








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (8): 221-232.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1326
Previous Articles Next Articles
SUN Bao-ting( ), QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong(
), QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong( ), JIA Shi-ru
), JIA Shi-ru
Received:2020-10-28
Online:2021-08-26
Published:2021-09-10
Contact:
CUI Jian-dong
E-mail:sunbaoting@mail.tust.edu.cn;jdcui@tust.edu.cn
SUN Bao-ting, QIU Meng-xia, WANG Zi-chen, WANG Zi-yuan, CUI Jian-dong, JIA Shi-ru. Preparation of @ZIF-8 Immobilized Enzyme by Using Cysteine as Auxiliary Reagent and Its Characterization[J]. Biotechnology Bulletin, 2021, 37(8): 221-232.
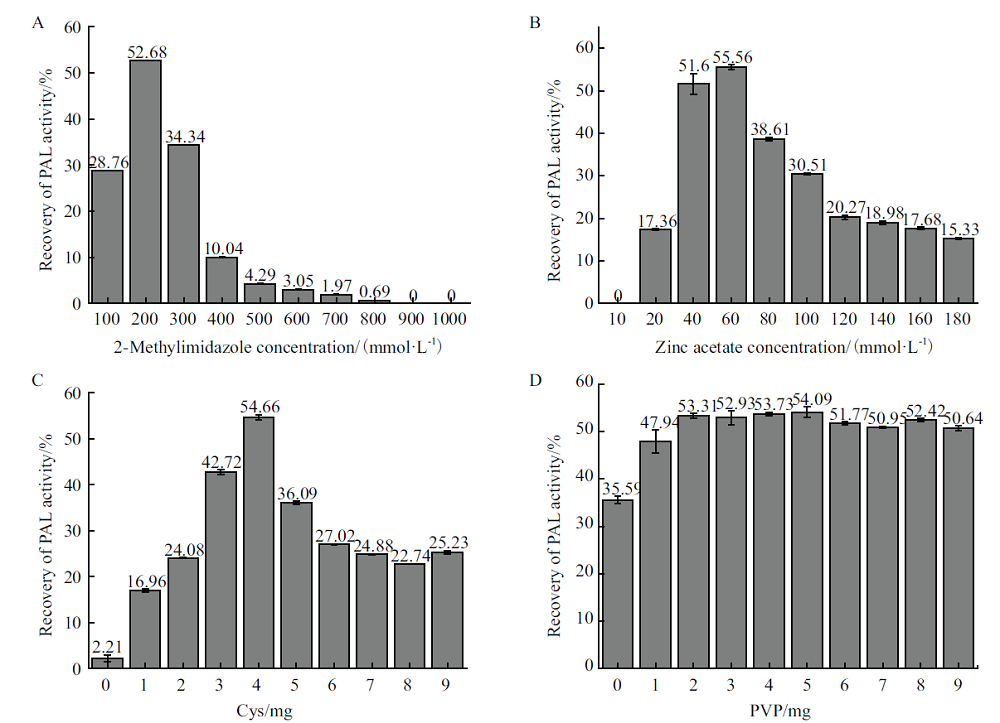
Fig.3 Influence of various factors on the recovery of PAL@ZIF-8 activity A: Effect of 2-methylimidazole concentration on the recovery of PAL@ZIF-8 activity. B: Effect of zinc acetate concentration on the recovery of PAL@ZIF-8 activity. C: Effect of addition of Cys on the recovery of PAL@ZIF-8 activity. D: Effect of addition of PVP on the recovery of PAL@ZIF-8 activity
| 编号 No. | 因素 Factor/(mmol·L-1) | 水平 Level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | 2-甲基咪唑 | 100 | 200 | 300 |
| B | 醋酸锌 | 40 | 60 | 80 |
| C | Cys | 3 | 4 | 5 |
Table 1 Test factors and level table of response surface
| 编号 No. | 因素 Factor/(mmol·L-1) | 水平 Level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | 2-甲基咪唑 | 100 | 200 | 300 |
| B | 醋酸锌 | 40 | 60 | 80 |
| C | Cys | 3 | 4 | 5 |
| 编号 No. | A | B | C | 酶活回收率 Recovery of PAL activity /% |
|---|---|---|---|---|
| 1 | 200 | 60 | 4 | 55.21 |
| 2 | 300 | 80 | 4 | 23.86 |
| 3 | 200 | 40 | 5 | 34.83 |
| 4 | 200 | 60 | 4 | 55.47 |
| 5 | 200 | 80 | 3 | 30.15 |
| 6 | 100 | 80 | 4 | 19.98 |
| 7 | 300 | 60 | 3 | 26.83 |
| 8 | 100 | 60 | 5 | 18.52 |
| 9 | 100 | 40 | 4 | 26.71 |
| 10 | 200 | 80 | 5 | 25.49 |
| 11 | 200 | 60 | 4 | 55.81 |
| 12 | 200 | 60 | 4 | 55.97 |
| 13 | 300 | 60 | 5 | 22.67 |
| 14 | 200 | 60 | 4 | 55.62 |
| 15 | 200 | 40 | 3 | 40.32 |
| 16 | 300 | 40 | 4 | 31.89 |
| 17 | 100 | 60 | 3 | 20.47 |
Table 2 Box-Behnken experimental design and response values
| 编号 No. | A | B | C | 酶活回收率 Recovery of PAL activity /% |
|---|---|---|---|---|
| 1 | 200 | 60 | 4 | 55.21 |
| 2 | 300 | 80 | 4 | 23.86 |
| 3 | 200 | 40 | 5 | 34.83 |
| 4 | 200 | 60 | 4 | 55.47 |
| 5 | 200 | 80 | 3 | 30.15 |
| 6 | 100 | 80 | 4 | 19.98 |
| 7 | 300 | 60 | 3 | 26.83 |
| 8 | 100 | 60 | 5 | 18.52 |
| 9 | 100 | 40 | 4 | 26.71 |
| 10 | 200 | 80 | 5 | 25.49 |
| 11 | 200 | 60 | 4 | 55.81 |
| 12 | 200 | 60 | 4 | 55.97 |
| 13 | 300 | 60 | 5 | 22.67 |
| 14 | 200 | 60 | 4 | 55.62 |
| 15 | 200 | 40 | 3 | 40.32 |
| 16 | 300 | 40 | 4 | 31.89 |
| 17 | 100 | 60 | 3 | 20.47 |
| 方差来源 Source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方差 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 3983.91 | 9 | 442.66 | 161.95 | < 0.0001 | ** |
| A | 47.78 | 1 | 47.78 | 17.48 | 0.0041 | ** |
| B | 107.24 | 1 | 107.24 | 39.23 | 0.0004 | ** |
| C | 21.58 | 1 | 21.58 | 7.90 | 0.0261 | * |
| AB | 4.02 | 1 | 4.02 | 1.47 | 0.2646 | |
| AC | 14.59 | 1 | 14.59 | 5.34 | 0.0541 | |
| BC | 23.23 | 1 | 23.23 | 8.50 | 0.0225 | * |
| A2 | 2247.70 | 1 | 2247.70 | 822.32 | < 0.0001 | ** |
| B2 | 352.11 | 1 | 352.11 | 128.82 | < 0.0001 | ** |
| C2 | 832.62 | 1 | 832.62 | 304.61 | < 0.0001 | ** |
| 残差误差 | 19.13 | 7 | 2.73 | |||
| 失拟 | 15.65 | 3 | 5.22 | 6.00 | 0.0581 | |
| 纯误差 | 3.48 | 4 | 0.8702 | |||
| 合计 | 4003.04 | 16 |
Table 3 Analysis for regression equation of Box-Behnken design
| 方差来源 Source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方差 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 3983.91 | 9 | 442.66 | 161.95 | < 0.0001 | ** |
| A | 47.78 | 1 | 47.78 | 17.48 | 0.0041 | ** |
| B | 107.24 | 1 | 107.24 | 39.23 | 0.0004 | ** |
| C | 21.58 | 1 | 21.58 | 7.90 | 0.0261 | * |
| AB | 4.02 | 1 | 4.02 | 1.47 | 0.2646 | |
| AC | 14.59 | 1 | 14.59 | 5.34 | 0.0541 | |
| BC | 23.23 | 1 | 23.23 | 8.50 | 0.0225 | * |
| A2 | 2247.70 | 1 | 2247.70 | 822.32 | < 0.0001 | ** |
| B2 | 352.11 | 1 | 352.11 | 128.82 | < 0.0001 | ** |
| C2 | 832.62 | 1 | 832.62 | 304.61 | < 0.0001 | ** |
| 残差误差 | 19.13 | 7 | 2.73 | |||
| 失拟 | 15.65 | 3 | 5.22 | 6.00 | 0.0581 | |
| 纯误差 | 3.48 | 4 | 0.8702 | |||
| 合计 | 4003.04 | 16 |
| Mean | 34.74 |
|---|---|
| R-square | 99.52% |
| Adj.R-square | 98.91% |
| Std. Dev. | 1.65 |
| CV | 4.76% |
Table 4 Reliability analysis of the model
| Mean | 34.74 |
|---|---|
| R-square | 99.52% |
| Adj.R-square | 98.91% |
| Std. Dev. | 1.65 |
| CV | 4.76% |

Fig.4 Response surface plot and contour plot of the effect of interaction of various factors on the recovery of PAL activity a:The effect of Cys addition on the recovery of PAL activity. b:Effect of zinc acetate concentration on the recovery of PAL activity. c:Effect of 2-methylimidazole concentration on the recovery of PAL activity
| Enzyme | Km | Vmax |
|---|---|---|
| PAL | 8.2756 ±0.67% | 0.0269 ±1.08% |
| PAL@ZIF-8 | 10.9602 ±0.58% | 0.0171±0.72% |
Table 5 Comparison of apparent kinetic parameters of free PAL and PAL@ZIF-8
| Enzyme | Km | Vmax |
|---|---|---|
| PAL | 8.2756 ±0.67% | 0.0269 ±1.08% |
| PAL@ZIF-8 | 10.9602 ±0.58% | 0.0171±0.72% |
| 分组 Group | 酶蛋白负载率 Enzyme load rate/% | 酶活回收率 Recovery of PAL activity/% |
|---|---|---|
| 传统PAL@ZIF-8 Traditional PAL@ZIF-8 | 82.11±0.27 | 13.38±0.12 |
| Cys辅助的PAL@ZIF-8 Cysteine-assisted PAL@ZIF-8 | 94.07±0.14 | 56.15±0.94 |
Table 6 Comparison of load rate and recovery of PAL activity
| 分组 Group | 酶蛋白负载率 Enzyme load rate/% | 酶活回收率 Recovery of PAL activity/% |
|---|---|---|
| 传统PAL@ZIF-8 Traditional PAL@ZIF-8 | 82.11±0.27 | 13.38±0.12 |
| Cys辅助的PAL@ZIF-8 Cysteine-assisted PAL@ZIF-8 | 94.07±0.14 | 56.15±0.94 |
| [1] |
Cui JD, Sun LM, Li LL. A simple technique of preparing stable CLEAs of phenylalanine ammonia lyase using co-aggregation with starch and bovine serum albumin[J]. Appl Biochem Biotechnol, 2013, 170(8):1827-1837.
doi: 10.1007/s12010-013-0317-9 URL |
| [2] |
Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease[J]. J Perinatol, 2007, 27(5):284-290.
pmid: 17344923 |
| [3] |
Westrup B. Newborn individualized developmental care and assessment program(NIDCAP)-family-centered developmentally supportive care[J]. Early Hum Dev, 2007, 83(7):443-449.
pmid: 17459617 |
| [4] |
Blau N, van Spronsen FJ, Levy HL. Phenylketonuria[J]. Lancet, 2010, 376(9750):1417-1427.
doi: 10.1016/S0140-6736(10)60961-0 URL |
| [5] |
Zhou HC, Kitagawa S. Metal-organic frameworks(MOFs)[J]. Chem Soc Rev, 2014, 43(16):5415-5418.
doi: 10.1039/C4CS90059F URL |
| [6] |
Meng J, Niu C, Xu L, et al. General oriented formation of carbon nanotubes from metal-organic frameworks[J]. J Am Chem Soc, 2017, 139(24):8212-8221.
doi: 10.1021/jacs.7b01942 URL |
| [7] |
Lin ZJ, Lü J, Hong MC, et al. Metal-organic frameworks based on flexible ligands(FL-MOFs):structures and applications[J]. Chem Soc Rev, 2014, 43(16):5867-5895.
doi: 10.1039/C3CS60483G URL |
| [8] |
Saliba D, Ammar M, Rammal M, et al. Crystal growth of ZIF-8, ZIF-67, and their mixed-metal derivatives[J]. J Am Chem Soc, 2018, 140(5):1812-1823.
doi: 10.1021/jacs.7b11589 URL |
| [9] | Yang X, Chen W, Bian H, et al. Synjournal of mesoporous ZIF-8 nanoribbons and their conversion into carbon nanoribbons for high-performance supercapacitors[J]. Chemistry, 2018, 24(43):11185-11192. |
| [10] |
Sun Q, Fu CW, Aguila B, et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks[J]. J Am Chem Soc, 2018, 140(3):984-992.
doi: 10.1021/jacs.7b10642 pmid: 29275637 |
| [11] |
Lei ZL, Liu JT, Lei L, et al. UiO-66-NH2@PMAA:A Hybrid Polymer-MOFs architecture for pectinase immobilization[J]. Industrial & Engineering Chemistry Research, 2018, 57(2):559-567.
doi: 10.1021/acs.iecr.7b03398 URL |
| [12] |
Li T, Qiu H, Liu N, et al. Construction of self-activated cascade metal-organic framework/enzyme hybrid nanoreactors as antibacterial agents[J]. Colloids Surf B Biointerfaces, 2020, 191:111001.
doi: 10.1016/j.colsurfb.2020.111001 URL |
| [13] |
Jeong GY, Ricco R, Liang K, et al. Bioactive MIL-88A framework hollow spheres via interfacial reaction in-droplet microfluidics for enzyme and nanoparticle encapsulation[J]. Chem Mater, 2015, 27(23):7903-7909.
doi: 10.1021/acs.chemmater.5b02847 URL |
| [14] |
Li P, Moon SY, Guelta MA, et al. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal-organic framework engenders thermal and long-term stability[J]. J Am Chem Soc, 2016, 138(26):8052-8055.
doi: 10.1021/jacs.6b03673 URL |
| [15] |
Liang K, Coghlan CJ, Bell SG, et al. Enzyme encapsulation in zeolitic imidazolate frameworks:a comparison between controlled co-precipitation and biomimetic mineralisation[J]. Chem Commun, 2016, 52(3):473-476.
doi: 10.1039/C5CC07577G URL |
| [16] |
Lin KYA, Chen YC, Phattarapattamawong S. Efficient demulsification of oil-in-water emulsions using a zeolitic imidazolate framework:Adsorptive removal of oil droplets from water[J]. J Colloid Interface Sci, 2016, 478:97-106.
doi: 10.1016/j.jcis.2016.05.057 URL |
| [17] | Xu LH, Zhao DY, Yang L, et al. Improvement of the electro-optical properties of nematic liquid crystals by doping with ZIF-8 materials[J]. Acta Phys Chimica Sin, 2016, 32(9):2377-2382. |
| [18] |
Dai XQ, Hang YH, Zhao X, et al. ZIF-8 as an adsorbent of aqueous phase for Eu and Tb ions[J]. Micro Nano Lett, 2017, 12(3):187-190.
doi: 10.1049/mna2.v12.3 URL |
| [19] | Wang Q, Xiong SS, Xiang ZH, et al. Dynamic separation of Xe and Kr by metal-organic framework and covalent-organic materials:a comparison with activated charcoal[J]. Science China Chem, 2016(5):643-650. |
| [20] |
Huang DD, Xin QP, Ni YZ, et al. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation[J]. RSC Adv, 2018, 8(11):6099-6109.
doi: 10.1039/C7RA09794H URL |
| [21] |
Yurderi M, Bulut A, Zahmakiran M, et al. Ruthenium(0) nanoparticles stabilized by metal-organic framework(ZIF-8):Highly efficient catalyst for the dehydrogenation of dimethylamine-borane and transfer hydrogenation of unsaturated hydrocarbons using dimethylamine-borane as hydrogen source[J]. Appl Catal B:Environ, 2014, 160/161:534-541.
doi: 10.1016/j.apcatb.2014.06.009 URL |
| [22] |
Zhang T, Li B, Zhang XF, et al. Pd nanoparticles immobilized in a microporous/mesoporous composite ZIF-8/MSS:a multifunctional catalyst for the hydrogenation of alkenes[J]. Microporous Mesoporous Mater, 2014, 197:324-330.
doi: 10.1016/j.micromeso.2014.07.002 URL |
| [23] |
Thanh MT, Thien TV, Du PD, et al. Iron doped zeolitic imidazolate framework(Fe-ZIF-8):synjournal and photocatalytic degradation of RDB dye in Fe-ZIF-8[J]. J Porous Mater, 2018, 25(3):857-869.
doi: 10.1007/s10934-017-0498-7 URL |
| [24] |
Wang X, Miao D, Liang X, et al. Nanocapsules engineered from polyhedral ZIF-8 templates for bone-targeted hydrophobic drug delivery[J]. Biomater Sci, 2017, 5(4):658-662.
doi: 10.1039/C6BM00915H URL |
| [25] |
Zheng CC, Wang Y, Phua SZF, et al. ZnO-DOX@ZIF-8 core-shell nanoparticles for pH-responsive drug delivery[J]. ACS Biomater Sci Eng, 2017, 3(10):2223-2229.
doi: 10.1021/acsbiomaterials.7b00435 URL |
| [26] |
Xiao YS, Huang W, Zhu DM, et al. Cancer cell membrane-camouflaged MOF nanoparticles for a potent dihydroartemisinin-based hepatocellular carcinoma therapy[J]. RSC Adv, 2020, 10(12):7194-7205.
doi: 10.1039/C9RA09233A URL |
| [27] |
Hu PP, Liu N, Wu KY, et al. Successive and specific detection of Hg2+ and i- by a DNA@MOF biosensor:experimental and simulation studies[J]. Inorg Chem, 2018, 57(14):8382-8389.
doi: 10.1021/acs.inorgchem.8b01051 URL |
| [28] |
Lian X, Fang Y, Joseph E, et al. Enzyme-MOF(metal-organic framework)composites[J]. Chem Soc Rev, 2017, 46(11):3386-3401.
doi: 10.1039/C7CS00058H URL |
| [29] |
Ran JY, Wang C, Zhang JJ, et al. New insight into Polydopamine@ZIF-8 nanohybrids:a zinc-releasing container for potential anticancer activity[J]. Polymers, 2018, 10(5):476.
doi: 10.3390/polym10050476 URL |
| [30] |
Chen GS, Huang SM, Kou XX, et al. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal-organic frameworks[J]. Angew Chem Int Ed, 2019, 58(5):1463-1467.
doi: 10.1002/anie.v58.5 URL |
| [31] |
Mohammad M, Razmjou A, Liang K, et al. Metal-organic-framework-based enzymatic microfluidic biosensor via surface patterning and biomineralization[J]. ACS Appl Mater Interfaces, 2019, 11(2):1807-1820.
doi: 10.1021/acsami.8b16837 URL |
| [32] |
Liang WB, Ricco R, Maddigan NK, et al. Control of structure topology and spatial distribution of biomacromolecules in Protein@ZIF-8 biocomposites[J]. Chem Mater, 2018, 30(3):1069-1077.
doi: 10.1021/acs.chemmater.7b04977 URL |
| [33] | Cui JD, Liu RL, Li LL. Imprinted cross-linked enzyme aggregate(iCLEA)of phenylalanine ammonia lyase:a new stable biocatalyst[M]// Advances in Applied Biotechnology. Heidelberg:Springer, 2015:223-231. |
| [34] |
Cui JD, Li LL, Bian HJ. Immobilization of cross-linked phenylalanine ammonia lyase aggregates in microporous silica gel[J]. PLoS One, 2013, 8(11):e80581.
doi: 10.1371/journal.pone.0080581 URL |
| [35] |
Chen WH, Luo GF, Vázquez-González M, et al. Glucose-responsive metal-organic-framework nanoparticles act as “smart” sense-and-treat carriers[J]. ACS Nano, 2018, 12(8):7538-7545.
doi: 10.1021/acsnano.8b03417 URL |
| [36] |
Feng Y, Zhong L, Hou Y, et al. Acid-resistant enzyme@MOF nanocomposites with mesoporous silica shells for enzymatic applications in acidic environments[J]. J Biotechnol, 2019, 306:54-61.
doi: 10.1016/j.jbiotec.2019.09.010 URL |
| [37] |
Peroza EA, Schmucki R, Güntert P, et al. The βE-domain of wheat ec-1 metallothionein:a metal-binding domain with a distinctive structure[J]. J Mol Biol, 2009, 387(1):207-218.
doi: 10.1016/j.jmb.2009.01.035 URL |
| [1] | DU Qing-jie, ZHOU Lu-yao, YANG Si-zhen, ZHANG Jia-xin, CHEN Chun-lin, LI Juan-qi, LI Meng, ZHAO Shi-wen, XIAO Huai-juan, WANG Ji-qing. Overexpression of CaCP1 Enhances Salt Stress Sensibility in Transgenic Tobacco [J]. Biotechnology Bulletin, 2023, 39(2): 172-182. |
| [2] | YIN Guo-ying, LIU Chang, CHANG Yong-chun, YU Wang-jie, WANG Bing, ZHANG Pan, GUO Yu-shuang. Identification of the Cysteine Protease Family and Corresponding miRNAs in Nicotiana tabacum L. and Their Responses to PVY [J]. Biotechnology Bulletin, 2023, 39(10): 184-196. |
| [3] | SU Yu, LI Zong-yun, HAN Yong-hua. Advances in Plant Vacuolar Processing Enzymes [J]. Biotechnology Bulletin, 2021, 37(6): 181-191. |
| [4] | MO Li-jie, LIU Xia-tong, LI Hui, LU Hai. On the Function of Plant Cysteine Protease in Plant Growth and Development [J]. Biotechnology Bulletin, 2021, 37(6): 202-212. |
| [5] | ZHANG Li, SUN Dui, WANG Xiao, ZHENG Chun-li. Research Progress on Cysteine Participation in Heavy Metal Resistance in Organism [J]. Biotechnology Bulletin, 2017, 33(5): 26-33. |
| [6] | ZHOU Wen-fei, BAI Juan ,GONG Chun-mei. Research Progress on the Oxidative Modification of Plant Proteins Mediated by Reactive Oxygen Species [J]. Biotechnology Bulletin, 2017, 33(4): 8-18. |
| [7] | GUO Tian-lu, ZHANG Huan-huan, DU Jian-zhong, HAO Yao-shan, WANG Yi-xue, SUN Yi. mRNA Expression and Bioinformatics Analysis of Cysteine Synthase in Allium sativum [J]. Biotechnology Bulletin, 2016, 32(8): 96-102. |
| [8] | ZHENG Chao, LI Deng-gao, BAI Wei. Advances on Cysteine-rich Receptor-like Kinases in Plants [J]. Biotechnology Bulletin, 2016, 32(11): 10-17. |
| [9] | Wang Dezheng, Wu Hui, Li Zhimin, Ye Qin. Optimizing the Addition of Amino Acids in the Production of Glutathione by Recombinant Escherichia coli [J]. Biotechnology Bulletin, 2015, 31(9): 197-203. |
| [10] | Zhao Jingnan, Li Zhimin, Ye Qin. Study on Fermentation and Conversion of L-cysteine by Pseudomonas sp. F-12 [J]. Biotechnology Bulletin, 2014, 0(10): 207-214. |
| [11] | Chen Jiali, Wu Liang, Wang Juan, Duan Xuehui. Glutathione Biosynthesis by Engineered Saccharomyces cerevisiae [J]. Biotechnology Bulletin, 2013, 0(8): 160-165. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||