








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (5): 254-266.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1080
Previous Articles Next Articles
ZHENG Huan1,2( ), LIN Dong-mei1, LIU Jun-yuan2, ZHANG Yin-lian2, LIN Biao-sheng3, LIN Zhan-xi1, LI Jing1(
), LIN Dong-mei1, LIU Jun-yuan2, ZHANG Yin-lian2, LIN Biao-sheng3, LIN Zhan-xi1, LI Jing1( )
)
Received:2022-09-01
Online:2023-05-26
Published:2023-06-08
Contact:
LI Jing
E-mail:2940251107@qq.com;13959197195@163.com
ZHENG Huan, LIN Dong-mei, LIU Jun-yuan, ZHANG Yin-lian, LIN Biao-sheng, LIN Zhan-xi, LI Jing. Analysis of Amino Acid Metabolism Difference Between Fruiting Body and Mycelium of Taiwanofungus camphoratus by LC-QTOF-MS Metabonomics[J]. Biotechnology Bulletin, 2023, 39(5): 254-266.
| 时间 Time/min | 流速 Row/(μL·min-1) | 流动相A A/% | 流动相B B/% |
|---|---|---|---|
| 0.00 | 300 | 15 | 85 |
| 1.00 | 300 | 15 | 85 |
| 12.00 | 300 | 35 | 65 |
| 12.10 | 300 | 60 | 40 |
| 15.00 | 300 | 60 | 40 |
| 15.10 | 300 | 15 | 85 |
| 20.00 | 300 | 15 | 85 |
Table 1 Gradient elution programs
| 时间 Time/min | 流速 Row/(μL·min-1) | 流动相A A/% | 流动相B B/% |
|---|---|---|---|
| 0.00 | 300 | 15 | 85 |
| 1.00 | 300 | 15 | 85 |
| 12.00 | 300 | 35 | 65 |
| 12.10 | 300 | 60 | 40 |
| 15.00 | 300 | 60 | 40 |
| 15.10 | 300 | 15 | 85 |
| 20.00 | 300 | 15 | 85 |

Fig. 4 Venn analysis of significant differential metabolites between every two of three groups of samples The numbers in the overlapping part indicate the number of common differential metabolites between the compared groups. The numbers in the non-overlapping part indicate the number of unique differential metabolites between the compared groups
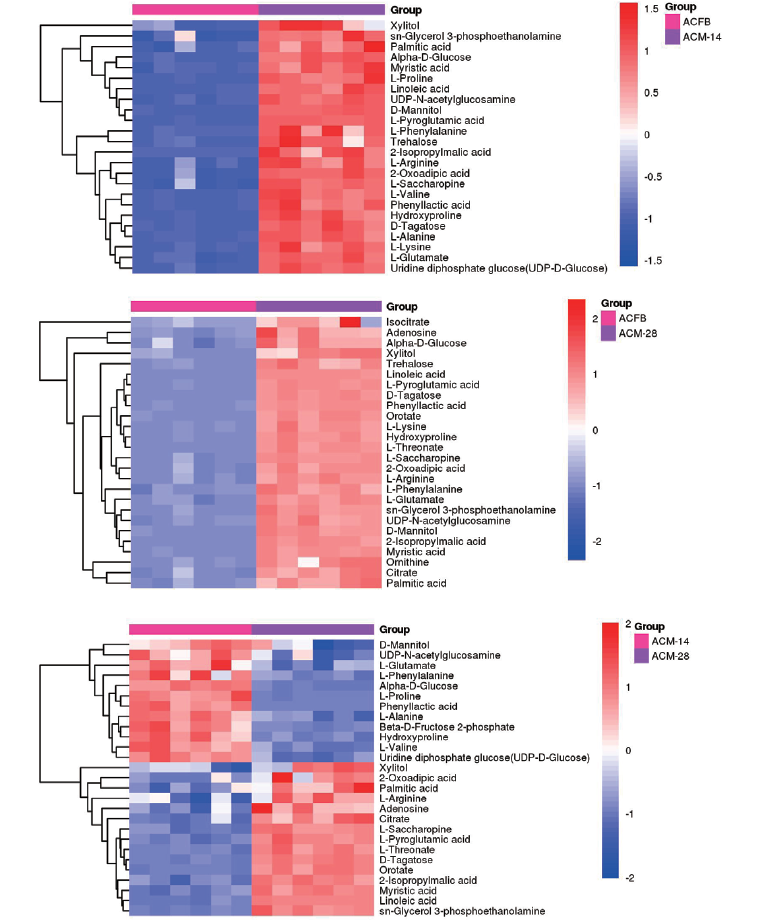
Fig. 5 Clustering analysis heatmap of significant differential metabolites between every two of three groups of samples The abscissa represents different groups. The ordinate represents the different metabolites compared in each group. The color patches at different positions represent the relative expression amounts of metabolites at the corresponding positions. The darker the red indicates the higher the expression content of the substance in the group, and the darker the blue indicates the lower the expression content of the substance in the group
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change及代谢趋势Log2 fold change and metabolic trend | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-精氨酸 L-Arginine | C7H16N4O2 | 919.066 | 3.0498 | 3.97E-02 | 2.12↑ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 2 | NG,NG'-二甲基-L-精氨酸NG,NG-dimethyl-L-arginine(ADMA) | C8H19ClN4O2 | 803.221 | 2.80174 | 4.89E-04 | -1.49 ↓ | 精氨酸代谢 |
| 3 | 甘氨酰-L-亮氨酸Glycyl-L-leucine | C8H16N2O3 | 247.711 | 1.17277 | 2.18E-04 | -2.09 ↓ | - |
| 4 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.27499 | 1.80E-05 | -2.41↓ | 酪氨酸代谢、异喹啉生物碱生物合成、碳水化合物代谢 |
| 5 | L-谷氨酰胺 L-Glutamate | C5H8NO4 | 523.018 | 2.31784 | 1.39E-10 | -2.47 ↓ | 精氨酸合成,嘌呤代谢,嘧啶代谢,丙氨酸、天冬氨酸和谷氨酸代谢、谷氨酸和谷氨酰胺代谢、氮代谢、氨酰合成 |
| 6 | D-焦谷氨酸 D-Proline | C5H9NO3 | 332.833 | 3.88710 | 4.12E-10 | -3.16 ↓ | 精氨酸和脯氨酸代谢 |
| 7 | L-酵母氨酸 L-Saccharopine | C11H19N2O6 | 657.875 | 1.07415 | 1.40E-07 | -3.49 ↓ | 赖氨酸合成 |
| 8 | L-苯丙氨酸 L-Phenylalanine | C9H11NO2 | 214.720 | 1.62936 | 1.07E-04 | -3.58 ↓ | 苯丙氨酸代谢、苯丙氨酸、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成 |
| 9 | L-高丝氨酸 L-Homoserine | C4H9NO3 | 414.936 | 1.00736 | 3.31E-10 | -4.14 ↓ | 甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、硫代谢 |
| 10 | L赖氨酸 L-Lysine | C6H14N2O2 | 815.743 | 1.10378 | 3.15E-06 | -4.87 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 11 | L-缬氨酸 L-Valine | C5H11NO2 | 305.713 | 1.36255 | 2.77E-06 | -4.88 ↓ | 缬氨酸、亮氨酸、异亮氨酸的生物合成与降解、青霉素和头孢菌素生物合成、泛酸盐和CoA生物合成 |
| 12 | L-丙氨酸 L-Alanine | C3H7NO2 | 399.437 | 1.24924 | 7.60E-07 | -5.03 ↓ | 丙氨酸、天冬氨酸和谷氨酸代谢、半胱氨酸和蛋氨酸代谢、牛磺酸代谢、丙氨酸代谢 |
| 13 | L-焦谷氨酸 L-Pyroglutamic acid | C5H7NO3 | 293.365 | 3.88261 | 5.43E-17 | -5.11 ↓ | 谷胱甘肽代谢 |
| 14 | L-脯氨酸L-Proline | C5H9NO2 | 334.343 | 1.61681 | 5.21E-06 | -5.72 ↓ | 精氨酸和普氨酸代谢、碳青霉烯合成 |
| 15 | 羟脯氨酸Hydroxyproline | C5H9NO3 | 398.864 | 1.84634 | 3.68E-06 | -7.25 ↓ | 精氨酸和脯氨酸代谢 |
Table 2 Component analysis of significantly differential amino acids between ACFB and ACM-14
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change及代谢趋势Log2 fold change and metabolic trend | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-精氨酸 L-Arginine | C7H16N4O2 | 919.066 | 3.0498 | 3.97E-02 | 2.12↑ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 2 | NG,NG'-二甲基-L-精氨酸NG,NG-dimethyl-L-arginine(ADMA) | C8H19ClN4O2 | 803.221 | 2.80174 | 4.89E-04 | -1.49 ↓ | 精氨酸代谢 |
| 3 | 甘氨酰-L-亮氨酸Glycyl-L-leucine | C8H16N2O3 | 247.711 | 1.17277 | 2.18E-04 | -2.09 ↓ | - |
| 4 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.27499 | 1.80E-05 | -2.41↓ | 酪氨酸代谢、异喹啉生物碱生物合成、碳水化合物代谢 |
| 5 | L-谷氨酰胺 L-Glutamate | C5H8NO4 | 523.018 | 2.31784 | 1.39E-10 | -2.47 ↓ | 精氨酸合成,嘌呤代谢,嘧啶代谢,丙氨酸、天冬氨酸和谷氨酸代谢、谷氨酸和谷氨酰胺代谢、氮代谢、氨酰合成 |
| 6 | D-焦谷氨酸 D-Proline | C5H9NO3 | 332.833 | 3.88710 | 4.12E-10 | -3.16 ↓ | 精氨酸和脯氨酸代谢 |
| 7 | L-酵母氨酸 L-Saccharopine | C11H19N2O6 | 657.875 | 1.07415 | 1.40E-07 | -3.49 ↓ | 赖氨酸合成 |
| 8 | L-苯丙氨酸 L-Phenylalanine | C9H11NO2 | 214.720 | 1.62936 | 1.07E-04 | -3.58 ↓ | 苯丙氨酸代谢、苯丙氨酸、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成 |
| 9 | L-高丝氨酸 L-Homoserine | C4H9NO3 | 414.936 | 1.00736 | 3.31E-10 | -4.14 ↓ | 甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、硫代谢 |
| 10 | L赖氨酸 L-Lysine | C6H14N2O2 | 815.743 | 1.10378 | 3.15E-06 | -4.87 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 11 | L-缬氨酸 L-Valine | C5H11NO2 | 305.713 | 1.36255 | 2.77E-06 | -4.88 ↓ | 缬氨酸、亮氨酸、异亮氨酸的生物合成与降解、青霉素和头孢菌素生物合成、泛酸盐和CoA生物合成 |
| 12 | L-丙氨酸 L-Alanine | C3H7NO2 | 399.437 | 1.24924 | 7.60E-07 | -5.03 ↓ | 丙氨酸、天冬氨酸和谷氨酸代谢、半胱氨酸和蛋氨酸代谢、牛磺酸代谢、丙氨酸代谢 |
| 13 | L-焦谷氨酸 L-Pyroglutamic acid | C5H7NO3 | 293.365 | 3.88261 | 5.43E-17 | -5.11 ↓ | 谷胱甘肽代谢 |
| 14 | L-脯氨酸L-Proline | C5H9NO2 | 334.343 | 1.61681 | 5.21E-06 | -5.72 ↓ | 精氨酸和普氨酸代谢、碳青霉烯合成 |
| 15 | 羟脯氨酸Hydroxyproline | C5H9NO3 | 398.864 | 1.84634 | 3.68E-06 | -7.25 ↓ | 精氨酸和脯氨酸代谢 |
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change 及代谢趋势Log2 fold change and metabolic trend | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-精氨酸 L-Arginine | C7H16N4O2 | 919.066 | 3.13864 | 3.43E-02 | 2.35 ↑ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 2 | D-脯氨酸 D-Proline | C5H10ClNO3 | 332.833 | 1.40639 | 6.85E-04 | -1.11 ↓ | 精氨酸和脯氨酸代谢 |
| 3 | NG,NG'-二甲基-L-精氨酸NG,NG-dimethyl-L-arginine(ADMA) | C8H19ClN4O2 | 803.221 | 2.50100 | 1.92E-03 | -1.35 ↓ | |
| 4 | L-谷氨酰胺 L-Glutamate | C5H10N2O3 | 523.018 | 2.09913 | 3.66E-10 | -2.21 ↓ | 精氨酸合成,嘌呤代谢,嘧啶代谢,丙氨酸、天冬氨酸和谷氨酸代谢、谷氨酸和谷氨酰胺代谢、氮代谢、氨酰合成 |
| 5 | L-苯丙氨酸L-Phenylalanine | C9H11NO2 | 214.720 | 1.11750 | 1.37E-08 | -2.56 ↓ | 苯丙氨酸代谢、苯丙氨酸、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成 |
| 6 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.54963 | 3.69E-11 | -2.87 ↓ | 酪氨酸代谢、异喹啉生物碱生物合成 |
| 7 | 鸟氨酸 Ornithine | C5H12N2O2 | 803.355 | 1.03339 | 1.06E-04 | -4.15 ↓ | 精氨酸合成、精氨酸和脯氨酸代谢、D-精氨酸和D-脯氨酸代谢、谷胱甘肽代谢 |
| 8 | L-酵母氨酸L-Saccharopine | C11H19N2O6 | 657.875 | 1.49179 | 3.91E-10 | -4.31 ↓ | 赖氨酸合成 |
| 9 | L-赖氨酸 L-Lysine | C6H14N2O2 | 815.7425 | 1.29183 | 5.70E-07 | -5.26 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 10 | L-焦谷氨酸L-Pyroglutamic acid | C5H7NO3 | 293.365 | 4.42017 | 6.95E-11 | -5.44 ↓ | 谷胱甘肽代谢 |
| 11 | 羟脯氨酸 Hydroxyproline | C5H9NO3 | 398.864 | 1.37226 | 1.53E-07 | -6.35 ↓ | 精氨酸和脯氨酸代谢 |
Table 3 Component analysis of significantly differential amino acids between ACFB and ACM-28
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change 及代谢趋势Log2 fold change and metabolic trend | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-精氨酸 L-Arginine | C7H16N4O2 | 919.066 | 3.13864 | 3.43E-02 | 2.35 ↑ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 2 | D-脯氨酸 D-Proline | C5H10ClNO3 | 332.833 | 1.40639 | 6.85E-04 | -1.11 ↓ | 精氨酸和脯氨酸代谢 |
| 3 | NG,NG'-二甲基-L-精氨酸NG,NG-dimethyl-L-arginine(ADMA) | C8H19ClN4O2 | 803.221 | 2.50100 | 1.92E-03 | -1.35 ↓ | |
| 4 | L-谷氨酰胺 L-Glutamate | C5H10N2O3 | 523.018 | 2.09913 | 3.66E-10 | -2.21 ↓ | 精氨酸合成,嘌呤代谢,嘧啶代谢,丙氨酸、天冬氨酸和谷氨酸代谢、谷氨酸和谷氨酰胺代谢、氮代谢、氨酰合成 |
| 5 | L-苯丙氨酸L-Phenylalanine | C9H11NO2 | 214.720 | 1.11750 | 1.37E-08 | -2.56 ↓ | 苯丙氨酸代谢、苯丙氨酸、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成 |
| 6 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.54963 | 3.69E-11 | -2.87 ↓ | 酪氨酸代谢、异喹啉生物碱生物合成 |
| 7 | 鸟氨酸 Ornithine | C5H12N2O2 | 803.355 | 1.03339 | 1.06E-04 | -4.15 ↓ | 精氨酸合成、精氨酸和脯氨酸代谢、D-精氨酸和D-脯氨酸代谢、谷胱甘肽代谢 |
| 8 | L-酵母氨酸L-Saccharopine | C11H19N2O6 | 657.875 | 1.49179 | 3.91E-10 | -4.31 ↓ | 赖氨酸合成 |
| 9 | L-赖氨酸 L-Lysine | C6H14N2O2 | 815.7425 | 1.29183 | 5.70E-07 | -5.26 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 10 | L-焦谷氨酸L-Pyroglutamic acid | C5H7NO3 | 293.365 | 4.42017 | 6.95E-11 | -5.44 ↓ | 谷胱甘肽代谢 |
| 11 | 羟脯氨酸 Hydroxyproline | C5H9NO3 | 398.864 | 1.37226 | 1.53E-07 | -6.35 ↓ | 精氨酸和脯氨酸代谢 |
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change 及代谢趋势Log2 fold change and metabolic tren | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-焦谷氨酸L-Pyroglutamic acid | C5H7NO3 | 521.6845 | 1.36714 | 4.51E-06 | 0.57 ↑ | 谷胱甘肽代谢 |
| 2 | L-丙氨酸 L-Alanine | C3H7NO2 | 399.437 | 1.1981 | 1.62E-06 | 0.90 ↑ | 丙氨酸、天冬氨酸和谷氨酸代谢、半胱氨酸和蛋氨酸代谢、牛磺酸代谢、丙氨酸代谢 |
| 3 | 羟脯氨酸Hydroxyproline | C5H9NO3 | 398.864 | 1.74395 | 6.67E-05 | 0.90 ↑ | 精氨酸和脯氨酸代谢 |
| 4 | L-缬氨酸 L-Valine | C5H11NO2 | 305.7125 | 1.33372 | 2.72E-06 | 0.95 ↑ | 缬氨酸、亮氨酸、异亮氨酸的生物合成与降解、青霉素和头孢菌素生物合成、泛酸盐和CoA生物合成 |
| 5 | L-高丝氨酸L-homoserine | C4H9NO3 | 414.936 | 1.13972 | 1.57E-08 | 1.44 ↑ | 甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、硫代谢 |
| 6 | 甘氨酸-L-亮氨酸Glycyl-L-leucine | C8H16N2O3 | 247.7105 | 1.30066 | 4.61E-04 | 1.13 ↑ | - |
| 7 | L-苯丙氨酸L-Phenylalanine | C9H11NO2 | 214.027 | 2.7225 | 1.64E-03 | 1.40 ↑ | 苯丙氨酸合成、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成、芥子油苷生物合成 |
| 8 | L-脯氨酸 L-Proline | C5H9NO2 | 334.343 | 1.9925 | 1.84E-05 | 2.01 ↑ | 精氨酸和脯氨酸代谢、碳青霉烯合成 |
| 9 | D-脯氨酸 D-Proline | C5H10ClNO3 | 332.833 | 4.98655 | 1.38E-06 | 2.05 ↑ | 精氨酸和脯氨酸代谢 |
| 10 | L-精氨酸L-Arginine | C7H16N4O2 | 806.1 | 1.64538 | 3.38E-03 | -0.33 ↓ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 11 | L-酵母氨酸L-Saccharopine | C11H19N2O6 | 658.6145 | 1.38022 | 1.96E-04 | -0.35 ↓ | 赖氨酸合成 |
| 12 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.12726 | 3.35E-04 | -0.46 ↓ | 酪氨酸代谢、异喹啉生物碱生物合成 |
| 13 | L-赖氨酸L-Saccharopine | C6H14N2O2 | 657.875 | 1.40151 | 2.54E-09 | -0.82 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 14 | L-苏氨酸L-Threonine | C4H9NO3 | 344.1635 | 1.22593 | 1.79E-09 | -1.08 ↓ | 甘氨酸、丝氨酸和苏氨酸代谢 |
Table 4 Component analysis of significantly different amino acids between ACM-14 and ACM-28
| 序号 No. | 物质名称 Name | 分子式 Molecular formula | 保留时间 Retention time/s | VIP | P-value | Log2 fold change 及代谢趋势Log2 fold change and metabolic tren | 代谢途径 Metabolic pathway |
|---|---|---|---|---|---|---|---|
| 1 | L-焦谷氨酸L-Pyroglutamic acid | C5H7NO3 | 521.6845 | 1.36714 | 4.51E-06 | 0.57 ↑ | 谷胱甘肽代谢 |
| 2 | L-丙氨酸 L-Alanine | C3H7NO2 | 399.437 | 1.1981 | 1.62E-06 | 0.90 ↑ | 丙氨酸、天冬氨酸和谷氨酸代谢、半胱氨酸和蛋氨酸代谢、牛磺酸代谢、丙氨酸代谢 |
| 3 | 羟脯氨酸Hydroxyproline | C5H9NO3 | 398.864 | 1.74395 | 6.67E-05 | 0.90 ↑ | 精氨酸和脯氨酸代谢 |
| 4 | L-缬氨酸 L-Valine | C5H11NO2 | 305.7125 | 1.33372 | 2.72E-06 | 0.95 ↑ | 缬氨酸、亮氨酸、异亮氨酸的生物合成与降解、青霉素和头孢菌素生物合成、泛酸盐和CoA生物合成 |
| 5 | L-高丝氨酸L-homoserine | C4H9NO3 | 414.936 | 1.13972 | 1.57E-08 | 1.44 ↑ | 甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、甘氨酸、丝氨酸和苏氨酸代谢、硫代谢 |
| 6 | 甘氨酸-L-亮氨酸Glycyl-L-leucine | C8H16N2O3 | 247.7105 | 1.30066 | 4.61E-04 | 1.13 ↑ | - |
| 7 | L-苯丙氨酸L-Phenylalanine | C9H11NO2 | 214.027 | 2.7225 | 1.64E-03 | 1.40 ↑ | 苯丙氨酸合成、酪氨酸和色氨酸生物合成、托烷、哌啶和吡啶生物碱生物合成、芥子油苷生物合成、芥子油苷生物合成 |
| 8 | L-脯氨酸 L-Proline | C5H9NO2 | 334.343 | 1.9925 | 1.84E-05 | 2.01 ↑ | 精氨酸和脯氨酸代谢、碳青霉烯合成 |
| 9 | D-脯氨酸 D-Proline | C5H10ClNO3 | 332.833 | 4.98655 | 1.38E-06 | 2.05 ↑ | 精氨酸和脯氨酸代谢 |
| 10 | L-精氨酸L-Arginine | C7H16N4O2 | 806.1 | 1.64538 | 3.38E-03 | -0.33 ↓ | 精氨酸代谢、单菌霉素代谢、克拉维酸生物合成、精氨酸和脯氨酸代谢 |
| 11 | L-酵母氨酸L-Saccharopine | C11H19N2O6 | 658.6145 | 1.38022 | 1.96E-04 | -0.35 ↓ | 赖氨酸合成 |
| 12 | 酪胺 Tyramine | C8H11NO | 179.924 | 1.12726 | 3.35E-04 | -0.46 ↓ | 酪氨酸代谢、异喹啉生物碱生物合成 |
| 13 | L-赖氨酸L-Saccharopine | C6H14N2O2 | 657.875 | 1.40151 | 2.54E-09 | -0.82 ↓ | 赖氨酸合成与降解、萜类、哌啶和吡啶生物碱生物合成、氨酰生物合成、生物碱合成 |
| 14 | L-苏氨酸L-Threonine | C4H9NO3 | 344.1635 | 1.22593 | 1.79E-09 | -1.08 ↓ | 甘氨酸、丝氨酸和苏氨酸代谢 |

Fig. 7 Regulatory network analysis between every two of three groups of samples Red indicates the metabolic pathway. Yellow indicates the material related regulatory enzyme information. Green indicates the background material of the metabolic pathway. Purple indicates the molecular module information of a class of substances. Blue dots indicates the chemical interaction reactions of substances. Green indicates the difference material obtained by comparison
| 名称 Amino acid name | ACFB | ACM-14 | ACM-28 |
|---|---|---|---|
| 天冬氨酸(Asp) | 0.75 | 1.03 | 1.07 |
| 苏氨酸(Thr) | 0.40 | 0.3 | 0.37 |
| 丝氨酸(Ser) | 0.50 | 0.45 | 0.49 |
| 谷氨酸(Glu) | 0.86 | 1.41 | 1.27 |
| 甘氨酸(Gly) | 0.43 | 1.62 | 1.4 |
| 丙氨酸(Ala) | 0.53 | 0.56 | 0.62 |
| 胱氨酸(Cystine) | 0.07 | 0.07 | 0.06 |
| 缬氨酸(Val) | 0.39 | 0.24 | 0.34 |
| 蛋氨酸(Met) | 0.00 | 1.02 | 1.48 |
| 异亮氨酸(Ice) | 0.33 | 0.11 | 0.18 |
| 亮氨酸(Leu) | 0.08 | 0.32 | 0.52 |
| 酪氨酸(Tyr) | 0.47 | 0.04 | 0.13 |
| 苯丙氨酸(Phe) | 0.75 | 0.19 | 0.29 |
| 赖氨酸(Lys) | 0.38 | 0.46 | 0.49 |
| 组氨酸(His) | 0.17 | 0.18 | 0.25 |
| 精氨酸(Arg) | 0.40 | 0.42 | 0.53 |
| 脯氨酸(Pro) | 0.37 | 1.12 | 0.99 |
| 总和 Total | 6.88 | 9.54 | 10.48 |
Table 5 Amino acid content of T. camphoratus in fruiting body and mycelium(g/100 g)
| 名称 Amino acid name | ACFB | ACM-14 | ACM-28 |
|---|---|---|---|
| 天冬氨酸(Asp) | 0.75 | 1.03 | 1.07 |
| 苏氨酸(Thr) | 0.40 | 0.3 | 0.37 |
| 丝氨酸(Ser) | 0.50 | 0.45 | 0.49 |
| 谷氨酸(Glu) | 0.86 | 1.41 | 1.27 |
| 甘氨酸(Gly) | 0.43 | 1.62 | 1.4 |
| 丙氨酸(Ala) | 0.53 | 0.56 | 0.62 |
| 胱氨酸(Cystine) | 0.07 | 0.07 | 0.06 |
| 缬氨酸(Val) | 0.39 | 0.24 | 0.34 |
| 蛋氨酸(Met) | 0.00 | 1.02 | 1.48 |
| 异亮氨酸(Ice) | 0.33 | 0.11 | 0.18 |
| 亮氨酸(Leu) | 0.08 | 0.32 | 0.52 |
| 酪氨酸(Tyr) | 0.47 | 0.04 | 0.13 |
| 苯丙氨酸(Phe) | 0.75 | 0.19 | 0.29 |
| 赖氨酸(Lys) | 0.38 | 0.46 | 0.49 |
| 组氨酸(His) | 0.17 | 0.18 | 0.25 |
| 精氨酸(Arg) | 0.40 | 0.42 | 0.53 |
| 脯氨酸(Pro) | 0.37 | 1.12 | 0.99 |
| 总和 Total | 6.88 | 9.54 | 10.48 |
| [1] |
Shivakumar N, Kashyap S, Kishore S, et al. Protein-quality evaluation of complementary foods in Indian children[J]. Am J Clin Nutr, 2019, 109(5): 1319-1327.
doi: 10.1093/ajcn/nqy265 pmid: 30920607 |
| [2] | 张勇, 李弘文, 曹晋良, 等. 栽培与野生羊肚菌营养成分及抗氧化性研究[J]. 食品科技, 2019, 44(1): 103-108. |
| Zhang Y, Li HW, Cao JL, et al. Study on nutrition and antioxidant of cultivated and wild Morchella esculenta[J]. Food Sci Technol, 2019, 44(1): 103-108. | |
| [3] |
Gobeil É, Maltais-Payette I, Taba N, et al. Mendelian randomization analysis identifies blood tyrosine levels as a biomarker of non-alcoholic fatty liver disease[J]. Metabolites, 2022, 12(5): 440.
doi: 10.3390/metabo12050440 URL |
| [4] | Huang L, He XY, Peng W, et al. Hyperuricemia induces liver injury by upregulating HIF-1α and inhibiting arginine biosynthesis pathway in mouse liver and human L02 hepatocytes[J]. Biochem Biophys Res Commun, 2022, 617(Pt 2): 55-61. |
| [5] | 周恩, 朱磊, 沈洪. 中医药对色氨酸代谢的研究进展[J]. 中医药临床杂志, 2022, 34(3): 580-585. |
| Zhou E, Zhu L, Shen H. Research progress of tryptophan metabolism in traditional Chinese medicine[J]. Clin J Tradit Chin Med, 2022, 34(3): 580-585. | |
| [6] | 唐翎, 魏伟, 赵勇. UPLC-MS/MS法测定猴头菌丝体中18种游离氨基酸的含量[J]. 药物分析杂志, 2018, 38(1): 112-117. |
| Tang L, Wei W, Zhao Y. Determination of free amino acids in the mycelium of Hericium by UPLC-MS/MS[J]. Chin J Pharm Anal, 2018, 38(1): 112-117. | |
| [7] |
Chang TT, Chou WN. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan[J]. Mycol Res, 1995, 99(6): 756-758.
doi: 10.1016/S0953-7562(09)80541-8 URL |
| [8] | 臧穆, 苏庆华. 我国台湾产灵芝属一新种——樟芝[J]. 云南植物研究, 1990, 12(4): 395-396. |
| Zang M, Su QH. Ganoderma comphoratum, a new taxon in genus Ganoderma from Taiwan, China[J]. Acta Bot Yunnanica, 1990, 12(4): 395-396. | |
| [9] |
李晶, 冯娜, 王升陽, 等. 牛樟芝化学成分及其药理作用研究进展[J]. 生物技术通报, 2021, 37(11): 14-31.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-1052 |
| Li J, Feng N, Wang SY, et al. Research progress in chemical constituents of Taiwanofungus camphoratum and its pharmacological activities[J]. Biotechnol Bull, 2021, 37(11): 14-31. | |
| [10] | Chang T, Chou W. Antrodia cinnamomea reconsidered and A. Salmonea sp. nov. on Cunninghamia konishii in Taiwan[J]. Bot Bull Acad Sin, 2004, 45: 347-352. |
| [11] | 张迅捷. 樟芝深层液体发酵工艺及其多糖特性的研究[D]. 福州: 福建农林大学, 2007. |
| Zhang XJ. Studies on submerged culture fermentation technology and polysaccharide prperties of Antrodia camphorata[D]. Fuzhou: Fujian Agriculture and Forestry University, 2007. | |
| [12] | 赵梦梦, 狄文玉, 康小红. 肿瘤代谢异常与靶向药物耐药[J]. 医学研究生学报, 2022, 35(7): 751-756. |
| Zhao MM, Di WY, Kang XH. Abnormal tumor metabolism and targeted drug resistance[J]. Journal of Medical Postgraduates, 2022, 35(7): 751-756. | |
| [13] |
Fu QY, Tong CY, Guo Y, et al. Flavonoid aglycone-oriented data-mining in high-performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry: efficient and targeted profiling of flavonoids in Scutellaria barbata[J]. Analytical and Bioanalytical Chemistry, 2020, 412(2): 321-333.
doi: 10.1007/s00216-019-02238-7 pmid: 31786643 |
| [14] | Nagai K, Uranbileg B, Chen Z, et al. Identification of novel biomarkers of hepatocellular carcinoma by high-definition mass spectrometry: Ultrahigh-performance liquid chromatography quadrupole time-of-flight mass spectrometry and desorption electrospray ionization mass spectrometry imaging[J]. Rapid Communications in Mass Spectrometry, 2020, 34(Suppl 1): e8551. |
| [15] | 黄琪, 刘琼, 张逢雨, 等. 基于超高效液相色谱-四极杆飞行时间质谱结合网络药理学探讨杜仲治疗糖尿病肾病的有效成分[J]. 中国新药与临床杂志, 2021, 40(6): 460-469. |
| Huang Q, Liu Q, Zhang FY, et al. Based on ultra high performance liquid chromatography quadrupole time-of-flight mass spectrometry combined with network pharmacology, the effective components of Eucommia ulmoides Oliv. in the treatment of diabetes nephropathy[J]. Chinese Journal of New Drugs and Clinical Medicine, 2021, 40(6): 460-469. | |
| [16] | 伍福仙, 张志清, 王瑾, 等. 超高效液相色谱串联四级杆飞行时间质谱法检测毒蘑菇中4种常见毒素含量[J]. 食品安全质量检测学报, 2019, 10(22): 7656-7664. |
| Wu FX, Zhang ZQ, Wang J, et al. Determination of 4 kinds of common toxins in poisonous mushroom by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry[J]. Journal of Food Safety and Quality Inspection, 2019, 10(22): 7656-7664. | |
| [17] | 薛莹, 徐先顺, 雍莉, 等. 磁性石墨烯固相萃取-UPLC/MS法分析蓝莓饮料成分[J]. 食品工业, 2018, 39(12): 294-298. |
| Xue Y, Xu XS, Yong L, et al. Rapid identification of active components in blueberry beverage by magnetic graphene solid phase extraction-UPLC/MS[J]. Food Industry, 2018, 39(12): 294-298. | |
| [18] | 武文帆. 牛樟芝利用差异代谢物液态发酵产4-乙酰基安卓奎诺尔B及其分离纯化工艺研究[D]. 北京: 北京化工大学, 2019. |
| Wu WF. Study on the production of 4-acetylandroquinol B by submerged fermentation of Ganoderma camphora with differential metabolites and its separation and purification process[D]. Beijing: Beijing University of Chemical Technology, 2019. | |
| [19] | 李晶. 牛樟芝MVA途径中基因克隆及转录组、代谢组研究[D]. 福州: 福建农林大学, 2016. |
| Li J. Molecular cloning of genes involved in MVA of Antrodia cinnamomea and its research on transcriptomics and metabolismics[D]. Fuzhou: Fujian Agriculture and Forestry University, 2016. | |
| [20] |
Wold S, Esbensen K, Geladi P. Principal component analysis[J]. Chemom Intell Lab Syst, 1987, 2(1/2/3): 37-52.
doi: 10.1016/0169-7439(87)80084-9 URL |
| [21] |
Lee J, Shin S, Choi JH, et al. Method to improve the classification accuracy of metal scraps by principal component analysis during laser-induced breakdown spectroscopy[J]. Trans Korean Soc Mech Eng B, 2019, 43(3): 193-199.
doi: 10.3795/KSME-B.2019.43.3.193 URL |
| [22] | Pérez-Enciso M, Tenenhaus M. Prediction of clinical outcome with microarray data: a partial least squares discriminant analysis(PLS-DA)approach[J]. Hum Genet, 2003, 112(5/6): 581-592. |
| [23] |
Trygg J, Wold S. Orthogonal projections to latent structures(O-PLS)[J]. J Chemom, 2002, 16(3): 119-128.
doi: 10.1002/(ISSN)1099-128X URL |
| [24] |
Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system[J]. Prog Neurobiol, 2001, 64(4): 365-391.
pmid: 11275358 |
| [25] | Li H. Regulatory role of arginase I and II in endothelial arginine metabolism and cell proliferation[D]. Texas: Texas A&M University, 2001. |
| [26] | 程功, 徐建中, 张伟国. L-精氨酸生物合成机制及其代谢工程育种研究进展[J]. 微生物学通报, 2016, 43(6): 1379-1387. |
| Cheng G, Xu JZ, Zhang WG. Progress in biosynthesis and metabolic engineering of L-Arginine producer[J]. Microbiol China, 2016, 43(6): 1379-1387. | |
| [27] |
闫洪波, 王威, 李令娣, 等. 原核细胞精氨酸生物合成途径的研究进展[J]. 生物技术通报, 2015, 31(1): 21-28.
doi: 10.13560/j.cnki.biotech.bull.1985.2015.01.003 |
|
Yan HB, Wang W, Li LD, et al. Research progress of the arginine biosynthetic pathway in prokaryotic cells[J]. Biotechnol Bull, 2015, 31(1): 21-28.
doi: 10.13560/j.cnki.biotech.bull.1985.2015.01.003 |
|
| [28] |
Fleck CB, Schöbel F, Brock M. Nutrient acquisition by pathogenic fungi: Nutrient availability, pathway regulation, and differences in substrate utilization[J]. Int J Med Microbiol, 2011, 301(5): 400-407.
doi: 10.1016/j.ijmm.2011.04.007 pmid: 21550848 |
| [29] | 刘爱民, 陈金风, 张金金, 等. 有机酸和氨基酸对双孢蘑菇2796菌丝生长的影响[J]. 中国农学通报, 2010, 26(3): 46-51. |
| Liu AM, Chen JF, Zhang JJ, et al. Effect of organic acid and amino acid on the mycelial growth of Agaricus bisporus 2796[J]. Chin Agric Sci Bull, 2010, 26(3): 46-51. | |
| [30] |
叶玉洁, 石光, 周正乙, 等. 桑黄菌丝体与子实体成分的比较分析[J]. 食品科学, 2019, 40(24): 246-251.
doi: 10.7506/spkx1002-6630-20181218-206 |
|
Ye YJ, Shi G, Zhou ZY, et al. Comparative chemical composition of mycelium and fruit body of Phellinus igniarius[J]. Food Sci, 2019, 40(24): 246-251.
doi: 10.1111/jfds.1975.40.issue-2 URL |
|
| [31] | 尹永刚, 郑雪平, 吴志忠, 等. 杏鲍菇菌丝体与子实体氨基酸的组成分析[J]. 食药用菌, 2014, 22(6): 329-330. |
| Yin YG, Zheng XP, Wu ZZ, et al. Amino acid composition analysis of Pleurotus eryngii mycelium and fruiting body[J]. Edible Med Mushrooms, 2014, 22(6): 329-330. | |
| [32] |
Yang YH, Yang J, Wang HL, et al. Analysis of primary metabolites of Morchella fruit bodies and mycelium based on widely targeted metabolomics[J]. Arch Microbiol, 2021, 204(1): 98.
doi: 10.1007/s00203-021-02612-z |
| [33] | 饶毅萍, 陈洁辉, 张冰娜, 等. 平菇菌丝体与子实体营养成分的分析比较[J]. 生物学杂志, 2011, 28(3): 94-96. |
| Rao YP, Chen JH, Zhang BN, et al. Analysis and comparison of nutritional contents of mycelium and fruitbody of Pleurotus ostreatus[J]. J Biol, 2011, 28(3): 94-96. | |
| [34] | 王培丹. 菌草菌糟栽培竹荪及其品质的研究[D]. 福州: 福建农林大学, 2015. |
| Wang PD. Studies on the cultivation by JUNCAO spent mushroom substrate, the character of Dictyophora indusiata[D]. Fuzhou: Fujian Agriculture and Forestry University, 2015. | |
| [35] | 周明, 申书婷. L-精氨酸对动物生殖机能的作用[J]. 饲料与畜牧, 2013(10): 5-6. |
| Zhou M, Shen ST. Effect of L-arginine on animal reproductive function[J]. Feed Husb, 2013(10): 5-6. | |
| [36] | 王斌. L-精氨酸的抗疲劳作用研究[J]. 氨基酸和生物资源, 2008, 30(2): 44-47. |
| Wang B. Study on antifatigue of L-arginine supplementation[J]. Amino Acids & Biotic Resour, 2008, 30(2): 44-47. | |
| [37] |
Lucas ML, Rhoden CR, Rhoden EL, et al. Effects of L-arginine and L-NAME on ischemia-reperfusion in rat liver[J]. Acta Cir Bras, 2015, 30(5): 345-352.
doi: 10.1590/S0102-865020150050000006 pmid: 26016934 |
| [38] | 刘志远, 谢纯良, 朱作华, 等. 食用菌菌丝体综合利用研究进展[J]. 中国食用菌, 2017, 36(3): 7-9. |
| Liu ZY, Xie CL, Zhu ZH, et al. Advances research on comprehensive utilization of edible fungus mycelium[J]. Edible Fungi China, 2017, 36(3): 7-9. | |
| [39] | 邓雅元, 游金坤, 华蓉, 等. 3种常见野生牛肝菌和3种大宗人工食用菌营养成分分析[J]. 中国食用菌, 2022, 41(3): 45-48. |
| Deng YY, You JK, Hua R, et al. Nutritional analysis of three common wild boltus spp. and three staple artificial edible fungi[J]. Edible Fungi China, 2022, 41(3): 45-48. | |
| [40] | 蒋滢. 氨基酸的应用[M]. 北京: 世界图书出版公司,1996. |
| Jiang Y. Application of amino acids[M]. Beijing: World Book Publishing Company, 1996. |
| [1] | MIAO Yong-mei, MIAO Cui-ping, YU Qing-cai. Properties of Bacillus subtilis Strain BBs-27 Fermentation Broth and the Inhibition of Lipopeptides Against Fusarium culmorum [J]. Biotechnology Bulletin, 2023, 39(9): 255-267. |
| [2] | LIU Yuan-yuan, WEI Chuan-zheng, XIE Yong-bo, TONG Zong-jun, HAN Xing, GAN Bing-cheng, XIE Bao-gui, YAN Jun-jie. Characteristics of Class II Peroxidase Gene Expression During Fruiting Body Development and Stress Response in Flammulina filiformis [J]. Biotechnology Bulletin, 2023, 39(11): 340-349. |
| [3] | WEI Jing-jing, ZHANG Hao-ran, WANG Zhi-ge, WANG Hui-chun. Effect of Morchella Mycelium on Soil Enzymatic Activity [J]. Biotechnology Bulletin, 2020, 36(9): 211-217. |
| [4] | ZHANG Ze-hua, GAO Wei, WU Xiang-li, HUANG Chen-yang, CHANG Ming-chang, ZHANG Jin-xia. Inheritance Study on Fruiting Body Color of Pleurotus djamor(Rumph. ex Fr.)Boedijn [J]. Biotechnology Bulletin, 2019, 35(5): 70-75. |
| [5] | HU Dong-dong, ZHAO Liang, FAN Li, LIU Xu-ping, DENG Xian-cun, MIU Shi-wei, TAN Wen-song. Effects of Yeast Extract on Cell Growth and Antibody Production in CHO Cell Culture [J]. Biotechnology Bulletin, 2017, 33(6): 162-169. |
| [6] | Zhao Chao, Zeng Feng, Huang Yifan, Liu Bin. Optimum Technique of Extracting Mycelium Polysaccharide from Auricularia auricular Using Box-Behnken Design-Response Surface Methodology [J]. Biotechnology Bulletin, 2013, 0(6): 188-193. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||