








生物技术通报 ›› 2025, Vol. 41 ›› Issue (10): 334-342.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0199
杨微1,2( ), 关海峰3, 任欣慧4, 彭金菊1, 陈志宝1(
), 关海峰3, 任欣慧4, 彭金菊1, 陈志宝1( )
)
收稿日期:2025-02-25
出版日期:2025-10-26
发布日期:2025-10-28
通讯作者:
陈志宝,男,博士,教授,研究方向 :兽医药理与毒理学;E-mail: chenzb@gdou.edu.cn作者简介:杨微,女,博士研究生,研究方向 :兽医药理与毒理学;E-mail: 15776581772@163.com
基金资助:
YANG Wei1,2( ), GUAN Hai-feng3, REN Xin-hui4, PENG Jin-ju1, CHEN Zhi-bao1(
), GUAN Hai-feng3, REN Xin-hui4, PENG Jin-ju1, CHEN Zhi-bao1( )
)
Received:2025-02-25
Published:2025-10-26
Online:2025-10-28
摘要:
目的 探究虾青素(astaxanthin, AST)对黄曲霉毒素B1(aflatoxin B1, AFB1)诱导肝损伤的保护作用及分子机制。 方法 构建AFB1诱导小鼠肝实质AML21细胞和C57BL/6小鼠模型,商品化试剂盒检测AST处理后生化指标(谷草转氨酶、谷丙转氨酶和碱性磷酸酶)、氧化指标(ROS、MDA、SOD、GSH和CAT)和炎症指标(IL-1β和IL-18)的水平,蛋白质免疫印迹(western blot, WB)方法检测AST对Nrf2和焦亡信号通路的影响。 结果 体内外结果一致表明,AST可有效缓解AFB1导致的生化指标、氧化指标和炎症指标的异常。AST激活Nrf2信号通路,显著升高抗氧化蛋白NQO1、HO-1、GCLC和GCLM的水平;Nrf2抑制剂ML385处理AML21细胞后,Nrf2、NQO1和HO-1蛋白表达水平显著降低,AST和ML385共同处理后,蛋白表达水平被显著逆转。另外,AST还能抑制焦亡途径,显著降低NLRP3、ASC、Caspase-1和GSDMD蛋白表达水平。 结论 AST通过激活Nrf2信号通路抑制细胞焦亡,从而抵抗氧化应激和炎症反应,缓解AFB1诱导肝损伤。
杨微, 关海峰, 任欣慧, 彭金菊, 陈志宝. 虾青素对黄曲霉毒素B1诱导肝损伤的缓解作用及机制[J]. 生物技术通报, 2025, 41(10): 334-342.
YANG Wei, GUAN Hai-feng, REN Xin-hui, PENG Jin-ju, CHEN Zhi-bao. Alleviating Effect of Astaxanthin on Liver Injury Induced by Aflatoxin B 1 and Its Mechanism[J]. Biotechnology Bulletin, 2025, 41(10): 334-342.

图1 AST对AFB1诱导AML21细胞损伤的影响A-C:生化指标;D-F:氧化指标;G-H:炎症因子;I-J:ROS水平及相对定量分析结果。与CON相比,#P<0.05;##P<0.01;###P<0.001。与AFB1相比,*P<0.05;**P<0.01;***P<0.001,下同
Fig. 1 Effect of AST on AFB1-induced AML21 cell damageA-C: Biochemical indicators. D-F: Antioxidation indexes. G-H: Inflammatory factors. I-J: ROS levels and relative quantitative analysis. Compared with CON, #P<0.05; ##P<0.01; ###P<0.001. Compared with AFB1, *P<0.05; **P<0.01; ***P<0.001, the same below

图2 AST对AFB1诱导AML21细胞Nrf2通路的影响A-B:AST对AFB1诱导AML21细胞中Nrf2、Keap1、NQO1、HO-1、GCLC和GCLM蛋白表达水平及蛋白相对定量分析;C-D:AST和Nrf2抑制剂ML385对AFB1诱导AML21细胞中Nrf2、NQO1、HO-1蛋白表达水平及蛋白相对定量分析
Fig. 2 Effects of AST on Nrf2 pathway in AFB1-induced AML21 cellsA-B: The results of AST on the protein expressions and relative protein quantitative analysis of Nrf2, Keap1, NQO1, HO-1, GCLC and GCLM in AFB1-induced AML21 cells. C-D: The results of AST and Nrf2 inhibitor ML385 on the protein expressions and relative protein quantitative analysis of Nrf2, NQO1, and HO-1 in AFB1-induced AML21 cells

图3 AST对AFB1诱导AML21细胞焦亡通路的影响A-B:AST对AFB1诱导AML21细胞中NLRP3、ASC、GSDMD和Caspase-1蛋白表达水平及蛋白相对定量分析
Fig. 3 Effect of AST on pyroptosis pathways in AFB1-induced AML21 cellsA-B: The results of AST on the protein expressions and relative protein quantitative analysis of NLRP3, ASC, GSDMD and Caspase-1 in AFB1-induced AML21 cells
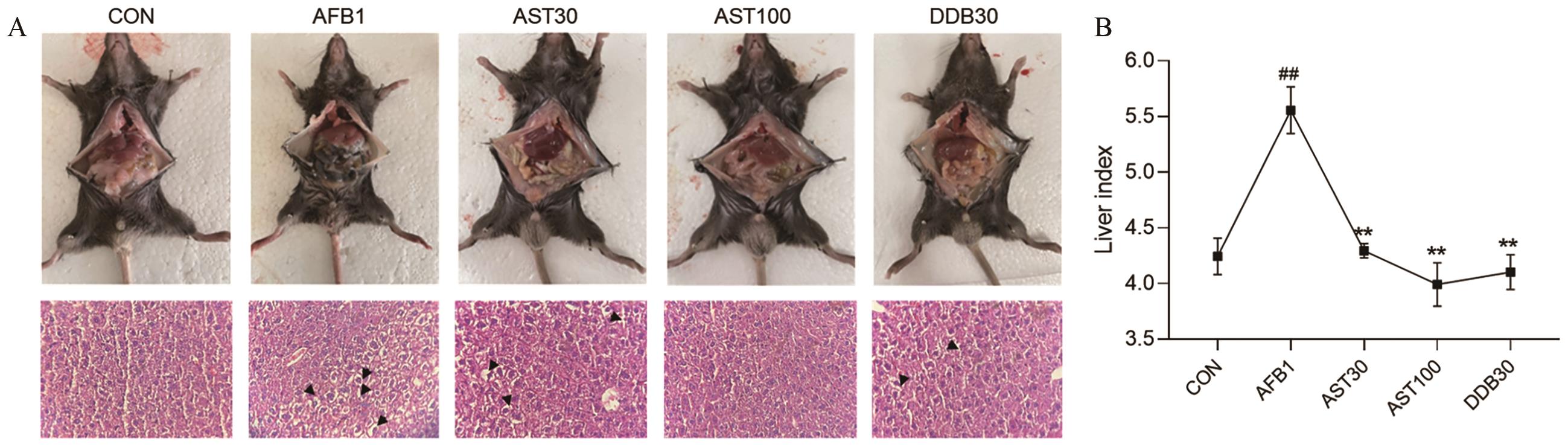
图4 AST对AFB1诱导小鼠肝脏病理损伤的影响A图上:小鼠肝脏损伤观察结果,黑色箭头代表发生肝细胞肿胀,空泡变性;A图下:小鼠肝脏组织HE染色(400×)
Fig. 4 Effect of AST on the liver pathological injury in AFB1-induced miceTop A: Observation of liver injury in mice, black arrows indicate the occurrence of hepatocyte swelling with vacuolar degeneration. Bottom A: HE staining of mouse liver tissue (400×)
组织 Tissue | 处理 Treatment | 谷草转氨酶 Glutamic oxalacetic transaminase(U/L) | 谷丙转氨酶 Glutamic-pyruvic transaminase(U/L) | 碱性磷酸酶 Alkaline phosphatase(U/L) |
|---|---|---|---|---|
肝脏 Liver | CON | 246.828±0.612 | 123.229±4.075 | 20.459±3.149 |
| AFB1 | 405.961±20.85## | 225.165±7.041## | 36.847±0.339## | |
| AST30 | 307.242±3.669** | 150.364±0.654** | 24.613±2.333** | |
| AST100 | 300.681±1.447** | 143.950±6.307** | 18.752±1.353** | |
| DDB30 | 330.726±4.687** | 155.653±2.001** | 27.069±0.352** | |
血清 Serum | CON | 16.944±0.307 | 22.708±0.676 | 30.801±0.408 |
| AFB1 | 35.599±1.417## | 62.137±0.378## | 74.568±1.569## | |
| AST30 | 16.465±0.109** | 32.139±0.577** | 46.452±1.645** | |
| AST100 | 11.187±0.392** | 21.456±1.185** | 31.701±4.681** | |
| DDB30 | 20.953±0.539* | 23.942±0.769** | 36.880±1.827** |
表1 肝脏和血清中肝功能指标分析
Table 1 Analysis of live function index in liver tissue and serum
组织 Tissue | 处理 Treatment | 谷草转氨酶 Glutamic oxalacetic transaminase(U/L) | 谷丙转氨酶 Glutamic-pyruvic transaminase(U/L) | 碱性磷酸酶 Alkaline phosphatase(U/L) |
|---|---|---|---|---|
肝脏 Liver | CON | 246.828±0.612 | 123.229±4.075 | 20.459±3.149 |
| AFB1 | 405.961±20.85## | 225.165±7.041## | 36.847±0.339## | |
| AST30 | 307.242±3.669** | 150.364±0.654** | 24.613±2.333** | |
| AST100 | 300.681±1.447** | 143.950±6.307** | 18.752±1.353** | |
| DDB30 | 330.726±4.687** | 155.653±2.001** | 27.069±0.352** | |
血清 Serum | CON | 16.944±0.307 | 22.708±0.676 | 30.801±0.408 |
| AFB1 | 35.599±1.417## | 62.137±0.378## | 74.568±1.569## | |
| AST30 | 16.465±0.109** | 32.139±0.577** | 46.452±1.645** | |
| AST100 | 11.187±0.392** | 21.456±1.185** | 31.701±4.681** | |
| DDB30 | 20.953±0.539* | 23.942±0.769** | 36.880±1.827** |
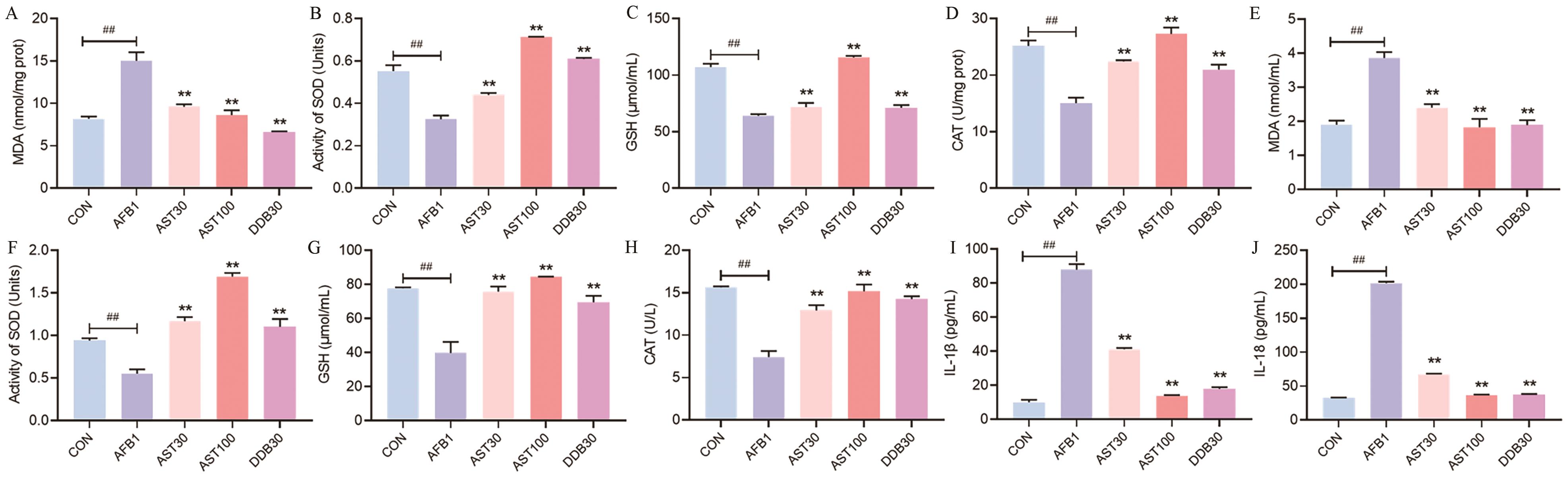
图5 AST对AFB1诱导小鼠氧化应激和炎症的影响A-D:AST对AFB1诱导小鼠肝组织氧化指标的影响;E-H:AST对AFB1诱导小鼠血清氧化指标的影响;I‒J:AST对AFB1诱导小鼠血清炎症因子的影响
Fig. 5 Effects of AST on the oxidative stress and inflammation in AFB1-induced miceA-D: Effect of AST on oxidative indices in AFB1-induced mouse liver tissue. E-H: Effect of AST on antioxidative markers in the serum of AFB1-induced mice. I-J: Effect of AST on inflammatory factors in the serum of AFB1-induced mice

图6 AST对AFB1诱导小鼠肝组织Nrf2通路的影响A-B:AST对AFB1诱导小鼠肝组织中Nrf2、Keap1、NQO1、HO-1、GCLC和GCLM蛋白表达水平及蛋白相对定量分析结果
Fig. 6 Effect of AST on AFB1 induced Nrf2 pathway in mice liver tissueA-B: The results of AST on the protein expressions and relative protein quantitative analysis of Nrf2, Keap1, NQO1, HO-1, GCLC and GCLM in AFB1-induced mouse liver tissue
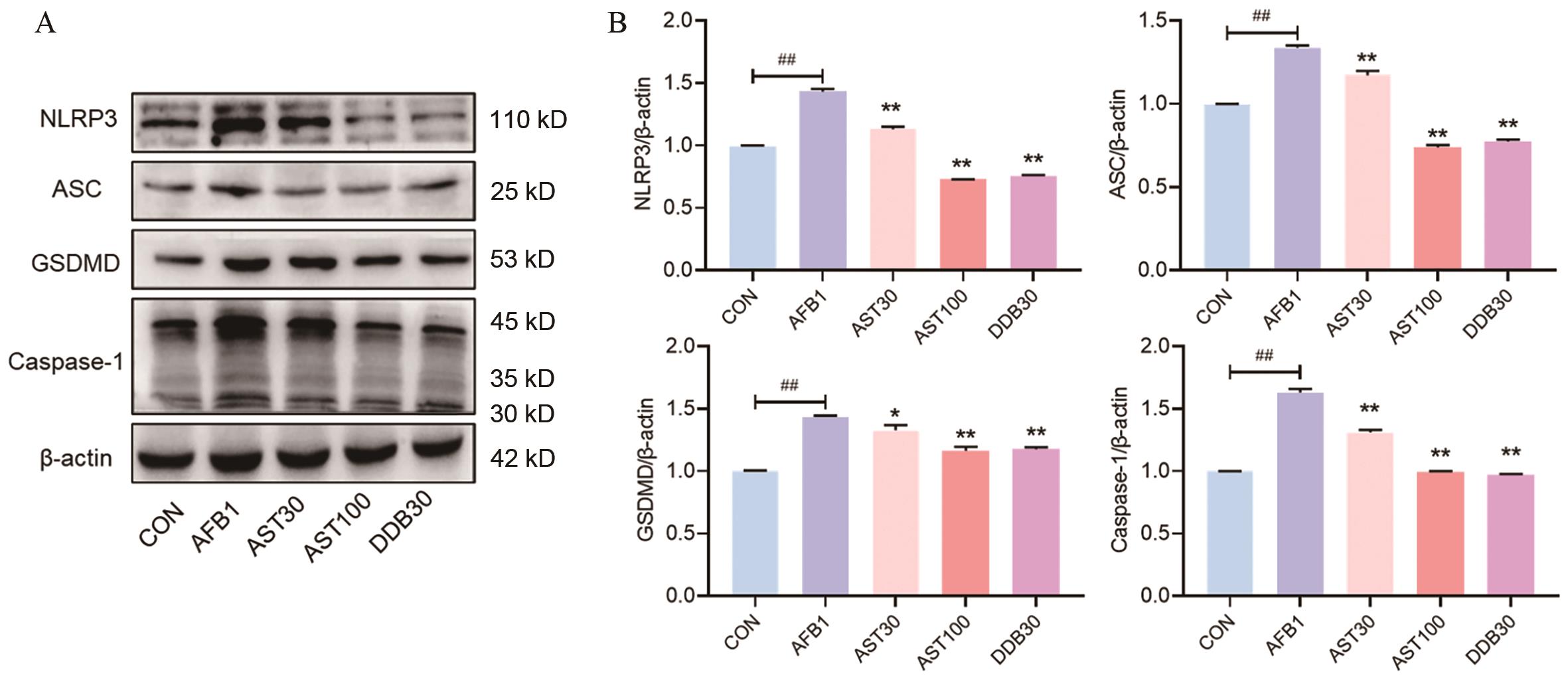
图7 AST对AFB1诱导小鼠肝组织焦亡通路的影响A-B:AST对AFB1诱导小鼠肝组织中NLRP3、ASC、GSDMD和Caspase-1蛋白表达水平及蛋白相对定量分析结果
Fig. 7 Effect of AST on AFB1 induced pyroptosis pathway in mice liver tissueA-B: The results of AST on the protein expressions and relative protein quantitative analysis of NLRP3, ASC, GSDMD and Caspase-1 in AFB1-induced mouse liver tissue
| [1] | Cao WY, Yu P, Yang KP, et al. Aflatoxin B1: metabolism, toxicology, and its involvement in oxidative stress and cancer development [J]. Toxicol Mech Methods, 2022, 32(6): 395-419. |
| [2] | 马慧慧. 寄生曲霉菌在不同饲料原料中产生黄曲霉毒素B1的差异性及其机制研究 [D]. 武汉:华中农业大学,2015. |
| Ma HH. Study on the difference and mechanism of aflatoxin B1 produced by parasitic Aspergillus in different feed materials [D]. Wuhan: Huazhong Agricultural University, 2015. | |
| [3] | Zhang L, Shi SW, Liu Y, et al. Aflatoxin B1 triggers apoptosis in rabbit hepatocytes via mediating oxidative stress and switching on the mitochondrial apoptosis pathway [J]. Ecotoxicol Environ Saf, 2023, 264: 115478. |
| [4] | Luo TY, Zhou XY, Qin MY, et al. Corilagin restrains NLRP3 inflammasome activation and pyroptosis through the ROS/TXNIP/NLRP3 pathway to prevent inflammation [J]. Oxid Med Cell Longev, 2022, 2022: 1652244. |
| [5] | Kohandel Z, Farkhondeh T, Aschner M, et al. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases [J]. Biomed Pharmacother, 2022, 145: 112179. |
| [6] | Si P, Zhu CK. Biological and neurological activities of astaxanthin (review) [J]. Mol Med Rep, 2022, 26(4): 300. |
| [7] | Cai XP, Hua SY, Deng JW, et al. Astaxanthin activated the Nrf2/HO-1 pathway to enhance autophagy and inhibit ferroptosis, ameliorating acetaminophen-induced liver injury [J]. ACS Appl Mater Interfaces, 2022, 14(38): 42887-42903. |
| [8] | Luo LX, Huang FF, Zhong SY, et al. Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1 signaling pathways in LPS-induced acute lung injury [J]. Life Sci, 2022, 311: 121091. |
| [9] | Song L, Yao S, Zheng D, et al. Astaxanthin attenuates contrast-induced acute kidney injury in rats via ROS/NLRP3 inflammasome [J]. Int Urol Nephrol, 2022, 54(6): 1355-1364. |
| [10] | 任欣慧, 姚强, 黄庆年, 等. 虾青素保护AFB1诱导AML12细胞损伤的最适浓度筛选 [J]. 黑龙江八一农垦大学学报, 2022, 34(2): 88-93. |
| Ren XH, Yao Q, Huang QN, et al. Screening of the optimal concentration of astaxanthin to protect AML12 cells from AFB1-induced damage [J]. Journal of Heilongjiang Bayi Agricultural University, 2022, 34(2): 88-93. | |
| [11] | Liu SP, Kang WL, Mao XR, et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice [J]. J Pineal Res, 2022, 73(2): e12812. |
| [12] | Cheng XY, Liang JH, Wu D, et al. Blunting ROS/TRPML1 pathway protects AFB1-induced porcine intestinal epithelial cells apoptosis by restoring impaired autophagic flux [J]. Ecotoxicol Environ Saf, 2023, 257: 114942. |
| [13] | Inoue Y, Shimazawa M, Nagano R, et al. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration [J]. J Pharmacol Sci, 2017, 134(3): 147-157. |
| [14] | Kurashige M, Okimasu E, Inoue M, et al. Inhibition of oxidative injury of biological membranes by astaxanthin [J]. Physiol Chem Phys Med NMR, 1990, 22(1): 27-38. |
| [15] | Zou YT, Zhang SY, Yang J, et al. Protective effects of astaxanthin on ochratoxin A-induced liver injury: effects of endoplasmic reticulum stress and mitochondrial fission-fusion balance [J]. Toxins, 2024, 16(2): 68. |
| [16] | Jelic MD, Mandic AD, Maricic SM, et al. Oxidative stress and its role in cancer [J]. J Cancer Res Ther, 2021, 17(1): 22-28. |
| [17] | Jomova K, Raptova R, Alomar SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging [J]. Arch Toxicol, 2023, 97(10): 2499-2574. |
| [18] | He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond [J]. Int J Mol Sci, 2020, 21(13): E4777. |
| [19] | Wu DY, Wu Y, Zhang M, et al. Aflatoxin B1 exposure triggers inflammation and premature skin aging via ERMCS/Ca2+/ROS signaling cascade [J]. Int Immunopharmacol, 2023, 124: 110961. |
| [20] | Rao ZP, Zhu YT, Yang P, et al. Pyroptosis in inflammatory diseases and cancer [J]. Theranostics, 2022, 12(9): 4310-4329. |
| [21] | Wang JS, Wu ZY, Zhu M, et al. ROS induced pyroptosis in inflammatory disease and cancer [J]. Front Immunol, 2024, 15: 1378990. |
| [22] | Feng SY, Wierzbowski MC, Hrovat-Schaale K, et al. Mechanisms of NLRP3 activation and inhibition elucidated by functional analysis of disease-associated variants [J]. Nat Immunol, 2025, 26(3): 511-523. |
| [23] | Zhang LY, Zhan DL, Chen YY, et al. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo and in vivo study [J]. Arch Toxicol, 2019, 93(11): 3305-3320. |
| [24] | Cao XJ, Cheng JX, Yang YS, et al. Arginine-derived carbon dots with antioxidant activity for treating aflatoxin B1-induced liver injury via Nrf2/Keap1 and NLRP3 pathways in mice [J]. Life Sci, 2025, 364: 123430. |
| [1] | 虎喜敏, 周冉, 王正兴, 李宇航, 罗仍卓么, 王兴平. 干扰lncRNA RNF5-AS1对奶牛乳腺上皮细胞炎症反应的影响[J]. 生物技术通报, 2025, 41(5): 333-342. |
| [2] | 黎伟华, 吴璟, 金学琴, 雷艳丽. 基于蛋白质组学方法探讨四氯化碳诱导的小鼠急性肝损伤的差异蛋白表达[J]. 生物技术通报, 2025, 41(2): 331-342. |
| [3] | 张春辉, 吉婧芳, 曹嘉敏, 马茜茜, 刘纹众, 季春丽, 张立涛, 李润植. 光呼吸对碳源诱导雨生红球藻积累虾青素的影响[J]. 生物技术通报, 2025, 41(10): 110-120. |
| [4] | 雷棋怡, 徐杨, 李鹏飞. 脆弱拟杆菌六型分泌系统对肠道屏障的影响及机制[J]. 生物技术通报, 2024, 40(3): 286-295. |
| [5] | 马文澳, 杨微, 李迎春, 朱彦彬, 陈志宝, 刘娜. 桦褐孔菌乙醇提取物对脂多糖诱导肠损伤的缓解作用及机制[J]. 生物技术通报, 2024, 40(12): 299-308. |
| [6] | 吴永娜, 滕文龙, 张磊, 王德富, 牛颜冰. 连翘叶茶对大鼠肝硬化的影响及其机理研究[J]. 生物技术通报, 2024, 40(11): 285-295. |
| [7] | 段子朋, 孙缦利, 陈彦锋, 邓同兴, 金少举, 范文娟, 陈旭东. 虾青素通过AMPK/mTOR信号通路促进鸡肌肉干细胞增殖与分化[J]. 生物技术通报, 2024, 40(11): 312-320. |
| [8] | 焦帅, 付域泽, 崔凯, 张吉贤, 王杰, 毕研亮, 刁其玉, 张建新, 张乃锋. 短小芽孢杆菌对羔羊肠道炎症和屏障功能的影响[J]. 生物技术通报, 2024, 40(1): 344-352. |
| [9] | 康凌云, 韩露露, 韩德平, 陈建胜, 甘瀚凌, 邢凯, 马友记, 崔凯. 褪黑素缓解空肠黏膜上皮细胞氧化损伤的效果研究[J]. 生物技术通报, 2023, 39(9): 291-299. |
| [10] | 陈彩萍, 任昊, 龙腾飞, 何冰, 鲁兆祥, 孙坚. 大肠杆菌Nissle 1917对炎症性肠病治疗作用的研究进展[J]. 生物技术通报, 2023, 39(6): 109-118. |
| [11] | 杨冬, 唐璎. 枯草芽孢杆菌WTX1胞外酶降解AFB1酶学特性及降解位点分析[J]. 生物技术通报, 2023, 39(4): 93-102. |
| [12] | 朱业胜, 伍国强, 魏明. 质膜Na+/H+逆向转运蛋白SOS1在植物离子稳态平衡中的作用[J]. 生物技术通报, 2023, 39(12): 16-32. |
| [13] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| [14] | 周琳, 梁轩铭, 赵磊. 天然类胡萝卜素的生物合成研究进展[J]. 生物技术通报, 2022, 38(7): 119-127. |
| [15] | 张小妮, 翁伊纯, 范奕浩, 王晓娟, 赵佳宇, 张云龙. Mito-OS-Timer:一种靶向监测线粒体氧化应激的荧光秒表[J]. 生物技术通报, 2022, 38(10): 97-105. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||