








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (6): 181-191.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1131
Previous Articles Next Articles
SU Yu( ), LI Zong-yun(
), LI Zong-yun( ), HAN Yong-hua
), HAN Yong-hua
Received:2020-09-06
Online:2021-06-26
Published:2021-07-08
Contact:
LI Zong-yun
E-mail:1586544642@qq.com;zongyunli@jsnu.edu.cn
SU Yu, LI Zong-yun, HAN Yong-hua. Advances in Plant Vacuolar Processing Enzymes[J]. Biotechnology Bulletin, 2021, 37(6): 181-191.
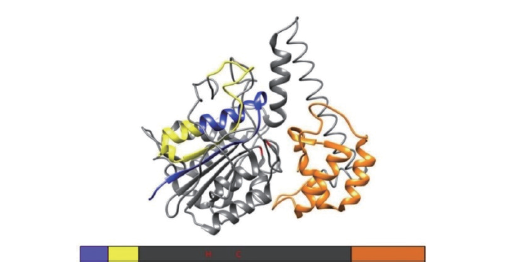
Fig. 1 Main three-dimensional structure of VPE Blue represents N-terminal signal peptide,yellow represents cleavable prepeptide,orange represents C-terminal prepeptide,i.e. LSAM domain,and gray represents mature protein. Refer to Barend et al[8]
| 物种 Species | 组织 Organization | VPE在不同类型PCD中的研究 Study on VPE in different types of PCD | 参考文献 References |
|---|---|---|---|
| 烟草 | 叶 | 液泡膜裂解介导的PCD,沉默VPE及caspase-1抑制剂能够抑制PCD及病毒增殖 | [9,34-35] |
| 叶 | VPE沉默诱导气孔关闭,抑制PCD | [36] | |
| 花瓣 | VPE基因表达的上调 | [37] | |
| 悬浮培养细胞 | 液泡膜裂解介导的PCD,VPE缺失及caspase-1抑制剂对PCD的抑制,VPE基因上调与VPE活性增加 | [38-39] | |
| 根 | Ced-9抑制铝诱导的PCD促进植物对铝的耐受 | [40] | |
| 拟南芥 | 花药 | γ VPE基因表达的上调 | [41] |
| 叶 | 液泡膜裂解介导的PCD,VPE缺失突变体及caspase-1抑制剂对PCD的抑制作用,重组p35蛋白对VPE活性的抑制作用 | [42-43] | |
| 叶 | VPE缺失突变体的卵子产孢量减少,卵子感染期间γ VPE活性的增加 | [44] | |
| 叶、侧根、悬浮培养细胞 | α VPE和γ VPE基因的上调 | [45-46] | |
| 叶原生质体 | caspase-1抑制剂和p35超表达对PCD的抑制作用 | [47] | |
| 种皮 | δ VPE突变体内珠被两层细胞PCD延迟 | [48] | |
| 水稻 | 胚珠 | VPE基因随类caspase活性的增加上调 | [49] |
| 种子 | caspase-1抑制剂对PCD的抑制使VPE活性提高 | [44] | |
| 土豆 | 块茎顶芽 分生组织 | caspase-1抑制剂抑制PCD使VPE活性提高 | [50] |
| 苹果 | 叶 | VPE基因的表达在HR过程中上调 | [51] |
| 番茄 | 叶 | Bcl-2过表达下调VPE基因表达,在抑制NaCl诱导的PCD过程中起重要作用 | [52-53] |
| 悬浮培养细胞 | caspase-1抑制剂对PCD的抑制作用 | [54] | |
| 大豆 | 叶原生质体 | VPE基因上调,鉴定两种VPE基因表达转录因子 | [55] |
| 甘薯 | 叶、根 | 参与发育、衰老、生殖过程中的PCD | [56] |
Table 1 VPE functions in plant PCD and the related processes
| 物种 Species | 组织 Organization | VPE在不同类型PCD中的研究 Study on VPE in different types of PCD | 参考文献 References |
|---|---|---|---|
| 烟草 | 叶 | 液泡膜裂解介导的PCD,沉默VPE及caspase-1抑制剂能够抑制PCD及病毒增殖 | [9,34-35] |
| 叶 | VPE沉默诱导气孔关闭,抑制PCD | [36] | |
| 花瓣 | VPE基因表达的上调 | [37] | |
| 悬浮培养细胞 | 液泡膜裂解介导的PCD,VPE缺失及caspase-1抑制剂对PCD的抑制,VPE基因上调与VPE活性增加 | [38-39] | |
| 根 | Ced-9抑制铝诱导的PCD促进植物对铝的耐受 | [40] | |
| 拟南芥 | 花药 | γ VPE基因表达的上调 | [41] |
| 叶 | 液泡膜裂解介导的PCD,VPE缺失突变体及caspase-1抑制剂对PCD的抑制作用,重组p35蛋白对VPE活性的抑制作用 | [42-43] | |
| 叶 | VPE缺失突变体的卵子产孢量减少,卵子感染期间γ VPE活性的增加 | [44] | |
| 叶、侧根、悬浮培养细胞 | α VPE和γ VPE基因的上调 | [45-46] | |
| 叶原生质体 | caspase-1抑制剂和p35超表达对PCD的抑制作用 | [47] | |
| 种皮 | δ VPE突变体内珠被两层细胞PCD延迟 | [48] | |
| 水稻 | 胚珠 | VPE基因随类caspase活性的增加上调 | [49] |
| 种子 | caspase-1抑制剂对PCD的抑制使VPE活性提高 | [44] | |
| 土豆 | 块茎顶芽 分生组织 | caspase-1抑制剂抑制PCD使VPE活性提高 | [50] |
| 苹果 | 叶 | VPE基因的表达在HR过程中上调 | [51] |
| 番茄 | 叶 | Bcl-2过表达下调VPE基因表达,在抑制NaCl诱导的PCD过程中起重要作用 | [52-53] |
| 悬浮培养细胞 | caspase-1抑制剂对PCD的抑制作用 | [54] | |
| 大豆 | 叶原生质体 | VPE基因上调,鉴定两种VPE基因表达转录因子 | [55] |
| 甘薯 | 叶、根 | 参与发育、衰老、生殖过程中的PCD | [56] |
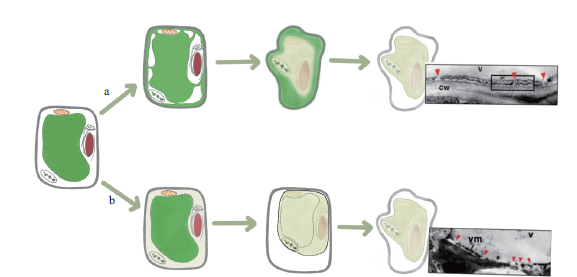
Fig. 2 Two different ways of vacuole-mediated cell death a represents the non-destructive way,b represents the non-destructive way,v represents vacuole,cw represents cell membrane,and vm represents vacuole membrane. Refer to Hara Nishimura et al[60]
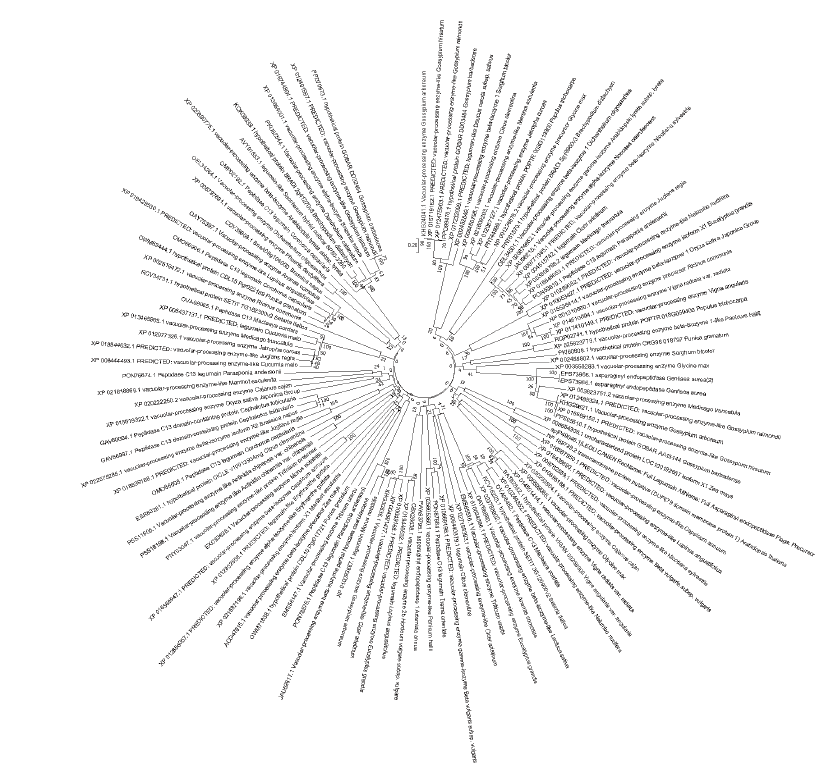
Fig. 3 Phylogenetic tree of vacuolase system in plants The analysis is involved 116 amino acid sequences. The tree is drawn to scale,with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. Refer to Kenji et al[80]
| [1] | Hara-Nishimura I. Plant legumain, asparaginyl endopeptidase, vacuolar processing enzyme[M]// Barrett AJ, Rawlings ND, Woessner JF. eds. Handbook of Proteolytic Enzymes 3rd Edition. London, UK: Academic Press, 2012:2314-2320. |
| [2] |
Hara-Nishimura I, Nishimura M. Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons[J]. Plant Physiology, 1987, 85:440-445.
doi: 10.1104/pp.85.2.440 URL |
| [3] |
Hara-Nishimura I, Inoue K, Nishimura M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms[J]. FEBS Letter, 1991, 294:89-93.
doi: 10.1016/0014-5793(91)81349-D URL |
| [4] |
Chen JM, Rawlings ND, Stevens RA, et al. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases[J]. FEBS Letter, 1998, 441:361-365.
doi: 10.1016/S0014-5793(98)01574-9 URL |
| [5] |
Kuroyanagi M, Nishimura M, Hara-Nishimura I. Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an autoinhibitory domain of the C-terminal propeptide[J]. Plant Cell Physiology, 2002, 43:143-151.
doi: 10.1093/pcp/pcf035 URL |
| [6] |
Hiraiwa N, Kondo M, Nishimura M, et al. An aspartic endopeptidase is involved in the breakdown of propeptides of storage proteins in proteinstorage vacuoles of plants[J]. Eur J Biochem, 1997, 246:133-141.
pmid: 9210475 |
| [7] |
Cohen GM. Caspase:the executioners of apoptosis[J]. Biochemical Journal, 1997, 326:1-16.
doi: 10.1042/bj3260001 URL |
| [8] |
Barend JV, Christopher A, Karl J. Plant vacuolar processing enzymes[J]. Front Plant Sci, 2019, 10:479.
doi: 10.3389/fpls.2019.00479 URL |
| [9] |
Hatsugai N, Kuroyanagi M, Yamada K, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death[J]. Science, 2004, 305:855-858.
doi: 10.1126/science.1099859 URL |
| [10] |
Saska I, Gillon AD, Hatsugai N, et al. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization[J]. Journal of Biological Chemistry, 2007, 282:29721-29728.
doi: 10.1074/jbc.M705185200 URL |
| [11] |
Min W, Jones DH. In-vitro splicing of concanavalin A is catalyzed by asparaginyl endopeptidase[J]. Nature Structural Biology, 1994, 1:502-504.
pmid: 7664074 |
| [12] |
Mylne JS, Colgrave ML, Daly NL, et al. Albumins and their processing machinery are hijacked for cyclic peptides in sunflower[J]. Nature Chemical Biology, 2011, 7:257-259.
doi: 10.1038/nchembio.542 URL |
| [13] |
Nguyen GKT, Wang SJ, Qiu YB, et al. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synjournal[J]. Nature Chemical Biology, 2014, 10:732-738.
doi: 10.1038/nchembio.1586 URL |
| [14] |
Bernath-Levin K, Nelson C, Elliott AG, et al. Peptide macrocyclization by a bifunctional endoprotease[J]. Chemistry & Biology, 2015, 22:571-582.
doi: 10.1016/j.chembiol.2015.04.010 URL |
| [15] |
Yang R, Wong YH, Nguyen GKT, et al. Engineering a catalytically efficient recombinant protein ligase[J]. Journal of the American Chemical Society, 2017, 139:5351-5358.
doi: 10.1021/jacs.6b12637 URL |
| [16] | Haywood J, Schmidberger JW, James AM, et al. Structural basis of ribosomal peptide macrocyclization in plants[J]. eLife, 2018, 7:32955. |
| [17] |
Jackson MA, Gilding EK, Shafee T, et al. Molecular basis for the production of cyclic peptides by plant asparaginyl endopeptidases[J]. Nature Communications, 2018, 9:2411.
doi: 10.1038/s41467-018-04669-9 pmid: 29925835 |
| [18] |
Shimada T, Takagi J, Ichino T, et al. Plant vacuoles[J]. Annu Rev Plant Biol, 2018, 69:123-145.
doi: 10.1146/annurev-arplant-042817-040508 pmid: 29561663 |
| [19] | Wang M, Han B, Zhao X, et al. The role of vacuolar processing enzyme[J]. Agricultural Science & Technology, 2012, 13(5):945-951. |
| [20] |
Yamada K, Shimada T, Nishimura M, et al. A VPE family supporting various vacuolar functions in plants[J]. Physiol Plantarum, 2005, 123:369-375.
doi: 10.1111/ppl.2005.123.issue-4 URL |
| [21] |
Hatsugai N, Kuroyanagi M, Nishimura M, et al. A cellular suicide strategy of plants:vacuole-mediated cell death[J]. Apoptosis, 2006, 11:905-911.
pmid: 16547592 |
| [22] |
Göransson U, Burman R, Gunasekera S, et al. Circular proteins from plants and fungi[J]. Journal of Biological Chemistry, 2012, 287:27001-27006.
doi: 10.1074/jbc.R111.300129 URL |
| [23] |
James AM, Haywood J, Mylne JS. Macrocyclization by asparaginyl endopeptidases[J]. New Phytologist, 2018, 218:923-928.
doi: 10.1111/nph.2018.218.issue-3 URL |
| [24] |
Sheldon PS, Keen JN, Bowles DJ. Post-translational peptide bond formation during concanavalin A processing in vitro[J]. Biochemical Journal, 1996, 320:865-870.
doi: 10.1042/bj3200865 URL |
| [25] |
Abe Y, Shirane K, Yokosawa H, et al. Asparaginyl endopeptidase of jack bean seeds. Purification, characterization, and high utility in protein-sequence analysis[J]. Journal of Biological Chemistry, 1993, 268:3525-3529.
doi: 10.1016/S0021-9258(18)53726-1 URL |
| [26] |
Takeda O, Miura Y, Mitta M, et al. Isolation and analysis of cDNA-encoding a precursor of canavalia-ensiformis asparaginyl endopeptidase(legumain)[J]. Journal of Biochemistry, 1994, 116:541-546.
doi: 10.1093/oxfordjournals.jbchem.a124559 URL |
| [27] |
Harris KS, Durek T, Kaas Q, et al. Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase[J]. Nature Communications, 2015, 6:10199.
doi: 10.1038/ncomms10199 pmid: 26680698 |
| [28] |
Franke B, James AM, Mobli M, et al. Two proteins for the price of one:Structural studies of the dual-destiny protein preproalbumin with sunflower trypsin inhibitor-1[J]. Journal of Biological Chemistry, 2017, 292:12398-12411.
doi: 10.1074/jbc.M117.776955 URL |
| [29] |
Zauner FB, Dall E, Regl C, et al. Crystal structure of plant legumain reveals a unique two-chain state with pH-dependent activity regulation[J]. The Plant Cell, 2018, 30:686-699.
doi: 10.1105/tpc.17.00963 URL |
| [30] |
James AM, Haywood J, Leroux J, et al. The macrocyclizing protease butelase 1 remains autocatalytic and reveals the structural basis for ligase activity[J]. The Plant Journal, 2019, 98:988-999.
doi: 10.1111/tpj.14293 pmid: 30790358 |
| [31] | Hemu X, El Sahili A, Hu S, et al. Structural determinants for peptide-bond formation by asparaginyl ligases[J]. PNAS, 2019, 116:11737-11746. |
| [32] |
Zheng Y, Zhang H, Deng X, et al. The relationship between vacuolation and initiation of PCD in rice(Oryza sativa)aleurone cells[J]. Sci Rep, 2017, 7:41245.
doi: 10.1038/srep41245 pmid: 28117452 |
| [33] | 熊园园, 邢达. 液泡信号通路依赖的细胞程序性死亡研究进展[J]. 激光生物学报, 2010, 19(3):419-422. |
| Xiong Y, Xing D. Progress in programmed cell death dependent on vacuole signaling pathway[J]. Acta Laser Biology Sinica, 2010, 19(3):419-422. | |
| [34] |
Urquhart W, Gunawardena AH, Moeder W, et al. The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner[J]. Plant Mol Biol, 2007, 65:747-761.
pmid: 17885810 |
| [35] |
Mase K, Mizuno T, Ishihama N, et al. Ethylene signaling pathway and MAPK cascades are required for AAL toxininduced programmed cell death[J]. Mol Plant Microbe Interact, 2012, 25:1015-1025.
doi: 10.1094/MPMI-02-12-0036-R URL |
| [36] |
Zhang H, Dong S, Wang M, et al. The role of vacuolar processing enzyme(VPE)from Nicotiana benthamiana in the elicitor-triggered hypersensitive response and stomatal closure[J]. J Exp Bot, 2010, 61:3799-3812.
doi: 10.1093/jxb/erq189 URL |
| [37] |
Muller GL, Drincovich MF, Andreo CS, et al. Role of photosynjournal and analysis of key enzymes involved in primary metabolism throughout the lifespan of the tobacco flower[J]. J Exp Bot, 2010, 61:3675-3688.
doi: 10.1093/jxb/erq187 URL |
| [38] |
Gauthier A, Lamotte O, Reboutier D, et al. Cryptogein-induced anion effluxes:electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitortriggered cell death[J]. Plant Signal Behav, 2007, 2:86-95.
pmid: 19516973 |
| [39] |
Kariya K, Demiral T, Sasaki T, et al. A novel mechanism of aluminum-induced cell death involving vacuolar processing enzyme and vacuolar collapse in tobacco cell line BY-2[J]. J Inorg Biochem, 2013, 128:196-201.
doi: 10.1016/j.jinorgbio.2013.07.001 pmid: 23891542 |
| [40] |
Wang W, Pan J, Zheng K, et al. Ced-9 inhibits Al-induced programmed cell death and promotes Al tolerance in tobacco[J]. Biochem Biophys Res Commun, 2009, 383:141-145.
doi: 10.1016/j.bbrc.2009.03.125 URL |
| [41] |
Li Z, Yue H, Xing D. MAP Kinase 6-mediated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis[J]. N Phytol, 2012, 195:85-96.
doi: 10.1111/j.1469-8137.2012.04131.x URL |
| [42] |
Kuroyanagi M, Yamada K, Hatsugai N, et al. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana[J]. Journal of Biological Chemistry, 2005, 280:32914-32920.
doi: 10.1074/jbc.M504476200 URL |
| [43] |
Qiang X, Zechmann B, Reitz MU, et al. The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death[J]. The Plant Cell, 2012, 24:794-809.
doi: 10.1105/tpc.111.093260 URL |
| [44] |
Misas-Villamil JC, Toenges G, Kolodziejek I, et al. Activity profiling of vacuolar processing enzymes reveals a role for VPE during oomycete infection[J]. The Plant Journal, 2013, 73:689-700.
doi: 10.1111/tpj.12062 pmid: 23134548 |
| [45] |
Kadono T, Tran D, Errakhi R, et al. Increased anion channel activity is an unavoidable event in ozone-induced programmed cell death[J]. PLoS One, 2010, 5:e13373.
doi: 10.1371/journal.pone.0013373 URL |
| [46] |
Kinoshita T, Yamada K, Hiraiwa N, et al. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions[J]. The Plant Journal, 1999, 19:43-53.
doi: 10.1046/j.1365-313X.1999.00497.x URL |
| [47] |
Danon A, Rotari VI, Gordon A, et al. Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and defender against apoptotic death[J]. J Biol Chem, 2004, 279:779-787.
doi: 10.1074/jbc.M304468200 URL |
| [48] |
Nakaune S, Yamada K, Kondo M, et al. A vacuolar processing enzyme, δ VPE, is involved in seed coat formation at the early stage of seed development[J]. The Plant Cell, 2005, 17:876-887.
doi: 10.1105/tpc.104.026872 URL |
| [49] |
Tran V, Weier D, Radchuk R, et al. Caspase-like activities accompany programmed cell death events in developing barley grains[J]. PLoS One, 2014, 9:e109426.
doi: 10.1371/journal.pone.0109426 URL |
| [50] |
Teper-Bamnolker P, Buskila Y, Lopesco Y, et al. Release of apical dominance in potato tuber is accompanied by programmed cell death in the apical bud meristem[J]. Plant Physiology, 2012, 158:2053-2067.
doi: 10.1104/pp.112.194076 URL |
| [51] |
Iakimova ET, Sobiczewski P, Michalczuk L, et al. Morphological and biochemical characterization of Erwinia amylovora-induced hypersensitive cell death in apple leaves[J]. Plant Physiol Biochem, 2013, 63:292-305.
doi: 10.1016/j.plaphy.2012.12.006 URL |
| [52] |
Deng M, Bian H, Xie Y, et al. Bcl-2 suppresses hydrogen peroxide-induced programmed cell death via OsVPE2 and OsVPE3, but not via OsVPE1 and OsVPE4, in rice[J]. FEBS J, 2011, 278:4797-4810.
doi: 10.1111/j.1742-4658.2011.08380.x URL |
| [53] |
Kim Y, Wang M, Bai Y, et al. Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice[J]. Plant Physiol Biochem, 2014, 80:168-175.
doi: 10.1016/j.plaphy.2014.04.002 URL |
| [54] |
Yakimova ET, Kapchina-Toteva VM, Laarhoven LJ, et al. Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells[J]. Plant Physiol Biochem, 2006, 44:581-589.
doi: 10.1016/j.plaphy.2006.09.003 URL |
| [55] |
Mendes GC, Reis PA, Calil IP, et al. GmNAC30 and GmNAC81 integrate the endoplasmic reticulum stress-and osmotic stress-induced cell death responses through a vacuolar processing enzyme[J]. PNAS, 2013, 110(48):19627-19632.
doi: 10.1073/pnas.1311729110 URL |
| [56] |
Jiang J, Hu J, Tan R, et al. Expression of IbVPE1 from sweet potato in Arabidopsis affects leaf development, flowering time and chlorophyll catabolism[J]. BMC Plant Biol, 2019, 19(1):184.
doi: 10.1186/s12870-019-1789-8 URL |
| [57] |
Hatsugai N, Iwasaki S, Tamura K, et al. A novel membrane fusionmediated plant immunity against bacterial pathogens[J]. Gene Dev, 2009, 23:2496-2506.
doi: 10.1101/gad.1825209 URL |
| [58] |
Zhang H, Zheng X, Zhang Z. The role of vacuolar processing enzymes in plant immunity[J]. Plant Signal Behav, 2010, 5(12):1565-1567.
doi: 10.4161/psb.5.12.13809 URL |
| [59] | 冉昆, 马怀宇, 杨洪强. 植物细胞程序性死亡中的类胱天蛋白酶研究进展[J]. 西北植物学报, 2008, 28(12):2564-2570. |
| Ran K, Ma HY, Yang HQ. Advances in caspases in programmed cell death in plants[J]. Acta Botanica Boreali-Occidentalia Sinica, 2008, 28(12):2564-2570. | |
| [60] |
Hara-Nishimura I, Hatsugai N. The role of vacuole in plant cell death[J]. Cell Death and Differentiation, 2011, 18:1298-1304.
doi: 10.1038/cdd.2011.70 URL |
| [61] | van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence[J]. J Exp Bo, 2008, 59:453-480. |
| [62] |
Cilliers M, van Wyk SG, van Heerden PDR, et al. Identification and changes of the drought-induced cysteine protease transcriptome in soybean(Glycine max)root nodules[J]. J Exp Environ Bot, 2018, 148:59-69.
doi: 10.1016/j.envexpbot.2017.12.005 URL |
| [63] |
Labudda M, Różańska E, Prabucka B, et al. Activity profiling of barley vacuolar processing enzymes provides new insights into the plant and cyst nematode interaction[J]. Mol Plant Pathol, 2020, 21(1):38-52.
doi: 10.1111/mpp.v21.1 URL |
| [64] |
Gong P, Yan L, Tang Y, et al. Vacuolar processing enzyme(Vvβ VPE)from Vitis vinifera, processes seed proteins during ovule development, and accelerates seed germination in VvβVPE heterologously over-expressed Arabidopsis[J]. Plant Science, 2018, 274:420-431.
doi: 10.1016/j.plantsci.2018.06.023 URL |
| [65] |
van Wyk SG, Du Plessis M, Cullis CA, et al. Cysteine protease and cystatin expression and activity during soybean nodule development and senescence[J]. BMC Plant Biol, 2014, 14:294.
doi: 10.1186/s12870-014-0294-3 URL |
| [66] |
Cheng Z, Guo X, Zhang J, et al. βVPE is involved in tapetal degradation and pollen development by activating proprotease maturation in Arabidopsis thaliana[J]. Journal of Experimental Botany, 2019, 71(6):1943-1955.
doi: 10.1093/jxb/erz560 URL |
| [67] |
Mino M, Murata N, Date S, et al. Cell death in seedlings of the interspecific hybrid of Nicotia nagossei and N. tabacum;possible role of knob-like bodies formed on tonoplast in vacuolar-collapse-mediated cell death[J]. Plant Cell Rep, 2007, 26:407-419.
doi: 10.1007/s00299-006-0261-z URL |
| [68] |
Khanna-Chopra R, Srivalli B, Ahlawat YS. Drought induces many forms of cysteine proteinases not observed during natural senescence[J]. Biochem Biophys Res Commun, 1999, 255:324-327.
doi: 10.1006/bbrc.1999.0195 URL |
| [69] |
Albertini A, Simeoni F, Galbiati M, et al. Involvement of the vacuolar processing enzyme γVPE in response of Arabidopsis thaliana to water stress[J]. Biol Plant, 2014, 58:531-538.
doi: 10.1007/s10535-014-0417-6 URL |
| [70] |
Lu W, Deng M, Guo F, et al. Suppression of OsVPE3 enhances salt tolerance by attenuating vacuole rupture during programmed cell death and affects stomata development in rice[J]. Rice, 2016, 9:65.
doi: 10.1186/s12284-016-0138-x URL |
| [71] |
Yakimova ET, Kapchina-Toteva VM, Woltering EJ. Signal transduction events in aluminum-induced cell death in tomato suspension cells[J]. J Plant Physiol, 2007, 164:702-708.
doi: 10.1016/j.jplph.2006.03.018 URL |
| [72] | Hatsugai N, Yamada K, Goto-Yamada S, et al. Vacuolar processing enzyme in plant programmed cell death[J]. Frontiers in Plant Science, 2015, 6:234. |
| [73] | Stennicke HR, Salvesen GS. Properties of the caspases[J]. Biochim Biophys, 1998, 1387:17-31. |
| [74] |
Kinoshita T, Nishimura M, Hara-Nishimura I. Homologues of a vacuolar processing enzyme that are expressed in different organs in Arabidopsis thaliana[J]. Plant Molecular Biology, 1995, 29:81-89.
doi: 10.1007/BF00019120 URL |
| [75] |
Kinoshita T, Nishimura M, Hara-Nishimura I. The sequence and expression of the γ-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana[J]. Plant and Cell Physiology, 1995, 36:1555-1562.
pmid: 8589932 |
| [76] |
Hara-Nishimura I, Kinoshita T, Hiraiwa N, et al. Vacuolar processing enzymes in protein-storage vacuoles and lytic vacuoles[J]. Journal of Plant Physiology, 1998, 152:668-674.
doi: 10.1016/S0176-1617(98)80028-X URL |
| [77] |
Gruis DF, Selinger DA, Curran JM, et al. Redundant proteolytic mechanisms process seed storage proteins in the absence of seed-type members of the vacuolar processing enzyme family of cysteine proteases[J]. The Plant Cell, 2002, 14(11):2863-2882.
doi: 10.1105/tpc.005009 URL |
| [78] |
Shimada T, Yamada K, Kataoka M, et al. Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana[J]. Journal of Biological Chemistry, 2003, 278(34):32292-32299.
doi: 10.1074/jbc.M305740200 URL |
| [79] |
Zakharov A, Müntz K. Seed legumains are expressed in stamens and vegetative legumains in seeds of Nicotiana tabacum L.[J]. Journal of Experimental Botany, 2004, 55:1593-1595.
doi: 10.1093/jxb/erh166 URL |
| [80] | Kenji Y, Arpan KB, Shino G, et al. Vacuolar processing enzymes in the plant life cycle[J]. New Phytol, 2019. |
| [81] | Poncet V, Scutt C, Tournebize R, et al. The Amborella vacuolar processing enzyme family[J]. Frontiers in Plant Science, 2015, 6:618. |
| [82] |
Gruis DF, Schulze J, Jung R. Storage protein accumulation in the absence of the vacuolar processing enzyme family of cysteine proteases[J]. The Plant Cell, 2004, 16:270-290.
doi: 10.1105/tpc.016378 URL |
| [83] |
Kumamaru T, Uemura Y, Inoue Y, et al. Vacuolar processing enzyme plays an essential role in the crystalline structure of glutelin in rice seed[J]. Plant and Cell Physiology, 2010, 51:38-46.
doi: 10.1093/pcp/pcp165 URL |
| [84] |
Friedman WE. The evolution of embryogeny in seed plants and the developmental origin and early history of endosperm[J]. American Journal of Botany, 1994, 81:1468-1486.
doi: 10.1002/j.1537-2197.1994.tb15633.x URL |
| [85] |
Ariizumi T, Higuchi K, Arakaki S, et al. Genetic suppression analysis in novel vacuolar processing enzymes reveals their roles in controlling sugar accumulation in tomato fruits[J]. Journal of Experimental Botany, 2011, 62:2773-2786.
doi: 10.1093/jxb/erq451 URL |
| [86] |
Wang W, Xiong H, Lin R, et al. A VPE-like protease NtTPE8 exclusively expresses in the integumentary tapetum and involves in seed development[J]. J Integr Plant Biol, 2019, 61(5):598-610.
doi: 10.1111/jipb.12766 |
| [87] |
Radchuk V, Weier D, Radchuk R, et al. Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell disintegration and coordinated with endosperm growth[J]. Journal of Experimental Botany, 2011, 62:1217-1227.
doi: 10.1093/jxb/erq348 URL |
| [88] |
Endo A, Tatematsu K, Hanada K, et al. Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynjournal and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds[J]. Plant Cell Physiol, 2012, 53:16-27.
doi: 10.1093/pcp/pcr171 URL |
| [89] |
Alpuerto JB, Mukherjee A, Kitazumi A, et al. Impaired expressions of the beta and delta isoforms of vacuolar processing enzymes compromise the basal defenses of Arabidopsis thaliana against the phloem-feeding insect Myzus persicae[J]. Acta Physiologiae Plantarum, 2017, 39:233.
doi: 10.1007/s11738-017-2529-z URL |
| [90] |
Enrique R, Jan Z, Clay C, et al. A unique mechanism for protein processing and degradation in Arabidopsis thaliana[J]. Proc Natl Acad Sci USA, 2003, 100(12):7389-94.
doi: 10.1073/pnas.1230987100 URL |
| [91] |
Lam E. Vacolar proteases livening up programmed cell death[J]. Trends Cell Biol, 2005, 15(3):124-127.
doi: 10.1016/j.tcb.2005.01.001 URL |
| [92] | 修林春, 张伟. 高等植物液泡加工酶VPE家族的生物信息学分析[J]. 南京农业大学学报, 2010, 33(3):19-25. |
| Xiu LC, Zhang W. Bioinformatic analysis of VPE family in higher plants[J]. Journal of Nanjing Agricultural University, 2010, 33(3):19-25. |
| [1] | HU Hai-lin, XU Li, LI Xiao-xu, WANG Chen-can, MEI Man, DING Wen-jing, ZHAO Yuan-yuan. Advances in the Regulation of Plant Growth, Development and Stress Physiology by Small Peptide Hormones [J]. Biotechnology Bulletin, 2023, 39(7): 13-25. |
| [2] | FENG Shan-shan, WANG Lu, ZHOU Yi, WANG You-ping, FANG Yu-jie. Research Progresses on WOX Family Genes in Regulating Plant Development and Abiotic Stress Response [J]. Biotechnology Bulletin, 2023, 39(5): 1-13. |
| [3] | XUE Jiao ZHU Qing-feng FENG Yan-zhao CHEN Pei LIU Wen-hua ZHANG Ai-xia LIU Qin-jian ZHANG Qi YU Yang. Advances in Upstream Open Reading Frame in Plant Genes [J]. Biotechnology Bulletin, 2023, 39(4): 157-165. |
| [4] | WEI Ming WANG Xin-yu WU Guo-qiang ZHAO Meng. The Role of NAD-dependent Deacetylase SRT in Plant Epigenetic Inheritance Regulation [J]. Biotechnology Bulletin, 2023, 39(4): 59-70. |
| [5] | SANG Tian, WANG Peng-cheng. Research Progress in Plant SUMOylation [J]. Biotechnology Bulletin, 2023, 39(3): 1-12. |
| [6] | HU Li-li, LIN Bo-rong, WANG Hong-hong, CHEN Jian-song, LIAO Jin-ling, ZHUO Kan. Transcriptome and Candidate Effectors Analysis of Pratylenchus brachyurus [J]. Biotechnology Bulletin, 2023, 39(3): 254-266. |
| [7] | SUN Yu-tong, LIU De-shuai, QI Xun, FENG Mei, HUANG Xu-zheng, YAO Wen-kong. Advances in Jasmonic Acid Regulating Plant Growth and Development as Well as Stress [J]. Biotechnology Bulletin, 2023, 39(11): 99-109. |
| [8] | AN Chang, LU Lin, SHEN Meng-qian, CHEN Sheng-zhen, YE Kang-zhuo, QIN Yuan, ZHENG Ping. Research Progress of bHLH Gene Family in Plants and Its Application Prospects in Medical Plants [J]. Biotechnology Bulletin, 2023, 39(10): 1-16. |
| [9] | YIN Guo-ying, LIU Chang, CHANG Yong-chun, YU Wang-jie, WANG Bing, ZHANG Pan, GUO Yu-shuang. Identification of the Cysteine Protease Family and Corresponding miRNAs in Nicotiana tabacum L. and Their Responses to PVY [J]. Biotechnology Bulletin, 2023, 39(10): 184-196. |
| [10] | TANG Qian-qian, LIN Chu-yu, TAO Zeng. Research Progress in Histone Demethylase in Plant [J]. Biotechnology Bulletin, 2022, 38(7): 13-22. |
| [11] | LI Ping, GUO Fa-ping, TIAN Min, SHUI Yang, XU Na-na, BAI Da-song, YU De-jin, ZHANG Jie, HU Yun-gao, PENG You-lin. Research Progress of Sterol in Regulating Plant Growth and Development [J]. Biotechnology Bulletin, 2022, 38(7): 90-98. |
| [12] | GU Pan, QI Xue-ying, LI Li, ZHANG Xi, SHAN Xiao-yi. Endocytosis of AtRGS1 Involved in the Regulation of G-protein-mediated Arabidopsis Development and Stress Responses [J]. Biotechnology Bulletin, 2022, 38(6): 34-42. |
| [13] | YUE Man-fang, ZHANG Chun, WU Zhong-yi. Research Progress in the Structural and Functional Analysis of Plant Transcription Factor AP2/ERF Protein Family [J]. Biotechnology Bulletin, 2022, 38(12): 11-26. |
| [14] | TANG Xiao-li, JIANG Fu-dong, ZHANG Hong-xia. Research Progress in the Functions of SINA E3 Ubiquitin Ligase in Plant [J]. Biotechnology Bulletin, 2022, 38(10): 10-17. |
| [15] | QIAN Jing-jie, LIN Su-meng, ZHANG Dong-ping, GAO Yong. Phytochrome Interacting Factors Involving in Auxin-regulated Plant Growth and Development [J]. Biotechnology Bulletin, 2022, 38(10): 29-33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||