








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 338-351.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0600
康恺1( ), 杨微2, 李迎春1, 谢为天1, 吴海燕1, 尤育品1, 陈志宝1,3(
), 杨微2, 李迎春1, 谢为天1, 吴海燕1, 尤育品1, 陈志宝1,3( )
)
收稿日期:2025-06-11
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
陈志宝,男,教授,研究方向 :分子药理学;E-mail: chenzb@gdou.edu.cn作者简介:康恺,女,副教授,研究方向 :细胞生物学与病原学;E-mail: kangk@gdou.edu.cn
基金资助:
KANG Kai1( ), YANG Wei2, LI Ying-chun1, XIE Wei-tian1, WU Hai-yan1, YOU Yu-pin1, CHEN Zhi-bao1,3(
), YANG Wei2, LI Ying-chun1, XIE Wei-tian1, WU Hai-yan1, YOU Yu-pin1, CHEN Zhi-bao1,3( )
)
Received:2025-06-11
Published:2026-01-26
Online:2026-02-04
摘要:
目的 桦褐孔菌醇(inotodiol, INO)是从药食两用真菌——桦褐孔菌中提取的三萜类化合物,具有抗氧化、抗炎等生物活性。通过体内外试验研究INO对黄曲霉毒素B1(aflatoxin B1, AFB1)肝毒性的保护效果。 方法 利用AFB1建立小鼠肝脏和肝实质细胞(AML12)损伤模型。制作肝组织切片,分析病理学变化,通过ELISA和抗氧化相关检测试剂盒分析肝组织、血清和AML12细胞中抗氧化水平;荧光显微镜观察活性氧(ROS)和线粒体膜电位(MMP),同时应用Western blot和qPCR实验方法检测肝脏和AML12细胞中Nrf2、PGC-1α、线粒体自噬信号通路蛋白表达情况。 结果 AFB1显著上调肝脏和AML12细胞中细胞色素P450酶(CYP450)基因表达,促进谷草转氨酶(AST)和谷丙转氨酶(ALT)的酶活力(P<0.01),INO预处理显著抑制AFB1诱导的上述指标的升高(P<0.01),且INO有效逆转AFB1诱导的抗氧化能力下降和线粒体功能障碍(P<0.01)。信号通路分析表明,INO可显著缓解AFB1诱导的Nrf2、PGC-1α和线粒体自噬信号通路的抑制,最终减轻AFB1引起的氧化损伤,恢复线粒体功能障碍。 结论 INO在体外和体内均能有效抑制AFB1诱导的肝细胞毒性。INO对肝脏的保护作用与激活Nrf2/PGC-1α信号通路和促进线粒体自噬密切相关。
康恺, 杨微, 李迎春, 谢为天, 吴海燕, 尤育品, 陈志宝. 桦褐孔菌醇通过激活Nrf2/PGC-1α/线粒体自噬防治AFB1诱导的小鼠肝损伤[J]. 生物技术通报, 2026, 42(1): 338-351.
KANG Kai, YANG Wei, LI Ying-chun, XIE Wei-tian, WU Hai-yan, YOU Yu-pin, CHEN Zhi-bao. Inotodiol Prevention of Aflatoxin B1-induced Liver Injury by Activating Nrf2/PGC-1α/Mitophagy[J]. Biotechnology Bulletin, 2026, 42(1): 338-351.
| 基因 Gene | 引物信息 Primer sequence | 产物长度 Product length (bp) | 基因序号 Gene ID |
|---|---|---|---|
| Nrf2 | F: GTGCTGCCAGAGGTCCTTAATGC R:CAGGAACAGTGAGGTGCCAGTAAC | 113 | NM_013693.1 |
| TNF⁃α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 113 | NM_013693.1 |
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC R: TCTGTTGGGAGTGGTATCCTCTGTG | 91 | NM_012589.2 |
| IL-1β | F: CACTACAGGCTCCGAGATGAACAA R: TGTCGTTGCTTGGTTCTCCTTGTAC | 145 | NM_008361.4 |
| HO-1 | F: GGAAATCATCCCTTGCACGC R: TGTTTGAACTTGGTGGGGCT | 91 | NM_012589.2 |
| NQO1 | F: GGTGAGCTGAAGGACTCGAA R: GCTCAGGCGTCCTTCCTTAT | 115 | NM_008361.4 |
| p62 | F: AGGAGGAGACGATGACTGGACAC R: TTGGTCTGTAGGAGCCTGGTGAG | 125 | NM_001411994.1 |
| LC3 | F: CTGTAAGGAGGTGCAGCAGAT R: TGCTTCTCACCCTTGTAGCGTA | 96 | NM_009741.5 |
| PGC-1α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 135 | NM_001330751.2 |
| PINK1 | F: CACTACAGGCTCCGAGATGAACAC R: TGTCGTTGCTTGGTTCTCCTTGTAC | 141 | NM_032409.3 |
| Parkin | F: CGTG AGCGGCTGCTTGTCTG R: ATGGTGAGCGAGGCGGTGAG | 124 | NM_168884.2 |
| CYP450 | F: TTTGCCACCTTCTGTCTCTTGTCAC R: AGTGTCGCCAGTGTTCTTAACCAT | 136 | NM_001327275.2 |
| β⁃actin | F: TATGCTCTCCCTCACGCCATCC R: GTCACGCACGATTTCCCTCTCAG | 129 | NM_011577.2 |
表1 RT-qPCR引物序列
Table 1 Sequences of primers used for RT-qPCR
| 基因 Gene | 引物信息 Primer sequence | 产物长度 Product length (bp) | 基因序号 Gene ID |
|---|---|---|---|
| Nrf2 | F: GTGCTGCCAGAGGTCCTTAATGC R:CAGGAACAGTGAGGTGCCAGTAAC | 113 | NM_013693.1 |
| TNF⁃α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 113 | NM_013693.1 |
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC R: TCTGTTGGGAGTGGTATCCTCTGTG | 91 | NM_012589.2 |
| IL-1β | F: CACTACAGGCTCCGAGATGAACAA R: TGTCGTTGCTTGGTTCTCCTTGTAC | 145 | NM_008361.4 |
| HO-1 | F: GGAAATCATCCCTTGCACGC R: TGTTTGAACTTGGTGGGGCT | 91 | NM_012589.2 |
| NQO1 | F: GGTGAGCTGAAGGACTCGAA R: GCTCAGGCGTCCTTCCTTAT | 115 | NM_008361.4 |
| p62 | F: AGGAGGAGACGATGACTGGACAC R: TTGGTCTGTAGGAGCCTGGTGAG | 125 | NM_001411994.1 |
| LC3 | F: CTGTAAGGAGGTGCAGCAGAT R: TGCTTCTCACCCTTGTAGCGTA | 96 | NM_009741.5 |
| PGC-1α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | 135 | NM_001330751.2 |
| PINK1 | F: CACTACAGGCTCCGAGATGAACAC R: TGTCGTTGCTTGGTTCTCCTTGTAC | 141 | NM_032409.3 |
| Parkin | F: CGTG AGCGGCTGCTTGTCTG R: ATGGTGAGCGAGGCGGTGAG | 124 | NM_168884.2 |
| CYP450 | F: TTTGCCACCTTCTGTCTCTTGTCAC R: AGTGTCGCCAGTGTTCTTAACCAT | 136 | NM_001327275.2 |
| β⁃actin | F: TATGCTCTCCCTCACGCCATCC R: GTCACGCACGATTTCCCTCTCAG | 129 | NM_011577.2 |
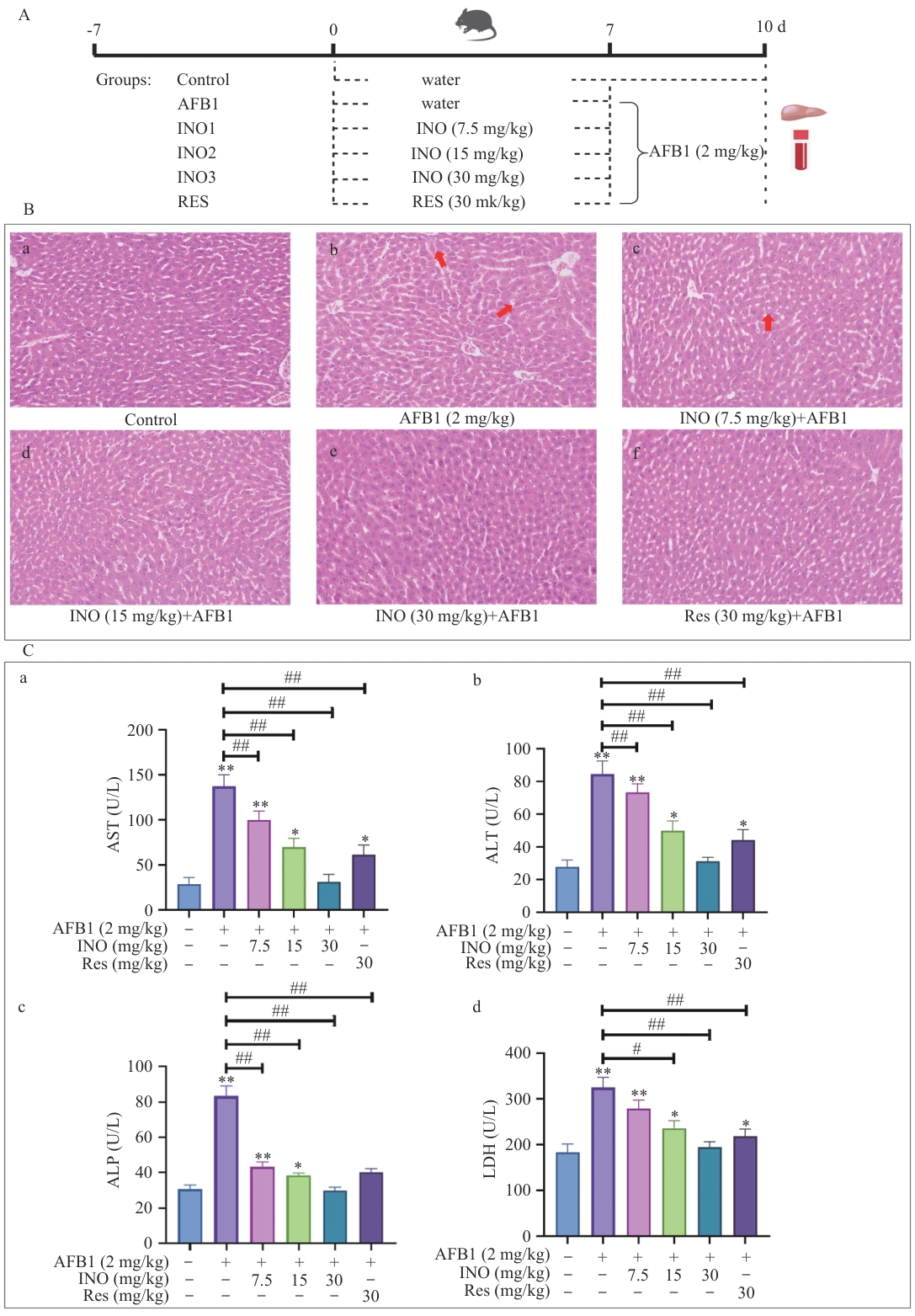
图1 INO保护小鼠肝脏免受AFB1诱导的损伤A:动物实验方案;B:小鼠肝脏组织切片及H&E染色;C:小鼠血清中AST、ALT、ALP和LDH酶活性检测。红色箭头表示肝窦扩张。B内标尺为50 μm。与对照组比较:*P<0.05;* *P<0.01。与AFB1组比较:#P<0.05;##P<0.01,下同
Fig. 1 INO protects liver of mice from AFB1-induced injuryA: Protocol of the animal experiments. B: Histological sectioning and H&E staining of mouse livers. C: Enzymatic activity of AST, ALT, ALP, and LDH in mouse serum was detected using a commercial kit. The red arrow indicates the hepatic sinusoidal dilatation. The bar in the B is 50 μm. Compared with the control group: *P<0.05; **P<0.01. Compared with the AFB1 group: #, P<0.05; ##, P<0.01. The same below
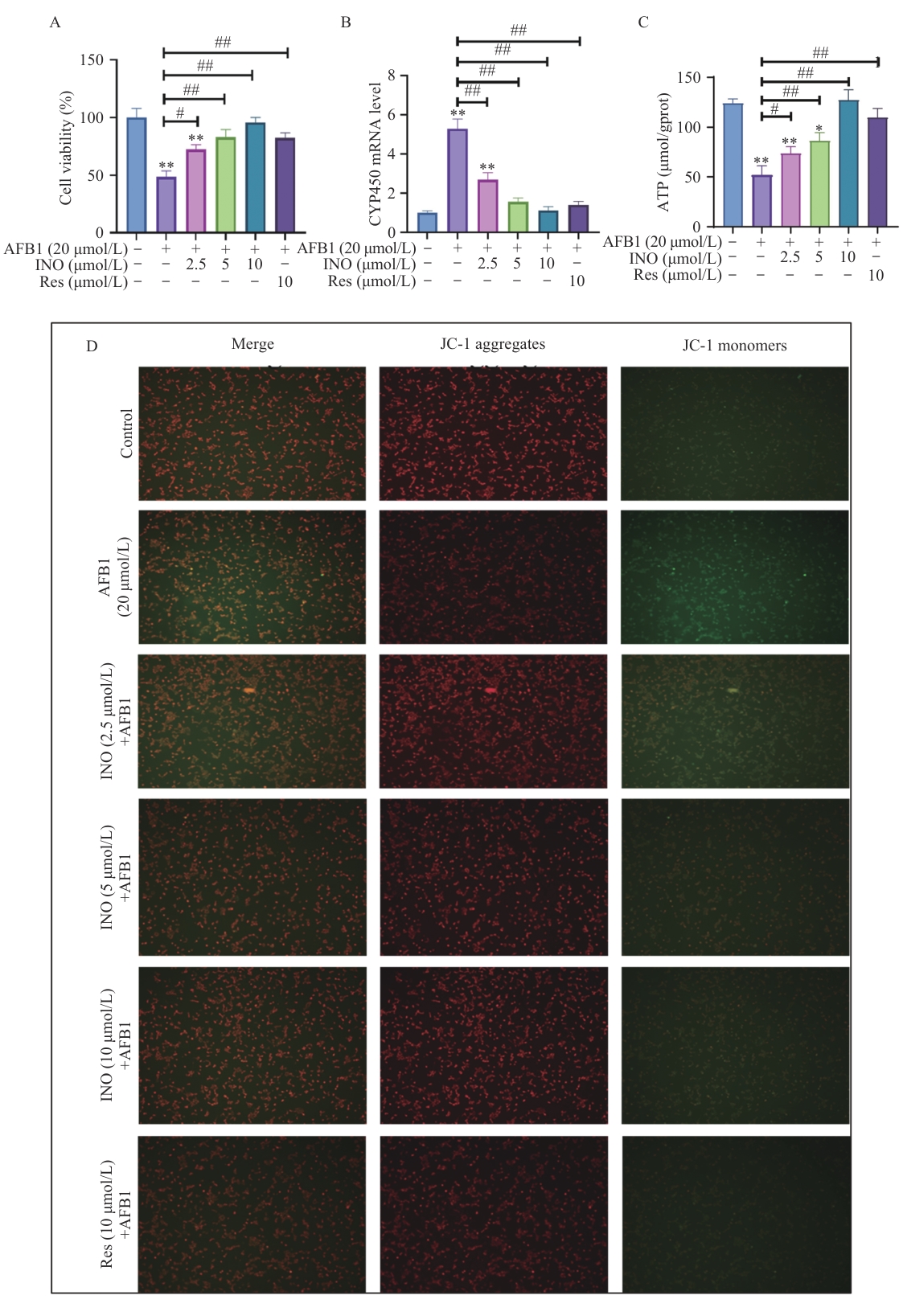
图3 INO防止AFB1诱导的AML12细胞活力下降和线粒体功能损伤A:CCK8检测AML12细胞活力;B:qPCR检测CYP450基因表达;C:ATP含量;D:线粒体膜电位
Fig. 3 INO prevents AFB1-induced AML12 cell viability decreases and mitochondrial function impairmentA: AML12 cell viability detected by CCK8. B: CYP450 gene expression detected using qPCR. C: ATP content. D: Mitochondrial membrane potential

图4 INO抑制AFB1诱导的AML12细胞氧化应激A:AST和ALT的酶活性检测;B:氧化应激相关蛋白的检测(a:根据图1-C统计的ROS荧光强度;b和c:GSH和MDA含量;d:SOD酶活性检测);C:AML12细胞中ROS含量。与对照组比较:*,P<0.05;**,P<0.01。与AFB1组比较:#,P<0.05;##,P<0.01
Fig. 4 INO inhibits AFB1-induced oxidative stress in AML12 cellsA: Enzymatic activity of AST and ALT. B: Detection of oxidative-stress-related proteins(a: Statistical results of fluorescence intensity for ROS according to Fig.1-C. b and c: GSH and MDA content. d: Enzymatic activity of SOD). C: The ROS in AML12 cells. Compared with the control group: *, P<0.05; **, P<0.01. Compared with the AFB1 group: #, P<0.05; ##, P<0.01
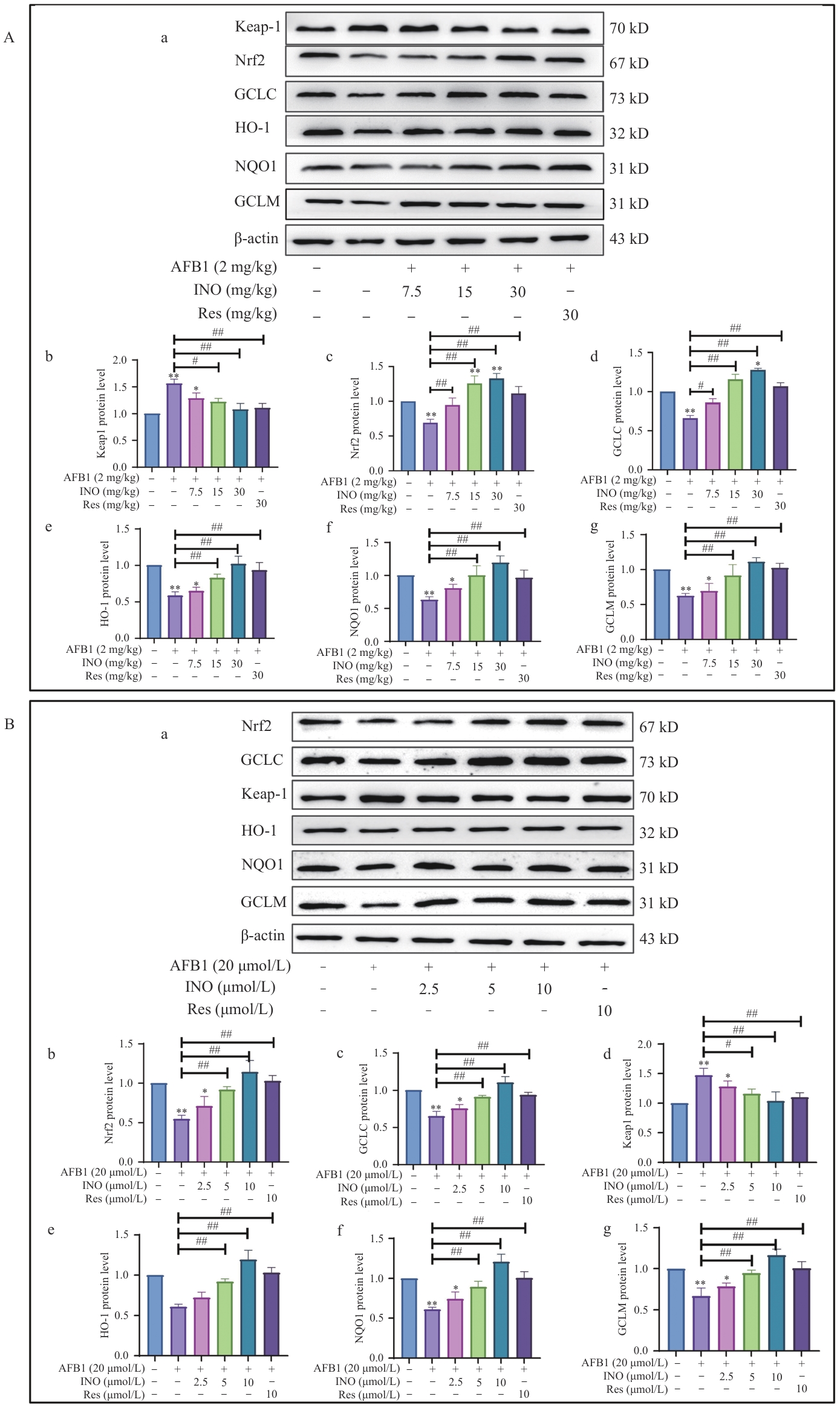
图5 INO在体内和体外均激活Keap1/Nrf2抗氧化信号通路A:INO激活小鼠肝脏中的Keap1/Nrf2抗氧化信号通路;B:INO激活AML12细胞中的Keap1/Nrf2抗氧化信号通路
Fig. 5 INO activates the Keap1/Nrf2 antioxidant signal pathway both in vivo and in vitroA: INO activates the Keap1/Nrf2 antioxidant signal pathway in mouse livers. B: INO activates the Keap1/Nrf2 antioxidant signal pathway in AML12 cells
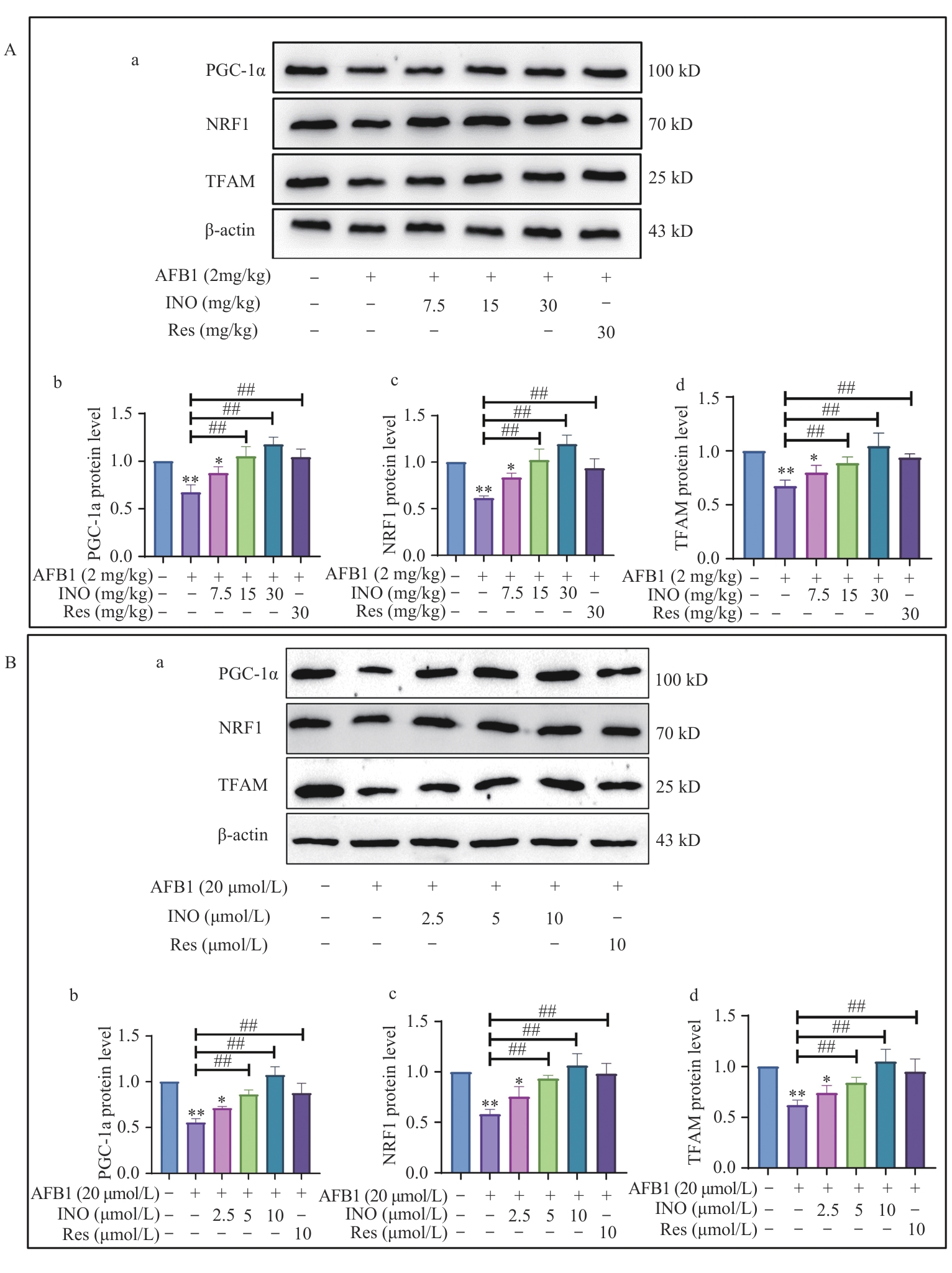
图6 INO在体内和体外均激活PGC-1α/NRF1信号通路A:INO激活小鼠肝脏中PGC-1α/NRF1信号通路;B:INO激活AML12细胞中的PGC-1α/NRF1抗氧化信号通路
Fig. 6 INO activates the PGC-1α/NRF1 signaling pathway both in vivo and in vitroA: INO activates the PGC-1α/NRF1 signaling pathway in mouse livers. B: INO activates the PGC-1α/NRF1 antioxidant signal pathway in AML12 cells
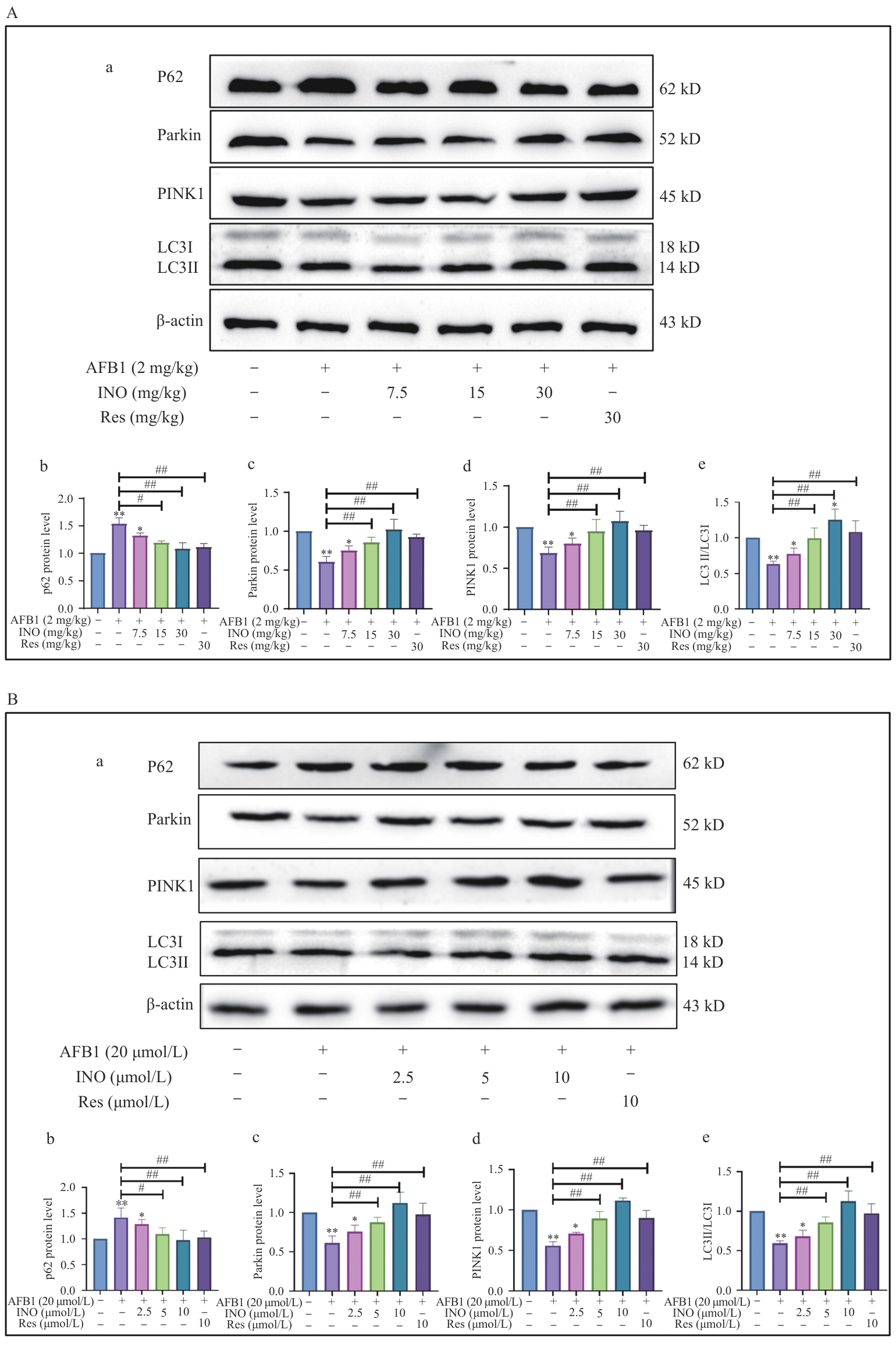
图7 INO在体内和体外激活线粒体自噬A:INO激活小鼠肝脏的线粒体自噬;B:INO激活AML12细胞的线粒体自噬
Fig. 7 INO activates mitophagy both in vivo and in vitroA: INO activates mitophagy in mouse livers. B: INO activates mitophagy in AML12 cell
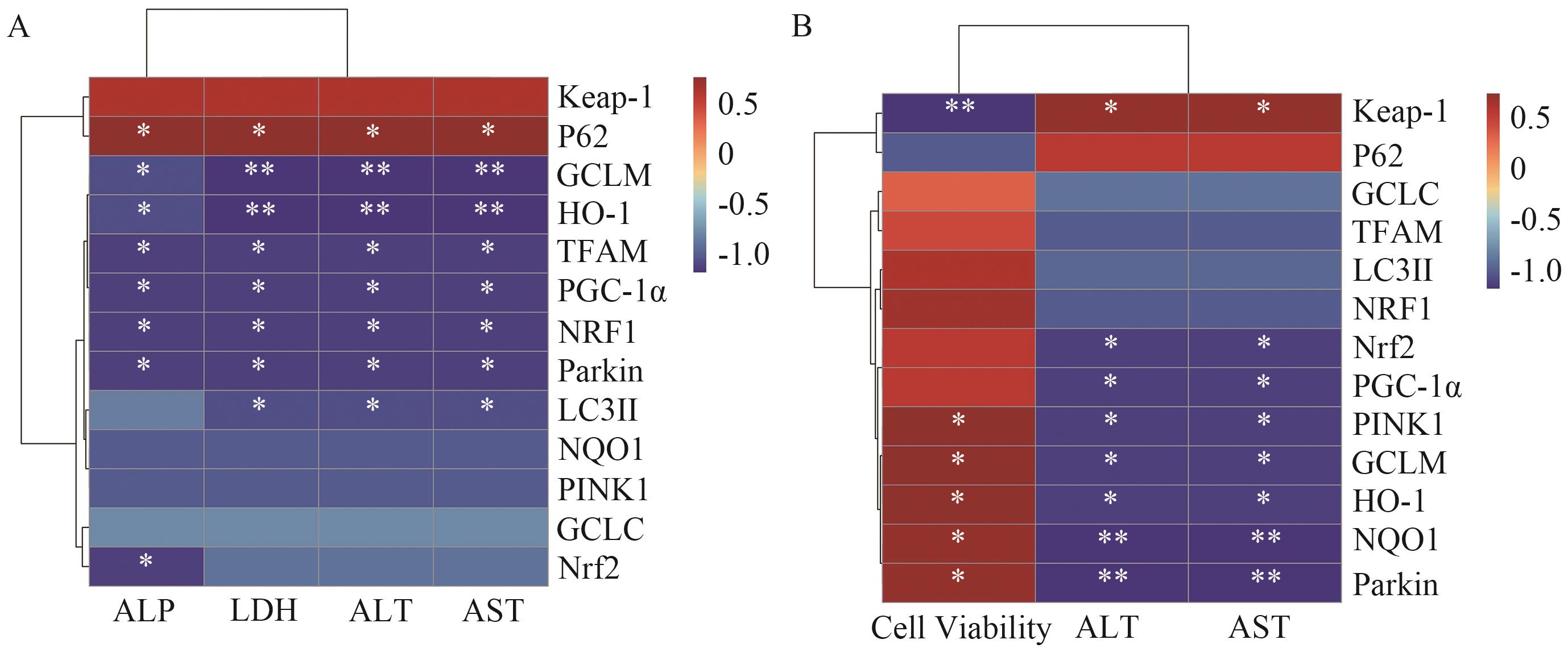
图8 对小鼠肝功能指数与信号通路蛋白相关性分析A:小鼠肝功能指数与信号通路蛋白表达;B:AML12细胞小鼠肝功能指数与信号通路蛋白相关性分析
Fig. 8 Correlation analysis between liver function index and signaling pathway proteins in miceA: Correlation analysis of liver function indices and expressions of signaling pathway proteins in mice. B: Correlation analysis of liver function indices and signaling pathway proteins in AML12 cells of mice
| [1] | 黄年来. 俄罗斯神秘的民间药用真菌——桦褐孔菌 [J]. 中国食用菌, 2002, 21(4): 7-8. |
| Huang NL. A mysterious folk medicinal A mysterious folk medicinal fungus in Russia—Inonotus obliquus [J]. Edible Fungi China, 2002, 21(4): 7-8. | |
| [2] | Zhao FQ, Mai QQ, Ma JH, et al. Triterpenoids from Inonotus obliquus and their antitumor activities [J]. Fitoterapia, 2015, 101: 34-40. |
| [3] | Yu SC, Lai ZX, Xue HM, et al. Inonotus obliquus aqueous extract inhibits intestinal inflammation and insulin metabolism defects in Drosophila [J]. Toxicol Mech Meth, 2024, 34(9): 970-984. |
| [4] | Endo T, Nakagomi Y, Kawaguchi E, et al. Anti-malarial activity in a Chinese herbal supplement containing Inonotus obliquus and Panax notoginseng [J]. Parasitol Int, 2022, 87: 102532. |
| [5] | 朱彦彬. 桦褐孔菌醇介导NF-κB/Nrf2通路促进自噬抑制LPS诱导小鼠肠损伤 [D]. 湛江: 广东海洋大学, 2023. |
| Zhu YB. Inotodiol promotes autophagy and inhibits LPS-induced intestinal injury in mice by regulating NF-κB/Nrf2 signal pathway [D]. Zhanjiang: Guangdong Ocean University, 2023. | |
| [6] | Marchese S, Polo A, Ariano A, et al. Aflatoxin B1 and M1: biological properties and their involvement in cancer development [J]. Toxins, 2018, 10(6): 214. |
| [7] | Gizachew D, Chang CH, Szonyi B, et al. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature [J]. Int J Food Microbiol, 2019, 296: 8-13. |
| [8] | Valencia-Quintana R, Milić M, Jakšić D, et al. Environment changes, aflatoxins, and health issues, a review [J]. Int J Environ Res Public Health, 2020, 17(21): 7850. |
| [9] | Qiao BX, He Y, Gao XL, et al. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage [J]. Food Chem Toxicol, 2023, 172: 113593. |
| [10] | Qiu Z, Wang HY, Li GQ, et al. Lactobacillus salivarius Ameliorates AFB1-induced hepatotoxicity via PINK1/Parkin-mediated mitophagy in Geese [J]. Ecotoxicol Environ Saf, 2024, 280: 116574. |
| [11] | Liu HY, He Y, Gao XL, et al. Curcumin alleviates AFB1-induced nephrotoxicity in ducks: regulating mitochondrial oxidative stress, ferritinophagy, and ferroptosis [J]. Mycotoxin Res, 2023, 39(4): 437-451. |
| [12] | Cook KL, Clarke PAG, Parmar J, et al. Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death [J]. FASEB J, 2014, 28(9): 3891-3905. |
| [13] | Jiang Y, Chen DK, Gong QM, et al. Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease [J]. Lipds Health Dis, 2021, 20(1): 40. |
| [14] | Gan ZY, Callegari S, Cobbold SA, et al. Activation mechanism of PINK1 [J]. Nature, 2022, 602(7896): 328-335. |
| [15] | Dagda RK, Cherra SJ, Kulich SM, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission [J]. J Biol Chem, 2009, 284(20): 13843-13855. |
| [16] | Chen XX, Che CP, Korolchuk VI, et al. Selenomethionine alleviates AFB1-induced damage in primary chicken hepatocytes by inhibiting CYP450 1A5 expression via upregulated SelW expression [J]. J Agric Food Chem, 2017, 65(12): 2495-2502. |
| [17] | Liu FJ, Wang YJ, Zhou X, et al. Resveratrol relieved acute liver damage in ducks (Anas platyrhynchos) induced by AFB1 via modulation of apoptosis and Nrf2 signaling pathways [J]. Animals, 2021, 11(12): 3516. |
| [18] | 魏艳梅, 陈惠琴, 杨理, 等. 桦褐孔菌化学成分的胆碱酯酶抑制和细胞毒活性研究 [J]. 天然产物研究与开发, 2020, 32(7): 1156-1163. |
| Wei YM, Chen HQ, Yang L, et al. Cholinesterase inhibitory and cytotoxic activity of chemical constituents from Inonotus obliquus [J]. Nat Prod Res Dev, 2020, 32(7): 1156-1163. | |
| [19] | Rotimi OA, Rotimi SO, Goodrich JM, et al. Time-course effects of acute aflatoxin B1 exposure on hepatic mitochondrial lipids and oxidative stress in rats [J]. Front Pharmacol, 2019, 10: 467. |
| [20] | Jiang XX, Liu HY, You YL, et al. Multi-omics reveals the protective effects of curcumin against AFB1-induced oxidative stress and inflammatory damage in duckling intestines [J]. Comp Biochem Physiol Part C Toxicol Pharmacol, 2024, 276: 109815. |
| [21] | He F, Ru XL, Wen T. NRF2, a transcription factor for stress response and beyond [J]. Int J Mol Sci, 2020, 21(13): 4777. |
| [22] | Bellezza I, Giambanco I, Minelli A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress [J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(5): 721-733. |
| [23] | Taguchi K, Takaku M, Egner PA, et al. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity [J]. Toxicol Sci, 2016, 152(1): 40-52. |
| [24] | Li HT, Sang R, Zhao X, et al. Research Note: Taraxasterol alleviates aflatoxin B1-induced oxidative stress in chicken primary hepatocytes [J]. Poult Sci, 2023, 102(1): 102286. |
| [25] | Zhao YX, Zheng WF. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus [J]. J Ethnopharmacol, 2021, 265: 113321. |
| [26] | Kou RW, Xia B, Han R, et al. Neuroprotective effects of a new triterpenoid from edible mushroom on oxidative stress and apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 signaling pathway in vitro and in vivo [J]. Food Funct, 2022, 13(23): 12121-12134. |
| [27] | Deng J, Zhao L, Zhang NY, et al. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents [J]. Mutat Res, 2018, 778: 79-89. |
| [28] | Ghallab A, Hassan R, Myllys M, et al. Subcellular spatio-temporal intravital kinetics of aflatoxin B1 and ochratoxin A in liver and kidney [J]. Arch Toxicol, 2021, 95(6): 2163-2177. |
| [29] | Qian X, Li XJ, Shi ZM, et al. KDM3A senses oxygen availability to regulate PGC-1α-mediated mitochondrial biogenesis [J]. Mol Cell, 2019, 76(6): 885-895.e7. |
| [30] | Wang Y, Chen XP, Baker JS, et al. Astaxanthin promotes mitochondrial biogenesis and antioxidant capacity in chronic high-intensity interval training [J]. Eur J Nutr, 2023, 62(3): 1453-1466. |
| [31] | Li PA, Hou XL, Hao SC. Mitochondrial biogenesis in neurodegeneration [J]. J Neurosci Res, 2017, 95(10): 2025-2029. |
| [32] | Xu FB, Li YF, Cao Z, et al. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition [J]. Ecotoxicol Environ Saf, 2021, 216: 112213. |
| [33] | Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs [J]. Cell Death Differ, 2015, 22(3): 377-388. |
| [34] | Bartolini D, Dallaglio K, Torquato P, et al. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma [J]. Transl Res, 2018, 193: 54-71. |
| [35] | Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy [J]. Mol Cell, 2013, 51(5): 618-631. |
| [36] | Connelly EM, Frankel KS, Shaw GS. Parkin and mitochondrial signalling [J]. Cell Signal, 2023, 106: 110631. |
| [37] | Yamada T, Dawson TM, Yanagawa T, et al. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy [J]. Autophagy, 2019, 15(11): 2012-2018. |
| [38] | Robertson I, Wai Hau T, Sami F, et al. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential [J]. Int J Pharm, 2022, 618: 121605. |
| [1] | 黎伟华, 吴璟, 金学琴, 雷艳丽. 基于蛋白质组学方法探讨四氯化碳诱导的小鼠急性肝损伤的差异蛋白表达[J]. 生物技术通报, 2025, 41(2): 331-342. |
| [2] | 杨微, 关海峰, 任欣慧, 彭金菊, 陈志宝. 虾青素对黄曲霉毒素B1诱导肝损伤的缓解作用及机制[J]. 生物技术通报, 2025, 41(10): 334-342. |
| [3] | 康凌云, 韩露露, 韩德平, 陈建胜, 甘瀚凌, 邢凯, 马友记, 崔凯. 褪黑素缓解空肠黏膜上皮细胞氧化损伤的效果研究[J]. 生物技术通报, 2023, 39(9): 291-299. |
| [4] | 朱业胜, 伍国强, 魏明. 质膜Na+/H+逆向转运蛋白SOS1在植物离子稳态平衡中的作用[J]. 生物技术通报, 2023, 39(12): 16-32. |
| [5] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| [6] | 张小妮, 翁伊纯, 范奕浩, 王晓娟, 赵佳宇, 张云龙. Mito-OS-Timer:一种靶向监测线粒体氧化应激的荧光秒表[J]. 生物技术通报, 2022, 38(10): 97-105. |
| [7] | 彭文超, 刘建新, 王迪铭. 哺乳动物低氧应激代谢成因的研究进展[J]. 生物技术通报, 2021, 37(1): 262-271. |
| [8] | 刘融, 崔凯, 白福恒, 刁其玉. 蛋氨酸调控畜禽氧化应激的研究进展[J]. 生物技术通报, 2020, 36(10): 207-214. |
| [9] | 吕鹏, 徐嘉擎, 王森, 闫艳春. 杀菌剂苯并异噻唑啉酮对斑马鱼胚胎的急性毒性和氧化应激效应研究[J]. 生物技术通报, 2018, 34(1): 172-182. |
| [10] | 饶磊, 饶敏, 仇红红, 李红辉, 刘静, 庄满娇. 纳米硒抗胰岛β细胞凋亡作用与机制的研究[J]. 生物技术通报, 2016, 32(11): 271-277. |
| [11] | 王志舒, 谭晓荣, 刘洹洹. 线粒体自噬调控机制研究进展[J]. 生物技术通报, 2015, 31(6): 42-47. |
| [12] | 李铁松,高杨,王颖,李庆伟. 抗增殖蛋白与氧化应激[J]. 生物技术通报, 2013, 29(10): 34-39. |
| [13] | 王潇;刘华;白洁;. 细胞色素P450调节肝脏药物代谢的途径[J]. , 2009, 0(07): 39-41. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||