








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (8): 252-260.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1422
Previous Articles Next Articles
WANG Yu-chen1,2( ), DING Zun-dan2, GUAN Fei-fei2, TIAN Jian2, LIU Guo-an1(
), DING Zun-dan2, GUAN Fei-fei2, TIAN Jian2, LIU Guo-an1( ), WU Ning-feng2(
), WU Ning-feng2( )
)
Received:2021-11-13
Online:2022-08-26
Published:2022-09-14
Contact:
LIU Guo-an,WU Ning-feng
E-mail:13519669461@163.com;liuguoan@nwnu.edu.cn;wuningfeng@caas.cn
WANG Yu-chen, DING Zun-dan, GUAN Fei-fei, TIAN Jian, LIU Guo-an, WU Ning-feng. Identification of the Thermostable Laccase Gene ba4 and Characterization of Its Enzymatic Properties[J]. Biotechnology Bulletin, 2022, 38(8): 252-260.
| 溶液 Solution | 加入量 Added amount/μL |
|---|---|
| A液:0.2 mol/L Na2HPO4-0.1 mol/L 柠檬酸(pH 6.0) | 750 |
| B液:5 mmol/L ABTS | 200 |
| BA4蛋白 | 50 |
Table 1 Determination system for laccase enzyme activity
| 溶液 Solution | 加入量 Added amount/μL |
|---|---|
| A液:0.2 mol/L Na2HPO4-0.1 mol/L 柠檬酸(pH 6.0) | 750 |
| B液:5 mmol/L ABTS | 200 |
| BA4蛋白 | 50 |
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 棉酚Gossypol/(mg·mL-1) | 0.00 | 0.05 | 0.1 | 0.15 | 0.2 |
Table 2 Concentrations of gossypol in marking the standa-rd curve
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 棉酚Gossypol/(mg·mL-1) | 0.00 | 0.05 | 0.1 | 0.15 | 0.2 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 玉米赤霉烯酮浓度 ZEN/(mg·mL-1) | 0.0000 | 0.0625 | 0.1250 | 0.2500 | 0.5000 | 1.0000 |
Table 3 Concentrations of ZEN in marking the standard curve
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 玉米赤霉烯酮浓度 ZEN/(mg·mL-1) | 0.0000 | 0.0625 | 0.1250 | 0.2500 | 0.5000 | 1.0000 |

Fig. 3 Expression of BA4 after micro aerobic fermentation for 20 h M:Protein marker;1:supernatant of crushed IPTG-induced laccase BA4;2:precipitation of crushed IPTG-induced laccase BA4;3:NTA-200 eluant
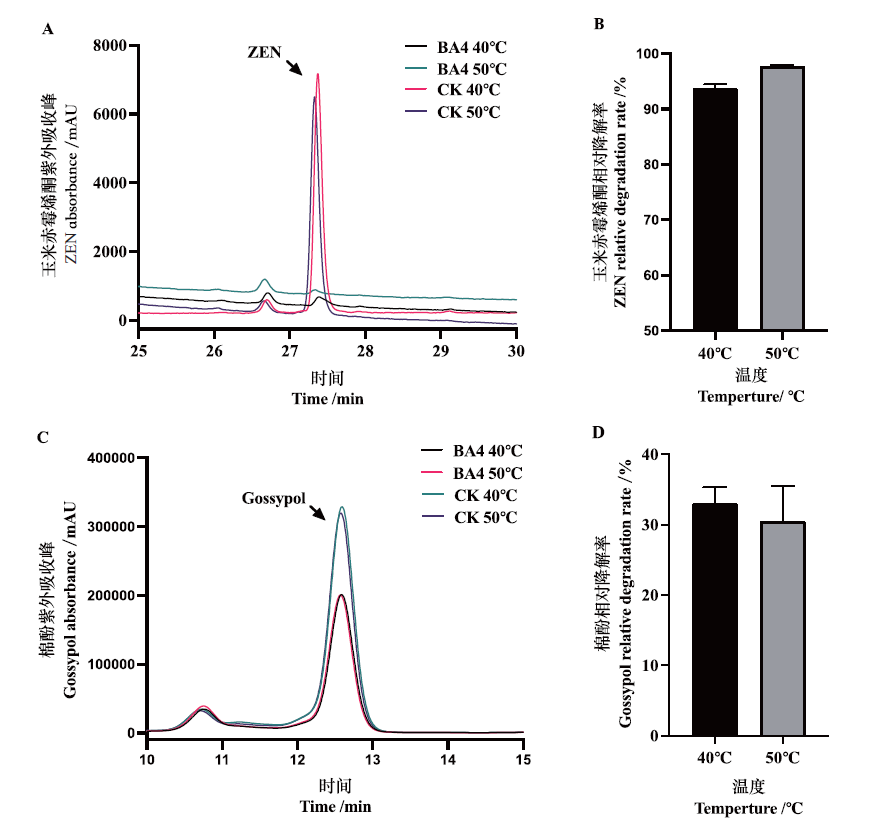
Fig. 6 Degradation of ZEN and gossypol by laccase BA4 A:Chromatogram. CK:ZEN reference substance;treatment group:laccase BA4 treating ZEN at 40℃ and 50℃,respectively. B:Relative degradation rate of ZEN. Treating ZEN with laccase BA4 at 40℃ and 50℃,respectively,and the relative degradation rate was calculated based on the peak area of the chromatogram. C:Chromatogram. CK:Gossypol reference substance. Treatment group:Laccase BA4 treated gossypol at 40℃ and 50℃,respectively. D:Relative degradation rate of gossypol. Laccase BA4 was treated with gossypol at 40℃ and 50℃,respectively,and the relative degradation rate was calculated based on the peak area of the chromatogram
| [1] |
Giardina P, Faraco V, Pezzella C, et al. Laccases:a never-ending story[J]. Cell Mol Life Sci, 2010, 67(3):369-385.
doi: 10.1007/s00018-009-0169-1 pmid: 19844659 |
| [2] |
Singh G, Bhalla A, Kaur P, et al. Laccase from prokaryotes:a new source for an old enzyme[J]. Rev Environ Sci Bio/technology, 2011, 10(4):309-326.
doi: 10.1007/s11157-011-9257-4 URL |
| [3] |
Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases[J]. Chem Rev, 1996, 96(7):2563-2606.
pmid: 11848837 |
| [4] |
Hidayat A, Yanto DHY. Biodegradation and metabolic pathway of phenanthrene by a new tropical fungus, Trametes hirsuta D7[J]. J Environ Chem Eng, 2018, 6(2):2454-2460.
doi: 10.1016/j.jece.2018.03.051 URL |
| [5] |
Dwivedi UN, Singh P, Pandey VP, et al. Structure-function relationship among bacterial, fungal and plant laccases[J]. J Mol Catal B Enzym, 2011, 68(2):117-128.
doi: 10.1016/j.molcatb.2010.11.002 URL |
| [6] |
Sharma B, Dangi AK, Shukla P. Contemporary enzyme based technologies for bioremediation:a review[J]. J Environ Manage, 2018, 210:10-22.
doi: 10.1016/j.jenvman.2017.12.075 URL |
| [7] |
Rodríguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases:a review[J]. Biotechnol Adv, 2006, 24(5):500-513.
pmid: 16716556 |
| [8] |
Galai S, Limam F, Marzouki MN. A new Stenotrophomonas maltophilia strain producing laccase. use in decolorization of synthetics dyes[J]. Appl Biochem Biotechnol, 2009, 158(2):416-431.
doi: 10.1007/s12010-008-8369-y pmid: 18931956 |
| [9] |
Knutson K, Ragauskas A. Laccase-mediator biobleaching applied to a direct yellow dyed paper[J]. Biotechnol Prog, 2004, 20(6):1893-1896.
doi: 10.1021/bp049833+ URL |
| [10] |
Santhanam N, Vivanco JM, Decker SR, et al. Expression of industrially relevant laccases:prokaryotic style[J]. Trends Biotechnol, 2011, 29(10):480-489.
doi: 10.1016/j.tibtech.2011.04.005 URL |
| [11] |
Alberts JF, Gelderblom WCA, Botha A, et al. Degradation of aflatoxin B(1)by fungal laccase enzymes[J]. Int J Food Microbiol, 2009, 135(1):47-52.
doi: 10.1016/j.ijfoodmicro.2009.07.022 pmid: 19683355 |
| [12] |
Zeinvand-Lorestani H, Sabzevari O, Setayesh N, et al. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products[J]. Chemosphere, 2015, 135:1-6.
doi: 10.1016/j.chemosphere.2015.03.036 pmid: 25876029 |
| [13] |
Zinedine A, Soriano JM, Moltó JC, et al. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of Zearalenone:an oestrogenic mycotoxin[J]. Food Chem Toxicol, 2007, 45(1):1-18.
doi: 10.1016/j.fct.2006.07.030 URL |
| [14] |
Maragos C. Zearalenone occurrence and human exposure[J]. World Mycotoxin J, 2010, 3(4):369-383.
doi: 10.3920/WMJ2010.1240 URL |
| [15] |
Richard JL. Some major mycotoxins and their mycotoxicoses—an overview[J]. Int J Food Microbiol, 2007, 119(1/2):3-10.
doi: 10.1016/j.ijfoodmicro.2007.07.019 URL |
| [16] |
Wu N, Ou W, Zhang ZD, et al. Recent advances in detoxification strategies for Zearalenone contamination in food and feed[J]. Chin J Chem Eng, 2021, 30:168-177.
doi: 10.1016/j.cjche.2020.11.011 URL |
| [17] |
Xu JH, Liu T, Chi JX, et al. Online high-efficient specific detection of Zearalenone in rice by using high-loading aptamer affinity hydrophilic monolithic column coupled with HPLC[J]. Talanta, 2020, 219:121309.
doi: 10.1016/j.talanta.2020.121309 URL |
| [18] |
Pack E, Stewart J, Rhoads M, et al. Quantification of Zearalenone and α-Zearalenol in swine liver and reproductive tissues using GC-MS[J]. Toxicon X, 2020, 8:100058.
doi: 10.1016/j.toxcx.2020.100058 URL |
| [19] |
Li CL, Deng CL, Zhou S, et al. High-throughput and sensitive determination of urinary Zearalenone and metabolites by UPLC-MS/MS and its application to a human exposure study[J]. Anal Bioanal Chem, 2018, 410(21):5301-5312.
doi: 10.1007/s00216-018-1186-4 URL |
| [20] |
Liu ZW, Hua QC, Wang J, et al. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals[J]. Biosens Bioelectron, 2020, 158:112178.
doi: 10.1016/j.bios.2020.112178 URL |
| [21] |
Wang L, Chen M, Luo XC, et al. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein[J]. Front Chem, 2020, 8:583176.
doi: 10.3389/fchem.2020.583176 URL |
| [22] |
Sunilkumar G, Campbell LM, Puckhaber L, et al. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol[J]. PNAS, 2006, 103(48):18054-18059.
pmid: 17110445 |
| [23] |
Adams R, Geissman TA, Edwards JD. Gossypol, a pigment of cottonseed[J]. Chem Rev, 1960, 60:555-574.
doi: 10.1021/cr60208a002 URL |
| [24] |
Yang J, Zhang F, Li JR, et al. Synthesis and antiviral activities of novel gossypol derivatives[J]. Bioorg Med Chem Lett, 2012, 22(3):1415-1420.
doi: 10.1016/j.bmcl.2011.12.076 URL |
| [25] |
Dodou K, Anderson RJ, Small DAP, et al. Investigations on gossypol:past and present developments[J]. Expert Opin Investig Drugs, 2005, 14(11):1419-1434.
doi: 10.1517/13543784.14.11.1419 URL |
| [26] |
Zhang L, Jiang HX, Cao XX, et al. Chiral gossypol derivatives:evaluation of their anticancer activity and molecular modeling[J]. Eur J Med Chem, 2009, 44(10):3961-3972.
doi: 10.1016/j.ejmech.2009.04.025 pmid: 19447525 |
| [27] |
Widsten P, Kandelbauer A. Laccase applications in the forest products industry:a review[J]. Enzyme Microb Technol, 2008, 42(4):293-307.
doi: 10.1016/j.enzmictec.2007.12.003 URL |
| [28] |
Fabbrini M, Galli C, Gentili P. Comparing the catalytic efficiency of some mediators of laccase[J]. J Mol Catal B Enzym, 2002, 16(5/6):231-240.
doi: 10.1016/S1381-1177(01)00067-4 URL |
| [29] | 罗爽, 谢天, 刘忠川, 等. 漆酶/介体系统研究进展[J]. 应用与环境生物学报, 2015, 21(6):987-995. |
| Luo S, Xie T, Liu ZC, et al. Laccase-mediator system:a reviewcase-mediator system:a review[J]. Chin J Appl Environ Biol, 2015, 21(6):987-995. | |
| [30] | 关菲菲, 田健, 伍宁丰, 等. 漆酶及其突变体和应用:CN113388591B[P], 2021-11-09. |
| Guan FF, Tian J, Wu NF, et al. Application of laccase and its mutants:CN113388591B[P], 2021-11-09. | |
| [31] | 国家卫生和计划生育委员会. 食品安全国家标准植物性食品中游离棉酚的测定:GB 5009. 148—2014[S]. 北京: 中国标准出版社, 2015. |
| National Food Safety Standard. Determination of Free Gossypol in Plant Food:GB 5009. 148—2014[S]. Beijing: Standards Press of China, 2015. | |
| [32] |
Lovley DR. Anaerobic benzene degradation[J]. Biodegradation, 2000, 11(2/3):107-116.
doi: 10.1023/A:1011191220463 URL |
| [33] |
Bosch R, García-Valdés E, Moore ER. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10[J]. Gene, 1999, 236(1):149-157.
pmid: 10433976 |
| [34] | Soana F, Sturini M, Cermenati L, et al. Titanium dioxide photocatalyzed oxygenation of naphthalene and some of its derivatives[J]. J Chem Soc, Perkin Trans 2, 2000(4):699-704. |
| [35] |
Wang XL, Bai YG, Huang HQ, et al. Degradation of aflatoxin B1 and Zearalenone by bacterial and fungal laccases in presence of structurally defined chemicals and complex natural mediators[J]. Toxins, 2019, 11(10):609.
doi: 10.3390/toxins11100609 URL |
| [36] |
Camarero S, Ibarra D, Martínez MJ, et al. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes[J]. Appl Environ Microbiol, 2005, 71(4):1775-1784.
doi: 10.1128/AEM.71.4.1775-1784.2005 URL |
| [1] | YANG Jun-zhao, ZHANG Xin-rui, ZHAO Guo-zhu, ZHENG Fei. Structure and Function Analysis of Novel GH5 Multi-domain Cellulase [J]. Biotechnology Bulletin, 2023, 39(4): 71-80. |
| [2] | FU Qiao, LIN Qi-lan, XUE Qiang, XIONG Hai-rong, WANG Ya-wei. Effects of CBM41 N-terminal Truncation on the Enzymological Properties of the Pullulanase from Bacillus subtilis 168 [J]. Biotechnology Bulletin, 2022, 38(6): 245-251. |
| [3] | JIA Chen-bo, SU Yi-huang, MA Xiu-mei, WANG Chun-li, XU Chun-yan. Medium Optimization for Laccase Production by Acrophialophora sp. Z45 and Its Decolorization of Dyes [J]. Biotechnology Bulletin, 2022, 38(6): 252-260. |
| [4] | MAO Guo-tao, WANG Jie, WANG Kai, WANG Fang-yuan, CAO Le-yan, ZHANG Hong-sen, SONG An-dong. Characterization of Laccase TaLac from Thermus aquaticus and Its Application in Removing Malachite Green Dye [J]. Biotechnology Bulletin, 2022, 38(4): 261-268. |
| [5] | WANG Xiao-tao, ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao. Heterologous Expression and Enzymatic Properties Analysis of Novel β-agarase Aga2 from Paraglaciecola hydrolytica [J]. Biotechnology Bulletin, 2022, 38(11): 258-268. |
| [6] | TIAN Jia-hui, FENG Jia-li, LU Jun-hua, MAO Lin-jing, HU Zhu-ran, WANG Ying, CHU Jie. Isolation,Purification and Characterization of Laccase LacT-1 from Cerrena unicolor [J]. Biotechnology Bulletin, 2021, 37(8): 186-194. |
| [7] | CHEN Ming-yu, NI Xuan, SI You-bin, SUN Kai. Advances in the Application of Immobilized Fungal Laccase for the Bioremediation of Environmental Organic Contamination [J]. Biotechnology Bulletin, 2021, 37(6): 244-258. |
| [8] | ZHANG Yao-xin, WANG Liang-jie, ZHENG Wen, XU Han-qin, ZHENG Lian, ZHONG Jing. Study on Enzyme Production of a Chitinase-producing Strain Achromobacter sp. ZWW8 by Fermentation and Its Enzymatic Characterization [J]. Biotechnology Bulletin, 2021, 37(4): 96-106. |
| [9] | XIONG Xue, LI Peng, ZHANG Gui-he, XIANG Zhun, TAO Wen-Guang, ZHOU Guang-yan, HE Yao-wei. Effects of Different Cultivation Substrates on the Laccase Activities of Lentinula edodes During Liquid Fermentation [J]. Biotechnology Bulletin, 2021, 37(12): 50-59. |
| [10] | WANG Hao, TANG Lu-xin, MA Hong-fei, QIAN Kun, SI Jing, CUI Bao-kai. Immobilization of Laccase from Trametes orientalis and Its Application for Decolorization of Multifarious Dyes [J]. Biotechnology Bulletin, 2021, 37(11): 142-157. |
| [11] | WANG Hui-lan, WU Jin-yong, CHEN Xiang-song, YUAN Li-xia, ZHU Wei-wei, YAO Jian-ming. Immobilization of N-acetylneuraminic Acid Aldolaseand Properties of the Immobilized Enzyme [J]. Biotechnology Bulletin, 2020, 36(6): 165-173. |
| [12] | CHEN Hui-ling, ZHANG Qing-yun, SUN Kai. Laccase-Mediated Oxidative Coupling of Phenolic Compounds in vivo:from Fundamentals to Multifunctional Applications in Green Synthesis [J]. Biotechnology Bulletin, 2020, 36(5): 193-204. |
| [13] | SUN Kai, CHEN Zheng-jie, WANG Deng-yang, SHU Ru-yu, WU Ji, WEI Fan. Removal of Bisphenol A in Wastewater by Immobilized Laccase [J]. Biotechnology Bulletin, 2020, 36(12): 188-198. |
| [14] | WU Yi, MA Hong-fei, CAO Yong-jia, SI Jing, CUI Bao-kai. Medium Optimization for the Laccase Production by White Rot Fungus Porodaedalea laricis and Its Dye Decolorizing Capacity [J]. Biotechnology Bulletin, 2020, 36(1): 45-59. |
| [15] | WU Yi, MA Hong-fei, CAO Yong-jia, SI Jing, CUI Bao-kai. Advances on Properties,Production,Purification and Immobilization of Fungal Laccase [J]. Biotechnology Bulletin, 2019, 35(9): 1-10. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||