








生物技术通报 ›› 2024, Vol. 40 ›› Issue (12): 45-52.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0324
田锦1,2( ), 张月秋1, 张华1, 陈子言1, 田璐2, 王颢潜1, 高芳瑞1, 梁晋刚1(
), 张月秋1, 张华1, 陈子言1, 田璐2, 王颢潜1, 高芳瑞1, 梁晋刚1( ), 陈红1(
), 陈红1( )
)
收稿日期:2024-04-03
出版日期:2024-12-26
发布日期:2025-01-15
通讯作者:
陈红,男,博士,研究员,研究方向:转基因检测技术;E-mail: chen1975hong@163.com;作者简介:田锦,女,硕士,教授,研究方向:分子遗传学;E-mail: 73466@bvca.edu.cn基金资助:
TIAN Jin1,2( ), ZHANG Yue-qiu1, ZHANG Hua1, CHEN Zi-yan1, TIAN Lu2, WANG Hao-qian1, GAO Fang-rui1, LIANG Jin-gang1(
), ZHANG Yue-qiu1, ZHANG Hua1, CHEN Zi-yan1, TIAN Lu2, WANG Hao-qian1, GAO Fang-rui1, LIANG Jin-gang1( ), CHEN Hong1(
), CHEN Hong1( )
)
Received:2024-04-03
Published:2024-12-26
Online:2025-01-15
摘要:
【目的】浙大瑞丰8是杭州瑞丰生物科技有限公司开发的抗虫转基因玉米,于2021年12月获得生产应用安全证书。研究浙大瑞丰8的特异性PCR检测方法,为市场监管和产业化推广提供技术支持。【方法】以浙大瑞丰8转化体外源基因插入端侧翼序列为靶标,在5'端(右侧翼)和3'端(左侧翼)分别设计特异性引物,以与玉米内标准基因zSSIIb一致的标准反应体系和反应程序,对左侧翼和右侧翼各20对引物进行了筛选,得到了特异性高、稳定性好的候选引物,通过改变退火温度和引物浓度这两个关键参数,初步建立了浙大瑞丰8转化体PCR检测方法。【结果】 筛选到的浙大瑞丰8 PCR扩增引物RF 8-RB-R4/F1可特异性扩增目标片段265 bp,建立了与玉米内标准基因zSSIIb一致的标准反应体系和反应程序,实现了批量检测的高通量。【结论】建立的特异性定性PCR检测方法稳定性及适应性好,对拷贝数分数为0.1%(约20个拷贝)能稳定检出,可精准、特异地检测出浙大瑞丰8转化体。
田锦, 张月秋, 张华, 陈子言, 田璐, 王颢潜, 高芳瑞, 梁晋刚, 陈红. 转基因玉米浙大瑞丰8特异性定性PCR检测方法研究[J]. 生物技术通报, 2024, 40(12): 45-52.
TIAN Jin, ZHANG Yue-qiu, ZHANG Hua, CHEN Zi-yan, TIAN Lu, WANG Hao-qian, GAO Fang-rui, LIANG Jin-gang, CHEN Hong. Specific Qualitative PCR Detection Method for Transgenic Maize Zheda Ruifeng 8[J]. Biotechnology Bulletin, 2024, 40(12): 45-52.
| 引物Primer | 序列 Sequence(5'-3') | 引物Primer | 序列Sequence(5'-3') | |
|---|---|---|---|---|
| RF 8-LB-F1 | GGGTTTCGCTCATGTGTTGAG | RF 8-RB-F1 | AGTTGGCGTCTCTCTGTTCG | |
| RF 8-LB-F2 | CGTCCGCAATGTGTTATTAAGTTGTCTA | RF 8-RB-F2 | GTCGTTACTGAAACCGGTCC | |
| RF 8-LB-F3 | AGGAGCTCGAGTTTCTCCAT | RF 8-RB-F3 | TCGTTACTGAAACCGGTCCAT | |
| RF 8-LB-F4 | AGCGTCAATTTGTTTACACCACA | RF 8-RB-F4 | ATCCCAACCATTGCCGTCTT | |
| RF 8-LB-F5 | ACTAAAATCCAGATCCCCCGAA | RF 8-RB-F5 | CCGTCTTGATGAGTTGGCGT | |
| RF 8-LB-F6 | GGTTTCGCTCATGTGTTGAG | RF 8-RB-F6 | TGCCGTCTTGATGAGTTGGC | |
| RF 8-LB-R1 | GTGTCGACGGCTGAGATGAA | RF 8-RB-R1 | AAAAACGTCCGCAATGTGTT | |
| RF 8-LB-R2 | AGTGCCAATACATACGCAACTGTTGCAG | RF 8-RB-R2 | CTGAGGCCACCGCCTAATA | |
| RF 8-LB-R3 | ATACGATCGGATTCGTGGGA | RF 8-RB-R3 | CTGAGGCCACCGCCTAAT | |
| RF 8-LB-R4 | GAACCAGAGCACTCTCACCA | RF 8-RB-R4 | CGTCCGCAATGTGTTATTAAGT | |
| RF 8-LB-R5 | GGCCCATGAGTCCAAGAACC | RF 8-RB-R5 | CATTAAAAACGTCCGCAATGTGT | |
| RF 8-LB-R6 | CAACTGGCGACACAGGGT | RF 8-RB-R6 | TTAAAAACGTCCGCAATGTGTT |
表1 用于PCR检测浙大瑞丰8转化体的候选引物
Table 1 Candidate primers for PCR detecting Zheda Ruifeng 8 event
| 引物Primer | 序列 Sequence(5'-3') | 引物Primer | 序列Sequence(5'-3') | |
|---|---|---|---|---|
| RF 8-LB-F1 | GGGTTTCGCTCATGTGTTGAG | RF 8-RB-F1 | AGTTGGCGTCTCTCTGTTCG | |
| RF 8-LB-F2 | CGTCCGCAATGTGTTATTAAGTTGTCTA | RF 8-RB-F2 | GTCGTTACTGAAACCGGTCC | |
| RF 8-LB-F3 | AGGAGCTCGAGTTTCTCCAT | RF 8-RB-F3 | TCGTTACTGAAACCGGTCCAT | |
| RF 8-LB-F4 | AGCGTCAATTTGTTTACACCACA | RF 8-RB-F4 | ATCCCAACCATTGCCGTCTT | |
| RF 8-LB-F5 | ACTAAAATCCAGATCCCCCGAA | RF 8-RB-F5 | CCGTCTTGATGAGTTGGCGT | |
| RF 8-LB-F6 | GGTTTCGCTCATGTGTTGAG | RF 8-RB-F6 | TGCCGTCTTGATGAGTTGGC | |
| RF 8-LB-R1 | GTGTCGACGGCTGAGATGAA | RF 8-RB-R1 | AAAAACGTCCGCAATGTGTT | |
| RF 8-LB-R2 | AGTGCCAATACATACGCAACTGTTGCAG | RF 8-RB-R2 | CTGAGGCCACCGCCTAATA | |
| RF 8-LB-R3 | ATACGATCGGATTCGTGGGA | RF 8-RB-R3 | CTGAGGCCACCGCCTAAT | |
| RF 8-LB-R4 | GAACCAGAGCACTCTCACCA | RF 8-RB-R4 | CGTCCGCAATGTGTTATTAAGT | |
| RF 8-LB-R5 | GGCCCATGAGTCCAAGAACC | RF 8-RB-R5 | CATTAAAAACGTCCGCAATGTGT | |
| RF 8-LB-R6 | CAACTGGCGACACAGGGT | RF 8-RB-R6 | TTAAAAACGTCCGCAATGTGTT |
| 序号 No. | 组合 Group | 产物大小 Amplication size/bp | 序号 No. | 组合 Group | 产物大小 Amplication size/bp | 序号 No. | 组合 Group | 产物大小 Amplication size/bp | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RF 8-RB-R1/F1 | 270 | 15 | RF 8-RB-R5/F5 | 285 | 28 | RF 8-LB-F2/R6 | 282 | ||
| 2 | RF 8-RB-R1/F4 | 294 | 16 | RF 8-RB-R5/F6 | 287 | 29 | RF 8-LB-F3/R3 | 299 | ||
| 3 | RF 8-RB-R1/F5 | 281 | 17 | RF 8-RB-R6/F1 | 272 | 30 | RF 8-LB-F4/R4 | 141 | ||
| 4 | RF 8-RB-R1/F6 | 283 | 18 | RF 8-RB-R6/F4 | 296 | 31 | RF 8-LB-F5/R1 | 149 | ||
| 5 | RF 8-RB-R2/F2 | 295 | 19 | RF 8-RB-R6/F5 | 283 | 32 | RF 8-LB-F5/R2 | 179 | ||
| 6 | RF 8-RB-R2/F3 | 294 | 20 | RF 8-RB-R6/F6 | 285 | 33 | RF 8-LB-F5/R3 | 128 | ||
| 7 | RF 8-RB-R3/F2 | 295 | 21 | RF 8-LB-F1/R1 | 254 | 34 | RF 8-LB-F5/R4 | 222 | ||
| 8 | RF 8-RB-R3/F3 | 294 | 22 | RF 8-LB-F1/R2 | 284 | 35 | RF 8-LB-F5/R5 | 237 | ||
| 9 | RF 8-RB-R4/F1 | 265 | 23 | RF 8-LB-F1/R3 | 233 | 36 | RF 8-LB-F5/R6 | 165 | ||
| 10 | RF 8-RB-R4/F4 | 289 | 24 | RF 8-LB-F1/R6 | 270 | 37 | RF 8-LB-F6/R1 | 253 | ||
| 11 | RF 8-RB-R4/F5 | 276 | 25 | RF 8-LB-F2/R1 | 266 | 38 | RF 8-LB-F6/R2 | 283 | ||
| 12 | RF 8-RB-R4/F6 | 278 | 26 | RF 8-LB-F2/R2 | 290 | 39 | RF 8-LB-F6/R3 | 232 | ||
| 13 | RF 8-RB-R5/F1 | 274 | 27 | RF 8-LB-F2/R3 | 245 | 40 | RF 8-LB-F6/R6 | 269 | ||
| 14 | RF 8-RB-R5/F4 | 298 |
表2 浙大瑞丰8转化体PCR检测的引物组合
Table 2 Primer pairs for PCR detecting Zheda Ruifeng 8 event
| 序号 No. | 组合 Group | 产物大小 Amplication size/bp | 序号 No. | 组合 Group | 产物大小 Amplication size/bp | 序号 No. | 组合 Group | 产物大小 Amplication size/bp | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RF 8-RB-R1/F1 | 270 | 15 | RF 8-RB-R5/F5 | 285 | 28 | RF 8-LB-F2/R6 | 282 | ||
| 2 | RF 8-RB-R1/F4 | 294 | 16 | RF 8-RB-R5/F6 | 287 | 29 | RF 8-LB-F3/R3 | 299 | ||
| 3 | RF 8-RB-R1/F5 | 281 | 17 | RF 8-RB-R6/F1 | 272 | 30 | RF 8-LB-F4/R4 | 141 | ||
| 4 | RF 8-RB-R1/F6 | 283 | 18 | RF 8-RB-R6/F4 | 296 | 31 | RF 8-LB-F5/R1 | 149 | ||
| 5 | RF 8-RB-R2/F2 | 295 | 19 | RF 8-RB-R6/F5 | 283 | 32 | RF 8-LB-F5/R2 | 179 | ||
| 6 | RF 8-RB-R2/F3 | 294 | 20 | RF 8-RB-R6/F6 | 285 | 33 | RF 8-LB-F5/R3 | 128 | ||
| 7 | RF 8-RB-R3/F2 | 295 | 21 | RF 8-LB-F1/R1 | 254 | 34 | RF 8-LB-F5/R4 | 222 | ||
| 8 | RF 8-RB-R3/F3 | 294 | 22 | RF 8-LB-F1/R2 | 284 | 35 | RF 8-LB-F5/R5 | 237 | ||
| 9 | RF 8-RB-R4/F1 | 265 | 23 | RF 8-LB-F1/R3 | 233 | 36 | RF 8-LB-F5/R6 | 165 | ||
| 10 | RF 8-RB-R4/F4 | 289 | 24 | RF 8-LB-F1/R6 | 270 | 37 | RF 8-LB-F6/R1 | 253 | ||
| 11 | RF 8-RB-R4/F5 | 276 | 25 | RF 8-LB-F2/R1 | 266 | 38 | RF 8-LB-F6/R2 | 283 | ||
| 12 | RF 8-RB-R4/F6 | 278 | 26 | RF 8-LB-F2/R2 | 290 | 39 | RF 8-LB-F6/R3 | 232 | ||
| 13 | RF 8-RB-R5/F1 | 274 | 27 | RF 8-LB-F2/R3 | 245 | 40 | RF 8-LB-F6/R6 | 269 | ||
| 14 | RF 8-RB-R5/F4 | 298 |
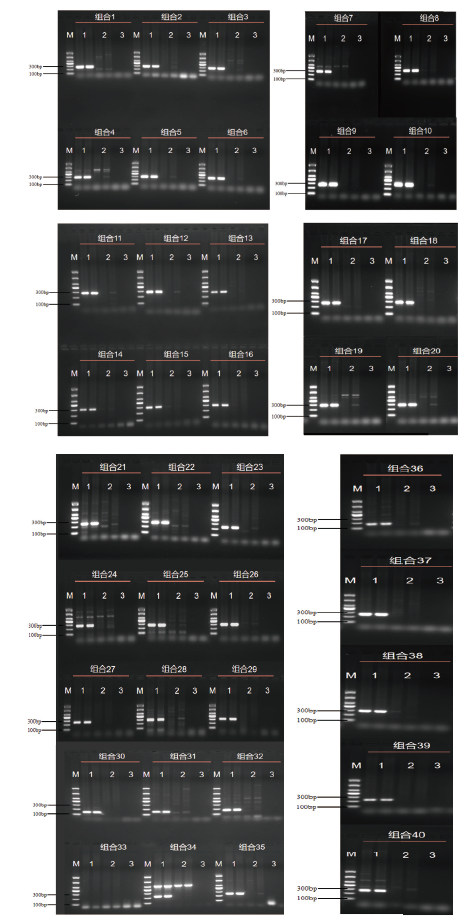
图1 浙大瑞丰8转化体PCR检测引物筛选 M:DL1000 marker;1:1%浙大瑞丰8;2:阴性对照;3:空白对照
Fig. 1 Screening of the primers for PCR detecting Zheda Ruifeng 8 event M:DL1000 marker; 1: 1% Zheda Ruifeng 8 event; 2: negative control; 3: blank control
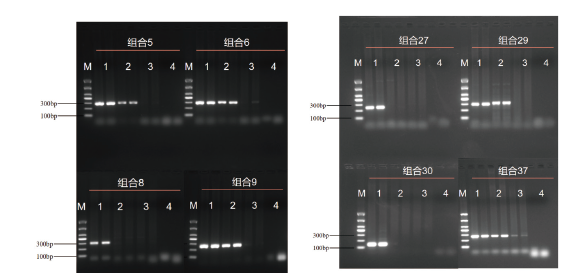
图2 浙大瑞丰8转化体PCR检测引物组合二次筛选 M:DL1000 marker;1:1%浙大瑞丰8;2:0.1%浙大瑞丰8;3:阴性对照(受体);4:空白对照
Fig. 2 Secondary screening of the primers for PCR detection of Zheda Ruifeng 8 event M: DL1000 marker; 1: 1% Zheda Ruifeng 8 event; 2: 0.1%; 3: negative control; 4: blank control
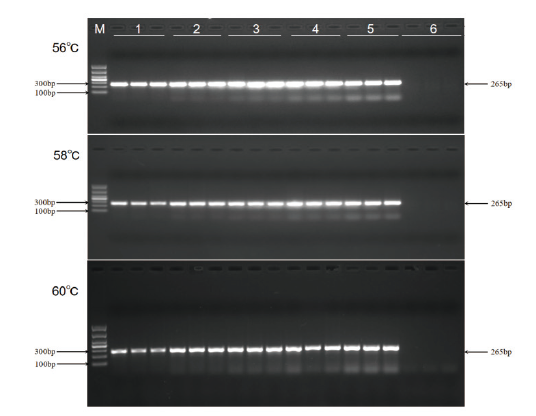
图3 浙大瑞丰8转化体引物反应浓度优化 M:DL1000 marker;1:引物浓度0.2 μmol/L;2:引物浓度0.4 μmol/L;3:引物浓度0.6 μmol/L;4:引物浓度0.8 μmol/L;5:引物浓度1.0 μmol/L;6:空白对照
Fig. 3 Optimization of primer concentration for Zheda Ruifeng 8 event M: DL1000 marker; 1: primer concentration 0.2 μmol/L; 2: primer concentration 0.4 μmol/L; 3: primer concentration 0.6 μmol/L; 4: primer concentration 0.8 μmol/L; 5: primer concentration 1.0 μmol/L; 6: blank control
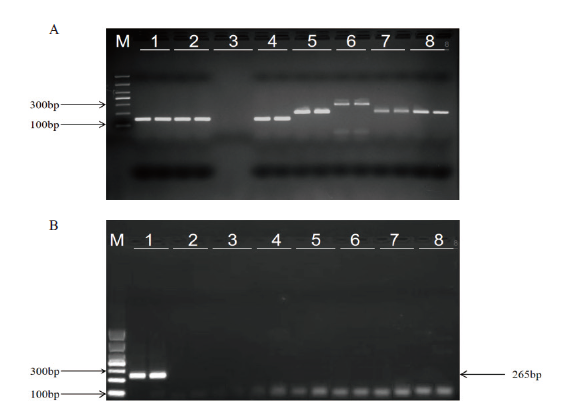
图4 浙大瑞丰8转化体PCR特异性测试 A:各被检样品内标准基因扩增结果。M:DL1000 marker;1-8分别对应B图1-8样品的内标准基因(其中玉米内标准基因zSSIIb扩增片段151 bp;大豆内标准基因Lectin扩增片段210 bp;水稻内标准基因SPS扩增片段287 bp;油菜内标准基因HMG I/Y扩增片段206 bp;棉花内标准基因ACP扩增片段210 bp);B:浙大瑞丰8特异性引物扩增结果。M:DL1000 marker;1:1% 浙大瑞丰8;2:受体浙大瑞丰1;3:空白对照;4:其他转基因玉米混样;5:转基因大豆混样;6:转基因水稻混样;7:转基因油菜混样;8:转基因棉花混样
Fig. 4 Specificity PCR test for Zheda Ruifeng 8 event A:Results of standard gene amplification in each sample. M:DL1000 marker;1-8 corresponds to the internal standard genes in figure B, respectively. B: Results of specific primer amplification by Zheda Ruifeng 8. M:DL1000 marker;1:1% Zheda Ruifeng 8;2:receptor maize of Ruifeng 1;3:blank control;4:other genetically modified(GM)corn mixed samples;5:GM soybean mixed samples;6:GM rice mixed samples;7:GM rape mixed samples;8:GM cotton mixed samples
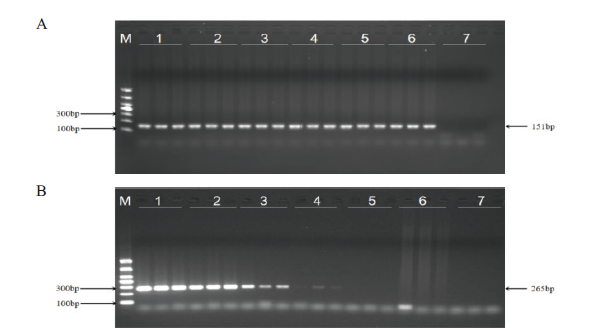
图5 浙大瑞丰8转化体PCR检测方法灵敏度测试 A:玉米内标准基因电泳图;B:浙大瑞丰8转化体电泳图。M:DL1000 marker;1:1%;2:0.5%;3:0.1%;4:0.05%;5:0.01%;6:阴性对照;7:空白对照
Fig. 5 Sensitivity test for PCR detection method of Zheda Ruifeng 8 event A: Electrophoresis diagram of standard gene in maize. B: Electrophoresis diagram of Zheda Ruifeng 8 event. M: DL1000 marker; 1: 1%; 2: 0.5%; 3: 0.1%; 4: 0.05%; 5: 0.01%; 6: negative control; 7: blank control
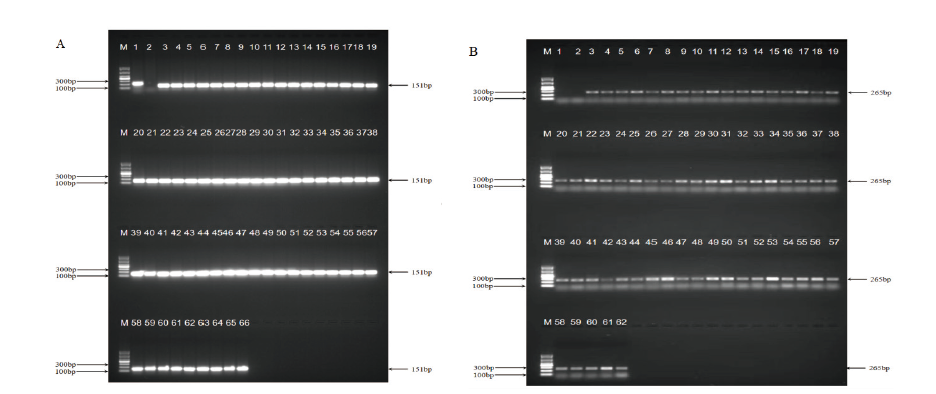
图6 浙大瑞丰8转化体检出限测试 A:玉米内标准基因电泳图;B:浙大瑞丰8转化体电泳图。M:DL1000 marker;1:阴性对照;2:空白对照;3-62:0.1%浙大瑞丰8
Fig. 6 Quantitative limit test for Zheda Ruifeng 8 event A: Electrophoresis diagram of standard gene in maize; B: electrophoresis diagram of Zheda Ruifeng 8 event. M: DL1000 marker; 1: negative control; 2: blank control; 3-62: 0.1% Zheda Ruifeng 8
| Lab | 样品名称/编号 Sample name/No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Lab1 - Lab8 | 阳性对照 Positive control | + | + | + | + | + | + |
| 阴性对照 Negative control | - | - | - | - | - | - | |
| 1%浙大瑞丰8 1% Zheda Ruifeng 8 | + | + | + | + | + | + | |
| 其他转基因玉米混样 Transgenic corn mixture samples | - | - | - | - | - | - | |
| 转基因大豆混样 Transgenic soybean mixture samples | - | - | - | - | - | - | |
| 转基因水稻混样 Transgenic rice mixture samples | - | - | - | - | - | - | |
| 转基因棉花混样 Transgenic cotton mixture samples | - | - | - | - | - | - | |
| 转基因油菜混样 Transgenic rape mixture samples | - | - | - | - | - | - | |
| 非转基因玉米混样 Non-transgenic maize mixture samples | - | - | - | - | - | - | |
| 0.1%浙大瑞丰8 0.1% Zheda Ruifeng 8 | + | + | + | + | + | + |
表3 浙大瑞丰8转化体特异性验证
Table 3 Event-specific verification for Zheda Ruifeng 8 event
| Lab | 样品名称/编号 Sample name/No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Lab1 - Lab8 | 阳性对照 Positive control | + | + | + | + | + | + |
| 阴性对照 Negative control | - | - | - | - | - | - | |
| 1%浙大瑞丰8 1% Zheda Ruifeng 8 | + | + | + | + | + | + | |
| 其他转基因玉米混样 Transgenic corn mixture samples | - | - | - | - | - | - | |
| 转基因大豆混样 Transgenic soybean mixture samples | - | - | - | - | - | - | |
| 转基因水稻混样 Transgenic rice mixture samples | - | - | - | - | - | - | |
| 转基因棉花混样 Transgenic cotton mixture samples | - | - | - | - | - | - | |
| 转基因油菜混样 Transgenic rape mixture samples | - | - | - | - | - | - | |
| 非转基因玉米混样 Non-transgenic maize mixture samples | - | - | - | - | - | - | |
| 0.1%浙大瑞丰8 0.1% Zheda Ruifeng 8 | + | + | + | + | + | + |
| Lab | 0.1%浙大瑞丰8 0.1% transgenic maize Zheda Ruifeng 8 | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Lab1 | + | + | + | + | + | + |
| Lab2 | + | + | + | + | + | + |
| Lab3 | + | + | + | + | + | + |
| Lab4 | + | + | + | + | + | + |
| Lab5 | + | + | + | + | + | + |
| Lab6 | + | + | + | + | + | + |
| Lab7 | + | + | + | + | + | + |
| Lab8 | + | + | + | + | + | + |
表4 浙大瑞丰8转化体检出限循环测试结果
Table 4 Cyclic verification for quantitative limit test for Zheda Ruifeng 8 event
| Lab | 0.1%浙大瑞丰8 0.1% transgenic maize Zheda Ruifeng 8 | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Lab1 | + | + | + | + | + | + |
| Lab2 | + | + | + | + | + | + |
| Lab3 | + | + | + | + | + | + |
| Lab4 | + | + | + | + | + | + |
| Lab5 | + | + | + | + | + | + |
| Lab6 | + | + | + | + | + | + |
| Lab7 | + | + | + | + | + | + |
| Lab8 | + | + | + | + | + | + |
| [1] | 黎裕, 徐辰武, 秦峰, 等. 玉米生物育种:现状与展望[J]. 中国基础科学, 2022, 24(4): 18-28. |
| Li Y, Xu CW, Qin F, et al. Maize breeding: advances and prospects[J]. China Basic Sci, 2022, 24(4): 18-28. | |
| [2] |
徐晓丽, 姜媛媛, 王鹏飞, 等. 表达Cry1Ab和Cry2Ab蛋白的转基因玉米GAB-3对四种主要鳞翅目害虫的抗性评价[J]. 中国农业科技导报, 2020, 22(12): 97-104.
doi: 10.13304/j.nykjdb.2020.0388 |
| Xu XL, Jiang YY, Wang PF, et al. Resistance evaluation of genetically modified maize GAB-3 expressing Cry1Ab and Cry2Ab against four major lepidopteran pests[J]. J Agric Sci Technol, 2020, 22(12): 97-104. | |
| [3] | 雷展, 王建成, 张晨, 等. 转基因油菜NS-B50027-4定性定量检测方法的建立[J]. 农业生物技术学报, 2021, 29(9): 1825-1835. |
| Lei Z, Wang JC, Zhang C, et al. Establishment of qualitative and quantitative detection method for transgenic Brassica napus NS-B50027-4[J]. J Agric Biotechnol, 2021, 29(9): 1825-1835. | |
| [4] | 斯能武, 李俊, 武玉花, 等. 数字PCR在转基因定量检测中的研究进展[J]. 中国油料作物学报, 2021, 43(1): 40-50. |
|
Si NW, Li J, Wu YH, et al. Research progress of digital PCR in quantitative detection of genetically modified organism[J]. Chin J Oil Crop Sci, 2021, 43(1): 40-50.
doi: 10.19802/j.issn.1007-9084.2020265 |
|
| [5] | 刘欣, 祝长青, 王毅谦, 等. 大豆转基因检测中DNA提取方法的比较研究[J]. 食品科学, 2013, 34(4): 199-203. |
| Liu X, Zhu CQ, Wang YQ, et al. Methodology comparison of DNA extraction from soybean for detection of genetically modified organism[J]. Food Sci, 2013, 34(4): 199-203. | |
| [6] |
Gryson N. Effect of food processing on plant DNA degradation and PCR-based GMO analysis: a review[J]. Anal Bioanal Chem, 2010, 396(6): 2003-2022.
doi: 10.1007/s00216-009-3343-2 pmid: 20012944 |
| [7] | 中华人民共和国农业部.转基因植物及其产品成分检测定性PCR方法制定指南: 农业部2259号公告-4—2015[S]. 北京: 中国农业出版社, 2015. |
| Ministry of Agriculture of the People's Republic of China.Detection of Genetically Modified Plants and Derived Products Guidelines for Establishing qualitative PCR Method: Department of Agriculture Notice 2259-4-2015[S]. Beijing: China Agriculture Press, 2015. | |
| [8] | 中华人民共和国农业部.转基因植物及其产品成分检测玉米内标准基因定性PCR方法: 农业部1861号公告-3—2012[S]. 北京: 中国农业出版社, 2013. |
| Ministry of Agriculture of the People's Republic of China.Detection of genetically modified plants and derived products. Target-taxon-specifis qualitative PCR method for maize: Department of Agriculture Notice 1861-3-2012[S]. Beijing: China Agriculture Press, 2013. | |
| [9] | 李凌燕, 张旭冬, 陈子言, 等. 转基因抗虫耐除草剂玉米MON87411精准定量检测方法的建立[J]. 生物安全学报, 2023, 32(1): 38-45. |
| Li LY, Zhang XD, Chen ZY, et al. Establishment of accurate quantitative detection method for insect-resistant and herbicide-tolerant maize MON87411[J]. J Biosaf, 2023, 32(1): 38-45. | |
| [10] | Gatto F, Savini C, Baillie C, et al. Definition of minimum performance requirements for analytical methods of GMO testing[J]. Luxembourg: Publications Office, 2023. |
| [11] | 阳丽, 操志林. 转基因玉米种子的PCR检测[J]. 江西农业学报, 2014, 26(4): 98-99, 106. |
| Yang L, Cao ZL. PCR detection of transgenic maize seeds[J]. Acta Agric Jiangxi, 2014, 26(4): 98-99, 106. | |
| [12] |
尹全, 李忆, 宋君, 等. 多重PCR筛查检测进口转基因玉米[J]. 核农学报, 2016, 30(6): 1045-1053.
doi: 10.11869/j.issn.100-8551.2016.06.1045 |
|
Yin Q, Li Y, Song J, et al. Multiplex PCR detection of exogenous gene in the imported genetically modified maize[J]. J Nucl Agric Sci, 2016, 30(6): 1045-1053.
doi: 10.11869/j.issn.100-8551.2016.06.1045 |
|
| [13] | 李葱葱, 王青山, 李飞武, 等. 用实时荧光PCR方法定量检测Bt176转基因玉米[J]. 吉林农业科学, 2007, 32(5): 24-27. |
| Li CC, Wang QS, Li FW, et al. Quantitative detection of the genetically modified Bt176 maize using real-time fluorescent PCR method[J]. J Jilin Agric Sci, 2007, 32(5): 24-27. | |
| [14] | 金永梅, 马瑞, 于志晶, 等. 转基因水稻吉生粳2号的外源基因旁侧序列分离及事件特异性PCR检测方法[J]. 东北农业科学, 2016, 41(1): 14-19. |
| Jin YM, Ma R, Yu ZJ, et al. Identification of the T-DNA flanking sequences and event-specific PCR detection of transgenic rice‘jishengjing 2’[J]. J Northeast Agric Sci, 2016, 41(1): 14-19. | |
| [15] | 郑子繁, 王颢潜, 高佳奇, 等. 耐除草剂转基因大豆SHZD32-1数字PCR绝对定量方法的建立[J]. 计量学报, 2023, 44(3): 415-421. |
| Zheng ZF, Wang HQ, Gao JQ, et al. Development of digital PCR absolute quantification method for herbicide-tolerant genetically modified soybean SHZD32-1[J]. Acta Metrol Sin, 2023, 44(3): 415-421. | |
| [16] | 林萍萍, 史云鹏, 安鹏天, 等. 影响实时荧光PCR检测转基因作物的因素分析[J]. 植物检疫, 2022, 36(5): 50-53. |
| Lin PP, Shi YP, An PT, et al. Analysis of influence factors on detection of transgenic crops by real-time PCR[J]. Plant Quar, 2022, 36(5): 50-53. |
| [1] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [2] | 李圣彦, 李香银, 李鹏程, 张明俊, 张杰, 郎志宏. 转基因玉米2HVB5的性状鉴定及遗传稳定性分析[J]. 生物技术通报, 2023, 39(1): 21-30. |
| [3] | 李鹏程, 张明俊, 王银晓, 李香银, 李圣彦, 郎志宏. 转基因玉米HGK60在不同遗传背景下抗虫性鉴定及农艺性状分析[J]. 生物技术通报, 2023, 39(1): 40-47. |
| [4] | 冯翠莲, 万玥, 王俊刚, 冯小艳, 赵婷婷, 王文治, 沈林波, 张树珍. 转Cry1Ac-2A-gna基因甘蔗BCG-17转化体特异性检测方法的建立[J]. 生物技术通报, 2021, 37(5): 248-258. |
| [5] | 焦悦, 韩宇, 杨桥, 黄耀辉, 安吉翠, 杨亚洲, 叶纪明. 全球转基因玉米商业化发展态势概述及启示[J]. 生物技术通报, 2021, 37(4): 164-176. |
| [6] | 温洪涛, 李夏莹, 杨洋, 陈子言, 丁一佳, 张秀杰, 张瑞英. 玉米转基因成分筛查策略[J]. 生物技术通报, 2020, 36(5): 39-47. |
| [7] | 王颢潜, 肖芳, 杨蕾, 缪青梅, 张旭冬, 张秀杰. 转基因玉米双抗12-5转化体特异性PCR方法验证结果分析[J]. 生物技术通报, 2020, 36(5): 48-55. |
| [8] | 杨镇州, 刘刚, 许丽. 基于RNAi技术的转基因玉米逆转录数字PCR检测方法[J]. 生物技术通报, 2020, 36(5): 56-63. |
| [9] | 李葱葱, 谢苹, 董立明, 夏蔚, 兰青阔, 闫伟, 龙丽坤, 李飞武. 抗虫耐除草剂玉米GH5112E-117C定性PCR检测方法[J]. 生物技术通报, 2020, 36(5): 64-67. |
| [10] | 王翠云, 刘艳, 刘允军. 外源基因在转基因玉米中的整合位点分析[J]. 生物技术通报, 2019, 35(3): 1-5. |
| [11] | 金永梅, 马瑞, 于志晶, 林秀峰. 转cry1C基因抗虫水稻吉生粳3号外源基因整合分析与品系特异性检测[J]. 生物技术通报, 2019, 35(3): 6-12. |
| [12] | 梁海生, 李梦桃, 李圣彦, 汪海, 张杰, 郎志宏. 转Bt基因抗虫玉米HGK60的农艺性状分析[J]. 生物技术通报, 2018, 34(7): 92-100. |
| [13] | 郑莹,刘家益,左璇,李圣彦,吴圣,聂凤英. 基于文献计量和内容挖掘的转基因玉米科研态势研究[J]. 生物技术通报, 2016, 32(12): 203-213. |
| [14] | 李欣竹, 耿丽丽, 高继国, 张杰. Cry1Ie蛋白的模拟胃肠液消化稳定性及热稳定性分析[J]. 生物技术通报, 2015, 31(11): 214-221. |
| [15] | 王建军,杨慧珍,刘佼. cryIAc基因在转基因玉米中的遗传规律及对抗虫性影响[J]. 生物技术通报, 2015, 31(1): 122-130. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||