牛, 李先民, 黄展文, 等. 利用流式细胞术鉴定茉莉花基因组大小和染色体倍性[J]. 热带作物学报, 2021, 42(5): 1231-1236.
doi: 10.3969/j.issn.1000-2561.2021.05.005 |
| Li CN, Li XM, Huang ZW, et al. Genome size estimation and ploidy identification of Jasminum sambac by flow cytometry[J]. Chin J Trop Crops, 2021, 42(5): 1231-1236. | |
| [25] | 杨丽, 孙浩元, 张俊环, 等. 利用流式细胞术鉴定杏及其部分近缘植物的倍性[J]. 西北农业学报, 2021, 30(10):1504-1513. |
| Yang L, Sun HY, Zhang JH, et al. Flow cytometry was used to identify ploidy of apricots and some of their relatives[J]. Northwest Journal of Agricultural Sciences, 2021, 30(10):1504-1513. | |
| [26] | 罗庆, 高建有, 李洁维, 等. 利用流式细胞仪对猕猴桃种质染色体倍性的鉴定[J]. 生物学杂志, 2022, 39(6): 66-72, 93. |
| Luo Q, Gao JY, Li JW, et al. Identification of chromosome ploidy in kiwifruit germplasm by flow cytometer[J]. J Biol, 2022, 39(6): 66-72, 93. | |
| [27] | Phurailatpam AK, Geetha KA, Maiti S. Ploidy distinction in male and female plants of betelvine(Piper betle L.): a study by flow cytometry[J]. Genet Resour Crop Evol, 2018, 65(6): 1565-1570. |
| [28] | 宋子琪, 卞国良, 林峰, 等. 流式细胞仪鉴定青钱柳倍性方法的建立及其应用[J]. 南京林业大学学报: 自然科学版, 2024, 48(2): 61-68. |
| Song ZQ, Bian GL, Lin F, et al. Establishment and application of flow cytometry for identification of ploidy of Cyclocarya paliurus[J]. J Nanjing For Univ Nat Sci Ed, 2024, 48(2): 61-68. | |
| [29] | 黄茂根, 沈凝, 刘雪羽, 等. 利用流式细胞术鉴定桦木染色体倍性和DNA含量[J]. 西南林业大学学报(自然科学), 2023, 43(1):49-57. |
| Huang MG, Shen N, Liu XY, et al Identification of chromosome ploidy and DNA content in birch by flow cytometry[J]. Journal of Southwest Forestry University(Natural Science), 2023, 43(1):49-57. | |
| [30] | 陆晓媚, 林强, 唐燕梅, 等. 植物染色体倍性鉴定方法及其在桑树研究中的应用[J]. 蚕学通讯, 2022, 42(1):30-36. |
| Lu XM, Lin Q, Tang YM, et al. Identification method of plant chromosome ploidy and its application in mulberry research[J]. Seriology Communication, 2022, 42(1): 30-36. | |
| [31] |
郑英转, 吕燕, 杨东旭, 等. 基于液氮研磨法的流式细胞术检测马铃薯倍性的研究[J]. 生物技术通报, 2021, 37(1): 282-288.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0478 |
| Zheng YZ, Lv Y, Yang DX, et al. Study on the identification of potato ploidy using flow cytometry based on liquid nitrogen grinding method[J]. Biotechnol Bull, 2021, 37(1): 282-288. | |
| [32] | 王婷婷, 张光海, 蔡汶玻, 等. 利用流式细胞法鉴定马铃薯倍性方法的优化[J]. 中国马铃薯, 2019, 33(1): 1-7. |
| Wang TT, Zhang GH, Cai WB, et al. Optimization of potato ploidy identification method using flow cytometry[J]. Chin Potato J, 2019, 33(1): 1-7. |
| [1] | 申鹏, 高雅彬, 丁红. 马铃薯SAT基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(9): 64-73. |
| [2] | 宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112. |
| [3] | 王超, 白如仟, 管俊梅, 罗稷林, 何雪姣, 迟绍轶, 马玲. 马铃薯块茎变绿中StHY5对龙葵素合成的促进作用[J]. 生物技术通报, 2024, 40(9): 113-122. |
| [4] | 夏士轩, 耿泽栋, 祝光涛, 张春芝, 李大伟. 基于深度学习的马铃薯花粉活力快速检测[J]. 生物技术通报, 2024, 40(9): 123-130. |
| [5] | 毛向红, 卢瑶, 范向斌, 杜培兵, 白小东. 基于SSR荧光标记毛细管电泳的马铃薯品种遗传多样性分析及分子身份证构建[J]. 生物技术通报, 2024, 40(9): 131-140. |
| [6] | 宋倩娜, 段永红, 冯瑞云. CRISPR/Cas9介导的高效四倍体马铃薯试管薯基因编辑体系的建立[J]. 生物技术通报, 2024, 40(9): 33-41. |
| [7] | 王柯然, 闫俊杰, 刘建凤, 高玉林. RNAi技术在马铃薯害虫防控中的应用和风险[J]. 生物技术通报, 2024, 40(9): 4-10. |
| [8] | 张小妹, 周南伶, 张赛行, 王超, 沈玉龙, 管俊梅, 马玲. 马铃薯StDREBs基因的克隆及其表达分析[J]. 生物技术通报, 2024, 40(9): 42-50. |
| [9] | 满全财, 孟姿诺, 李伟, 蔡心汝, 苏润东, 付长青, 高顺娟, 崔江慧. 马铃薯AQP基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 51-63. |
| [10] | 吴娟, 武小娟, 王沛捷, 谢锐, 聂虎帅, 李楠, 马艳红. 彩色马铃薯花青素合成相关ERF基因筛选及表达分析[J]. 生物技术通报, 2024, 40(9): 82-91. |
| [11] | 乔岩, 杨芳, 任盼荣, 祁伟亮, 安沛沛, 李茜, 李丹, 肖俊飞. 马铃薯野生种烯酰水合酶超家族基因ScDHNS的克隆与功能分析[J]. 生物技术通报, 2024, 40(9): 92-103. |
| [12] | 张震, 李清, 徐菁, 陈凯园, 张春芝, 祝光涛. 马铃薯线粒体靶向表达载体的构建与应用[J]. 生物技术通报, 2024, 40(5): 66-73. |
| [13] | 张玉, 石磊, 巩檑, 聂峰杰, 杨江伟, 刘璇, 杨文静, 张国辉, 颉瑞霞, 张丽. 马铃薯WOX基因家族的鉴定及在离体再生和非生物胁迫中的表达分析[J]. 生物技术通报, 2024, 40(3): 170-180. |
| [14] | 梅显军, 宋慧洋, 李京昊, 梅超, 宋倩娜, 冯瑞云, 陈喜明. 马铃薯StDof5的克隆及表达分析[J]. 生物技术通报, 2024, 40(3): 181-192. |
| [15] | 邵雪花, 李桂兰, 肖维强, 赖多, 庄庆礼, 秦健. 基于流式细胞仪鉴定番石榴倍性方法的建立及应用[J]. 生物技术通报, 2024, 40(2): 48-54. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||