








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (4): 269-277.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0148
Previous Articles Next Articles
WANG Yue( ), GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing
), GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing
Received:2021-02-04
Online:2022-04-26
Published:2022-05-06
WANG Yue, GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing. Expression of Pyranose Oxidase with Optimized Codon in Pichia pastoris[J]. Biotechnology Bulletin, 2022, 38(4): 269-277.

Fig. 1 Gene sequence after the optimization of codons The boxed sequences indicate restriction sites EcoR Iand Not I. The underlined sequences show 6×His-tag

Fig. 7 Production of P2O by high-density fermentation in 10 L bioreactor A:Growth curve of P2O activity. B:SDS-PAGE result. M:Protein marker. 1-12:Accumulation of recombinant protein after 0,12,24,36,48,60,72,84,96,108,120 and 132 h

Fig. 8 Optimal pH and pH stability A:Relative activity at different pH conditions at 30℃. B:The relative enzyme activity of P2O kept in the same temperature for 24 h at different pH conditions at 30℃. The used buffers were 100 mmol/L sodium citrate(■,black line),potassium phosphate(●,red line),Tris-HCl(▲,blue line)and glycine-NaOH(▼,pink line)
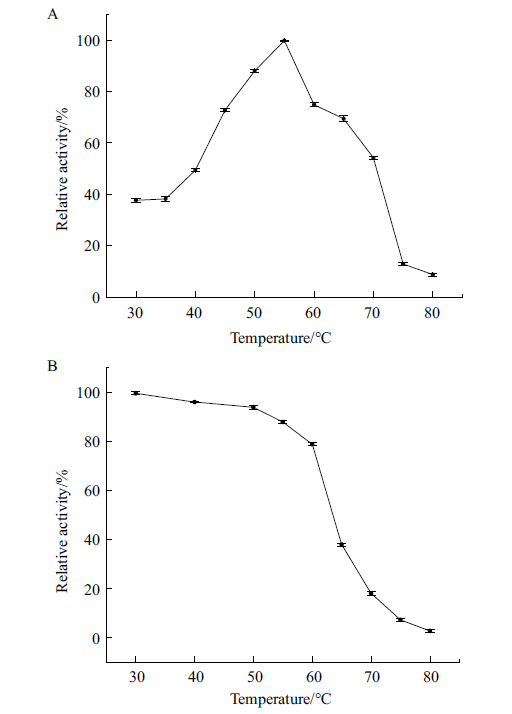
Fig. 9 Optimal temperature and temperature stability A:Relative activity of P2O at different temperatures at pH 6.5. B:The relative enzyme activity of P2O kept in the different temperatures for 30 min at pH 6.5
| [1] |
Ai MQ, Wang FF, Zhang YZ, et al. Purification of pyranose oxidase from the white rot fungus Irpex lacteus and its cooperation with laccase in lignin degradation[J]. Process Biochem, 2014, 49(12):2191-2198.
doi: 10.1016/j.procbio.2014.10.001 URL |
| [2] |
Wongnate T, Chaiyen P. The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily[J]. FEBS J, 2013, 280(13):3009-3027.
doi: 10.1111/febs.12280 URL |
| [3] |
Albrecht M, Lengauer T. Pyranose oxidase identified as a member of the GMC oxidoreductase family[J]. Bioinformatics, 2003, 19(10):1216-1220.
pmid: 12835264 |
| [4] |
Leitner C, Volc J, Haltrich D. Purification and characterization of pyranose oxidase from the white rot fungus Trametes multicolor[J]. Appl Environ Microbiol, 2001, 67(8):3636-3644.
doi: 10.1128/AEM.67.8.3636-3644.2001 URL |
| [5] |
Giffhorn F. Fungal pyranose oxidases:occurrence, properties and biotechnical applications in carbohydrate chemistry[J]. Appl Microbiol Biotechnol, 2000, 54(6):727-740.
pmid: 11152063 |
| [6] | 刘敏, 王彦. 1, 5-脱水葡萄糖醇与糖尿病及其并发症[J]. 国际内分泌代谢杂志, 2018, 38(2):106-109. |
| Liu M, Wang Y. 1,5-anhydroglucitol in diabetes and its compllications[J]. Int J Endocrinol Metab, 2018, 38(2):106-109. | |
| [7] |
Bannwarth M, Bastian S, Heckmann-Pohl D, et al. Crystal structure of pyranose 2-oxidase from the white-rot fungus Peniophora sp[J]. Biochemistry, 2004, 43(37):11683-11690.
pmid: 15362852 |
| [8] |
Vecerek B, Maresová H, Kocanová M, et al. Molecular cloning and expression of the pyranose 2-oxidase cDNA from Trametes ochracea MB49 in Escherichia coli[J]. Appl Microbiol Biotechnol, 2004, 64(4):525-530.
pmid: 14689250 |
| [9] |
de Koker TH, Mozuch MD, Cullen D, et al. Isolation and purification of pyranose 2-oxidase from Phanerochaete chrysosporium and characterization of gene structure and regulation[J]. Appl Environ Microbiol, 2004, 70(10):5794-5800.
doi: 10.1128/AEM.70.10.5794-5800.2004 URL |
| [10] |
Nishimura I, Okada K, Koyama Y. Cloning and expression of pyranose oxidase cDNA from Coriolus versicolor in Escherichia coli[J]. J Biotechnol, 1996, 52(1):11-20.
pmid: 9025322 |
| [11] |
Dietrich D, Crooks C. Gene cloning and heterologous expression of pyranose 2-oxidase from the brown-rot fungus, Gloeophyllum trabeum[J]. Biotechnol Lett, 2009, 31(8):1223-1228.
doi: 10.1007/s10529-009-9983-7 pmid: 19343506 |
| [12] |
Takakura Y, Kuwata S. Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the basidiomycete, Tricholoma matsutake[J]. Biosci Biotechnol Biochem, 2003, 67(12):2598-2607.
doi: 10.1271/bbb.67.2598 URL |
| [13] | 杨运桂, 童芹, 郑卫东, 等. 分子伴侣过量表达对蛋白质分泌及可溶性的影响[J]. 中国生物化学与分子生物学报, 2000, 16(3):382-387. |
| Yang YG, Tong Q, Zheng WD, et al. The effect of over-expression of chaperones on the secretion and the solubility of proteins[J]. Chin J Biochem Mol Biol, 2000, 16(3):382-387. | |
| [14] | 陆海, 吴薇, 曾庆银, 等. 大肠杆菌BL21(DE3)中表达重组蛋白的研究[J]. 北京林业大学学报, 2001, 23(6):1-4. |
| Lu H, Wu W, Zeng QY, et al. The expression analysis of recombinant protein in Escherichia coli BL21(DE3)[J]. J Beijing For Univ, 2001, 23(6):1-4. | |
| [15] | 彭凌, 朱必凤, 刘主. 包涵体复性研究[J]. 韶关学院学报, 2007, 28(9):89-93. |
| Peng L, Zhu BF, Liu Z. Refolding of genetic engineering protein expressed as inclusion bodies[J]. J Shaoguan Univ, 2007, 28(9):89-93. | |
| [16] | Palmer I, Wingfield PT. Preparation and extraction of insoluble(inclusion-body)proteins from Escherichia coli[J]. Curr Protoc Protein Sci, 2012, Chapter 6: Unit6. 3. |
| [17] | 郭井利, 彭永刚, 刘鹏, 等. 重组蛋白在大肠杆菌中包涵体蛋白的表达及其复性[J]. 畜牧兽医科技信息, 2005(8):66-68. |
| Guo JL, Peng YG, Liu P, et al. Expression and renaturation of recombinant inclusion body protein in Escherichia coli[J]. Sci Inf Animal Husbamdry Vet Mecicine, 2005(8):66-68. | |
| [18] | 邝爱丽, 陈圆圆, 彭志峰, 等. 包涵体的形成原因及其处理方法[J]. 上海畜牧兽医通讯, 2009(1):62-63. |
| Kuang AL, Chen YY, Peng ZF, et al. The formation of inclusion body and its treatment[J]. Shanghai J Animal Husb Vet Med, 2009(1):62-63. | |
| [19] |
Wu PC, Chien MS, Tseng YY, et al. Expression of the porcine Circovirus type 2 capsid protein subunits and application to an indirect ELISA[J]. J Biotechnol, 2008, 133(1):58-64.
doi: 10.1016/j.jbiotec.2007.09.015 URL |
| [20] | Selleck W, Tan S. Recombinant protein complex expression in E. coli[J]. Curr Protoc Protein Sci, 2008, Chapter 5:Unit 5. 21. |
| [21] | 郜赵伟. 葡萄糖氧化酶基因密码子优化及其在毕赤酵母中的高效表达[D]. 重庆:西南大学, 2010. |
| Gao ZW. Codon optimization and high level expression in Pichia pastoris of glucose oxidase[D]. Chongqing:Southwest University, 2010. | |
| [22] | 孙风敏, 韩焱, 李文利. 基于密码子优化的蛋白酶K在毕赤酵母中的表达及分离纯化[J]. 微生物学通报, 2014, 41(11):2198-2207. |
| Sun FM, Han Y, Li WL. Expression and purification of Codon optimized proteinase K in Pichia pastoris[J]. Microbiol China, 2014, 41(11):2198-2207. | |
| [23] |
高庆华, 胡美荣, 吴芳彤, 等. 点青霉葡萄糖氧化酶基因的克隆及其酶学性质研究[J]. 生物技术通报, 2016, 32(7):152-159.
doi: 10.13560/j.cnki.biotech.bull.1985.2016.07.023 |
| Gao QH, Hu MR, Wu FT, et al. Cloning of gene for a glucose oxidase from Penicillium notatum and its enzymatic properties[J]. Biotechnol Bull, 2016, 32(7):152-159. | |
| [24] | 张可可. 一种新的细菌吡喃糖氧化酶的表达及其催化性质研究[D]. 长沙:湖南大学, 2017. |
| Zhang KK. Expression and catalytic characterization of a new bacterial pyranose oxidase[D]. Changsha:Hunan University, 2017. | |
| [25] |
Hallberg BM, Leitner C, Haltrich D, et al. Crystal structure of the 270 kDa homotetrameric lignin-degrading enzyme pyranose 2-oxidase[J]. J Mol Biol, 2004, 341(3):781-796.
pmid: 15288786 |
| [26] | 周亚萍, 于海燕, 朱钰峰, 等. 吡喃糖氧化酶及其表达纯化方法和应用:CN105861456A[P]. 2016-08-17. |
| Zhou YP, Yu HY, Shu YF. et al. Pyranose oxidase and expression and purification method and application thereof:CN105861456A[P]. 2016-08-17. | |
| [27] |
Danneel HJ, Rössner E, Zeeck A, et al. Purification and characterization of a pyranose oxidase from the basidiomycete Peniophora gigantea and chemical analyses of its reaction products[J]. Eur J Biochem, 1993, 214(3):795-802.
pmid: 8319689 |
| [28] |
Schneider K, Dorscheid S, Witte K, et al. Controlled feeding of hydrogen peroxide as oxygen source improves production of 5-ketofructose from L-sorbose using engineered pyranose 2-oxidase from Peniophora gigantea[J]. Biotechnol Bioeng, 2012, 109(11):2941-2945.
doi: 10.1002/bit.24572 URL |
| [29] |
Tamaki T, Ito T, Yamaguchi T. Immobilization of hydroquinone through a spacer to polymer grafted on carbon black for a high-surface-area biofuel cell electrode[J]. J Phys Chem B, 2007, 111(34):10312-10319.
doi: 10.1021/jp074334n URL |
| [30] | 陈颖, 李莎, 胡婷, 等. 吡喃糖氧化酶法检测1, 5-脱水葡萄糖醇的空白限、检出限和功能灵敏度的建立及评价[J]. 检验医学与临床, 2019, 16(15):2131-2133. |
| Chen Y, Li S, Hu T, et al. Establishment and evaluation of blank limit, detection limit and function sensitivity of pyranose oxidase method for detecting 1, 5-anhydroglucitol[J]. Lab Med Clin, 2019, 16(15):2131-2133. | |
| [31] | 王海生, 周亚萍, 于海燕, 等. 吡喃糖氧化酶的原核表达及初步实验应用[J]. 国际检验医学杂志, 2019, 40(7):774-777. |
| Wang HS, Zhou YP, Yu HY, et al. Prokaryotic expression and initial experimental application of pyranose oxidase[J]. Int J Lab Med, 2019, 40(7):774-777. |
| [1] | ZHAO Si-jia, WANG Xiao-lu, SUN Ji-lu, TIAN Jian, ZHANG Jie. Modification of Pichia pastoris for Erythritol Production by Metabolic Engineering [J]. Biotechnology Bulletin, 2023, 39(8): 137-147. |
| [2] | DONG Cong, GAO Qing-hua, WANG Yue, LUO Tong-yang, WANG Qing-qing. Increasing the Expression of FAD-dependent Glucose Dehydrogenase by Recombinant Pichia pastoris Using a Combined Strategy [J]. Biotechnology Bulletin, 2023, 39(6): 316-324. |
| [3] | YANG Yue, TAO Yan, XIE Jing, QIAN Yun-fang. Biosynthesis of Ctenopharyngodon idella C-type Lysozyme Based on Recombinant Pichia pastoris and Its Antibacterial Activity [J]. Biotechnology Bulletin, 2021, 37(12): 169-179. |
| [4] | LIN Mei-xuan, ZHOU Xiao-man, GUAN Feng, CUI Wen-jing. Heterologous Expression and Application of Phosphatidylinositol-specific Phospholipase C [J]. Biotechnology Bulletin, 2020, 36(1): 81-87. |
| [5] | DONG Cong, GAO Qing-hua, WANG Yue, LUO Tong-yang. Expression and Enzymatic Characterization of Codon-optimized FAD-dependent Glucose Dehydrogenase in Pichia pastoris [J]. Biotechnology Bulletin, 2019, 35(7): 114-120. |
| [6] | JIN Yong-mei, CHEN Mo-jun, LIU Xiao-xiao, LIN Xiu-feng. Antigenic Epitope Analysis and Preparation of Polyclonal Antibody of Lepidopteran Pest-resistant Gene cry1C [J]. Biotechnology Bulletin, 2018, 34(9): 224-229. |
| [7] | CHEN Jian-jun, LIU Liang-tao, CAO Xiang-lin. Cloning,Expression and Enzyme Production of Laccase Gene lac1680 in Phanerochaete chrysosporium [J]. Biotechnology Bulletin, 2018, 34(4): 214-220. |
| [8] | LIU Yi-jun, JIA Yu-kun, WANG Ling-fang, LIU Hong-xing, YANG Xian-yu. Prokaryotic Expression,Purification and Antiserum Preparation of Recombinant EDF-1 of Bufo gargarizans [J]. Biotechnology Bulletin, 2018, 34(10): 129-134. |
| [9] | CHEN Ling-yan,CHEN Xian-jin,JIANG Ye,Lü Tun. Research on the Expression of Porcine Circovirus Capsid Protein in the Bacillus subtilis [J]. Biotechnology Bulletin, 2016, 32(5): 140-145. |
| [10] | Wang Yan, Li Xiao, Chen Yong, Wu Yun. Expression of a Xylanase Gene Originated from Rumen Anaerobic Fungi Neocallimastix frontalis in Pichia pastoris [J]. Biotechnology Bulletin, 2015, 31(5): 186-193. |
| [11] | Lü Xiaomeng, Hu Tong, Yu Ting, Cui Yanhua. Advances of Heterogeneous Expression of Antimicrobial Peptides in Bacteria [J]. Biotechnology Bulletin, 2014, 0(5): 37-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||