








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (6): 97-107.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1353
Previous Articles Next Articles
LIAO Zhao-min1( ), CAI Jun1,2,3(
), CAI Jun1,2,3( ), LIN Jian-guo1, DU Xin1, WANG Chang-gao1
), LIN Jian-guo1, DU Xin1, WANG Chang-gao1
Received:2020-11-03
Online:2021-06-26
Published:2021-07-08
Contact:
CAI Jun
E-mail:hgliaozhaomin@163.com;hgdcaijun@hbut.edu.cn
LIAO Zhao-min, CAI Jun, LIN Jian-guo, DU Xin, WANG Chang-gao. Expression of Glucose Oxidase Gene from Aspergillus niger in Pichia pastoris and Optimization of Enzyme Production Conditions[J]. Biotechnology Bulletin, 2021, 37(6): 97-107.

Fig.5 Construction of recombinant plasmid pPICZαA-GOD and identification by double restriction endonuclease digestion A: The plasmid map of the recombinant expression vector. B: The results of double restriction endonuclease digestion. M: DNA marker. 1: The product of double restriction endonuclease digestion of pPICZαA-GOD
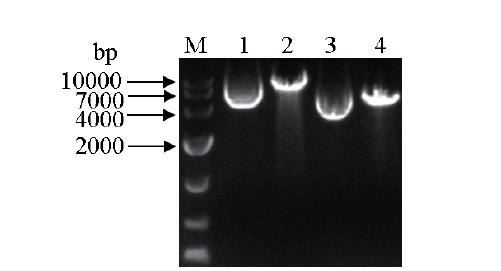
Fig.6 Agarose gel electrophoresis of the linearized products of pPICZαA-GOD and pPICZαA M: DNA marker. 1: pPICZαA-GOD; 2: pPICZαA-GOD linearization product;3: pPICZαA; 4: pPICZαA linearization product
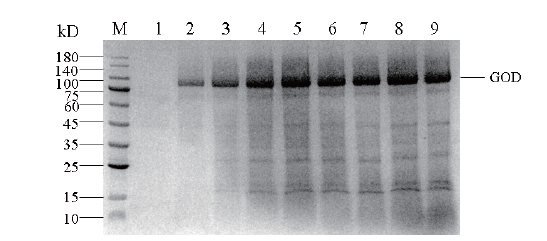
Fig.12 SDS-PAGE of glucose oxidase protein expressed in recombinant P. pastoris in 30 L bioreactor M: Protein Maker; 1-9: the expression of glucose oxidase was induced by methanol at 0, 12, 24, 36, 48, 60, 72, 84 and 96 h
| [1] |
Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases:a bird’s eye view of glucose sensing enzymes[J]. J Diabetes Sci Technol, 2011, 5(5):1068-1076.
doi: 10.1177/193229681100500507 URL |
| [2] |
Wang HC, Lee AR. Recent developments in blood glucose sensors[J]. J Food Drug Anal, 2015, 23(2):191-200.
doi: 10.1016/j.jfda.2014.12.001 URL |
| [3] | Cichello SA. Oxygen absorbers in food preservation:a review[J]. Journal of Food Science & Technology, 2015, 52(4):1889-1895. |
| [4] |
Kriaa M, Ouhibi R, Graba H, et al. Synergistic effect of Aspergillus tubingensis CTM 507 glucose oxidase in presence of ascorbic acid and alpha amylase on dough properties, baking quality and shelf life of bread[J]. J Food Sci Technol, 2016, 53(2):1259-1268.
doi: 10.1007/s13197-015-2092-9 URL |
| [5] | 汤海鸥, 高秀华, 李学军, 等. 葡萄糖氧化酶对仔猪生长性能、粪便菌群和血清指标的影响[J]. 动物营养学报, 2014, 26(12):3781-3786. |
| Tang HO, Gao XH, Li XJ, et al. Effects of glucose oxidase on growth performance, faecal microflora and serum parameters of piglets[J]. Chinese Journal of Animal Nutrition, 2014, 26(12):3781-3786. | |
| [6] | 廖兆民, 蔡俊, 林建国. 微生物葡萄糖氧化酶的研究进展[J]. 食品与发酵工业, 2018, 44(7):308-315. |
| Liao ZM, Cai J, Lin JG, Research progress of microbial glucose oxidase[J]. Food and Fermentation Industries, 2018, 44(7):308-315. | |
| [7] | 范新蕾, 肖成建, 顾秋亚, 等. ARTP诱变选育葡萄糖氧化酶高产菌株及发酵条件优化[J]. 工业微生物, 2015(1):15-19. |
| Fan XL, Xiao CJ, Gu QY, et al. ARTP mutation breeding of glucose oxidase-producing strains and optimization of fermentation conditions[J]. Industrial Microbiology, 2015(1):15-19. | |
| [8] | Zehra A, Dubey MK, Ttwari A, et al. Fungal biomolecules and their implications[M]. John Wiley & Sons, Ltd, 2015. |
| [9] | Damasceno LM, Huang CJ, Batt CA. Protein secretion in Pichia pastoris and advances in protein production[J]. Applied Microbiology & Biotechnology, 2012, 93(1):31-39. |
| [10] | 高庆华, 胡美荣, 吴芳彤, 等. 点青霉葡萄糖氧化酶基因的克隆及其酶学性质研究[J]. 生物技术通报, 2016, 32(7):152-159. |
| Gao QH, Hu MR, Wu FT, et al. Cloning of gene for a glucose oxidase from Penicillium notatum and its enzymatic properties[J]. Biotechnology Bulletin, 2016, 32(7):152-159. | |
| [11] | 闵兆升, 郭会明, 颜旭, 等. 巴斯德毕赤酵母(P. pastoris)高密度发酵研究进展[J]. 生物技术通报, 2014(3):42-49. |
| Min ZS, Guo HM, Yan X, et al. Progress of Pichia pastoris engineering bacteria on high-density fermentation[J]. Biotechnology Bulletin, 2014, 30(3):42-49. | |
| [12] | 高立云. 一种快速葡萄糖氧化酶活性测定方法与应用效果研究[D]. 济南:齐鲁工业大学, 2017. |
| Gao LY. Study of rapid activity analytic assay and application performance evaluation of glucose oxidase[D]. Ji’nan:Qilu University of Technology, 2017. | |
| [13] | 陈楠, 肖成建, 范新蕾, 等. 黑曲霉葡萄糖氧化酶基因在毕赤酵母SMD1168中的表达[J]. 食品与生物技术学报, 2017, 36(9):975-981. |
| Chen N, Xiao CJ, Fan XL, et al. Expression of Aspergillus niger glucose oxidase gene in Pichia pastoris SMD1168[J]. Journal of Food Science and Biotechnology, 2017, 36(9):975-981. | |
| [14] | 顾磊. Aspergillus niger葡萄糖氧化酶的异源分泌表达、分子改造和发酵生产[D]. 无锡:江南大学, 2014. |
| Gu L. Heterologous expression, molecular molecular modification, and fermentation of glucose oxidase from Aspergillus niger[D]. Wuxi:Jiangnan University, 2014. | |
| [15] | 郝杰清, 王帅坤, 师慧, 等. 重组毕赤酵母葡萄糖氧化酶的纯化和性质[J]. 食品科学, 2013, 34(9):159-163. |
| Hao JQ, Wang SK, Shi H, et al. Purification and characterization of recombinant glucose oxidase from Pichia pastoris[J]. Food Science, 2013, 34(9):159-163. | |
| [16] | 闻一凡, 顾磊, 张娟, 等. 定点突变提高毕赤酵母产葡萄糖氧化酶的氧化稳定性[J]. 食品与生物技术学报, 2016, 35(12):1260-1267. |
| Wen YF, Gu L, Zhang J, et al. Enhancing oxidative stability of glucose oxidase from Aspergillus niger by site-directed mutagenesis[J]. Journal of Food Science and Biotechnology, 2016, 35(12):1260-1267. | |
| [17] |
Cregg JM. Recombinant protein expression in Pichia pastoris[J]. Molecular Biotechnology, 2000, 16(1):23-52.
doi: 10.1385/MB:16:1 URL |
| [18] | Veenhuis M, Dijken JPV, Harder W. The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts[J]. Advances in Microbial Physiology, 1983, 24:1-82. |
| [19] |
Wegner G. Emerging applications of the methylotrophic yeasts[J]. FEMS Microbiol Rev, 1990, 7(3/4):279-283.
doi: 10.1111/fml.1980.7.issue-4 URL |
| [20] |
Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris[J]. Nature Biotechnology, 1993, 11(8):905-910.
doi: 10.1038/nbt0893-905 URL |
| [21] | 关波. 改良人血清白蛋白融合蛋白在毕赤酵母中分泌表达的研究[D]. 无锡:江南大学, 2014. |
| Guan B. Improvement of secretory expression of human serum albumin fusion protein in Pichia pastoris[D]. Wuxi:Jiangnan University, 2014. | |
| [22] | 刘瑜. 高效表达黑曲霉葡萄糖氧化酶基因工程菌的构建[D]. 济南:齐鲁工业大学, 2013. |
| Liu Y. Cloning and high efficiency expression of glucose oxidase from Aspergillus niger[D]. Ji’nan:Qilu University of Technology, 2013. | |
| [23] | 周亚凤, 张先恩, 刘虹, 等. 黑曲霉葡萄糖氧化酶基因的克隆及其在酵母中的高效表达[J]. 生物工程学报, 2001, 17(4):400-405. |
| Zhou YF, Zhang XE, Liu H, et al. Cloning and expression of Aspergillus niger glucose oxidase gene in methylotrophic yeast[J]. Chinese Journal of Biotechnology, 2001, 17(4):400-405. | |
| [24] | Crognale S, Petruccioli M, Fenice M, et al. Fed-batch gluconic acid production from Penicillium variabile P16 under different feeding strategies[J]. Enzyme & Microbial Technology, 2008, 42(5):445-449. |
| [25] | 郭瑶. Aspergillus niger Z-25葡萄糖氧化酶基因在毕赤酵母中的表达[D]. 南京:南京农业大学, 2010. |
| Guo Y. Expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris[D]. Nanjing:Nanjing Agricultural University, 2010. | |
| [26] | Crognale S, Pulci V, Brozzoli V, et al. Expression of Penicillium variabile P16 glucose oxidase gene in Pichia pastoris and characterization of the recombinant enzyme[J]. Enzyme & Microbial Technology, 2006, 39(6):1230-1235. |
| [1] | ZHAO Si-jia, WANG Xiao-lu, SUN Ji-lu, TIAN Jian, ZHANG Jie. Modification of Pichia pastoris for Erythritol Production by Metabolic Engineering [J]. Biotechnology Bulletin, 2023, 39(8): 137-147. |
| [2] | WANG Shuai, FENG Yu-mei, BAI Miao, DU Wei-jun, YUE Ai-qin. Functional Analysis of Soybean Gene GmHMGR Responding to Exogenous Hormones and Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(7): 131-142. |
| [3] | DONG Cong, GAO Qing-hua, WANG Yue, LUO Tong-yang, WANG Qing-qing. Increasing the Expression of FAD-dependent Glucose Dehydrogenase by Recombinant Pichia pastoris Using a Combined Strategy [J]. Biotechnology Bulletin, 2023, 39(6): 316-324. |
| [4] | MA Yu-qian, SUN Dong-hui, YUE Hao-feng, XIN Jia-yu, LIU Ning, CAO Zhi-yan. Identification, Heterologous Expression and Functional Analysis of a GH61 Family Glycoside Hydrolase from Setosphaeria turcica with the Assisting Function in Degrading Cellulose [J]. Biotechnology Bulletin, 2023, 39(4): 124-135. |
| [5] | CHEN Nan-nan, WANG Chun-lai, JIANG Zhen-zhong, JIAO Peng, GUAN Shu-yan, MA Yi-yong. Genetic Transformation and Chilling Resistance Analysis of Maize ZmDHN15 Gene in Tobacco [J]. Biotechnology Bulletin, 2023, 39(4): 259-267. |
| [6] | NIU Xin, ZHANG Ying, WANG Mao-jun, LIU Wen-long, LU Fu-ping, LI Yu. Effects of Different Integration Sites on the Expression of Exogenous Alkaline Protease in Bacillus amyloliquefaciens [J]. Biotechnology Bulletin, 2022, 38(4): 253-260. |
| [7] | WANG Yue, GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing. Expression of Pyranose Oxidase with Optimized Codon in Pichia pastoris [J]. Biotechnology Bulletin, 2022, 38(4): 269-277. |
| [8] | WANG Bo-ya, JIANG Yong, HUANG Yan, CAO Ying, HU Shang-lian. Cloning and Functional Analysis of BeCesA4 in Bambusa emeiensis [J]. Biotechnology Bulletin, 2022, 38(11): 185-193. |
| [9] | WANG Xiao-tao, ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao. Heterologous Expression and Enzymatic Properties Analysis of Novel β-agarase Aga2 from Paraglaciecola hydrolytica [J]. Biotechnology Bulletin, 2022, 38(11): 258-268. |
| [10] | ZHANG Tong-tong, ZHENG Deng-yu, WU Zhong-yi, ZHANG Zhong-bao, YU Rong. Functional Analysis of ZmNF-YB13 Responding to Drought and Salt Stress [J]. Biotechnology Bulletin, 2022, 38(10): 115-123. |
| [11] | YANG Wei, WU Xi, CHENG Jian-guo, LUO Yan, WANG Yin, YANG Ze-xiao, YAO Xue-ping. Cloning,Expression and Transcriptional Regulation of Interferon-α in Forest Musk Deer [J]. Biotechnology Bulletin, 2022, 38(1): 194-204. |
| [12] | YANG Yue, TAO Yan, XIE Jing, QIAN Yun-fang. Biosynthesis of Ctenopharyngodon idella C-type Lysozyme Based on Recombinant Pichia pastoris and Its Antibacterial Activity [J]. Biotechnology Bulletin, 2021, 37(12): 169-179. |
| [13] | LIU Shan, YE Wei, ZHU Mu-zi, LI Sai-ni, DENG Zhang-shuang, ZHANG Wei-min. Cloning,Expression and Characterization of a Novel Acyltransferase GPAT [J]. Biotechnology Bulletin, 2021, 37(11): 257-266. |
| [14] | ZHAO Hai-yan, SONG Chen-bin, LIU Zheng-ya, MA Xing-rong, SHANG Hui-hui, LI An-hua, GUAN Xian-jun, WANG Jian-she. Cloning,Recombinant Expression and Enzymatic Properties of α-Amylase Gene from Laceyella sp. [J]. Biotechnology Bulletin, 2020, 36(8): 23-33. |
| [15] | ZHAO Zhen, WANG Sha-sha, LÜ Xing-xing, TAO Yan, XIE Jing, QIAN Yun-fang. Heterologous Expression of Cyclina sinensis Mytimacin Antibacterial Peptide Based on Recombinant Pichia pastoris [J]. Biotechnology Bulletin, 2020, 36(5): 150-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||