








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (8): 92-100.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1499
Previous Articles Next Articles
CHEN Guang1( ), LI Jia2, DU Rui-ying1, WANG Xu1(
), LI Jia2, DU Rui-ying1, WANG Xu1( )
)
Received:2021-12-03
Online:2022-08-26
Published:2022-09-14
Contact:
WANG Xu
E-mail:chenguang0066@126.com;wangxuguangzhou@126.com
CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. pOsHAK1:OsFLN2 Expression Enhances the Drought Tolerance by Altering Sugar Metabolism in Rice[J]. Biotechnology Bulletin, 2022, 38(8): 92-100.
| 基因名称Gene name | 正向引物Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsFLN2 | CCGAATGGCTTCTCTTCTTCTC | GGCTCCTGATTGAGTTGGTACTACA |
| SGR | GCAATGTCGCCAAATGACG | GCTCACCACACTCATTCCCTAAAG |
| OsATG8a | AGCCCAGAAAAGGCCATCTT | CATCCTTGTTCTCTTCGTAGATTGC |
| OsSNAC1 | GTCAAGACTGATTGGATCATGC | CCAATCATCCAACCTGAGAGA |
| OsMYB2 | GAGCAGCGAGGAGGAGGT | TGTAGTTGACGAGCAGGAGGT |
Table 1 Primers used for RT-qPCR assays
| 基因名称Gene name | 正向引物Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsFLN2 | CCGAATGGCTTCTCTTCTTCTC | GGCTCCTGATTGAGTTGGTACTACA |
| SGR | GCAATGTCGCCAAATGACG | GCTCACCACACTCATTCCCTAAAG |
| OsATG8a | AGCCCAGAAAAGGCCATCTT | CATCCTTGTTCTCTTCGTAGATTGC |
| OsSNAC1 | GTCAAGACTGATTGGATCATGC | CCAATCATCCAACCTGAGAGA |
| OsMYB2 | GAGCAGCGAGGAGGAGGT | TGTAGTTGACGAGCAGGAGGT |
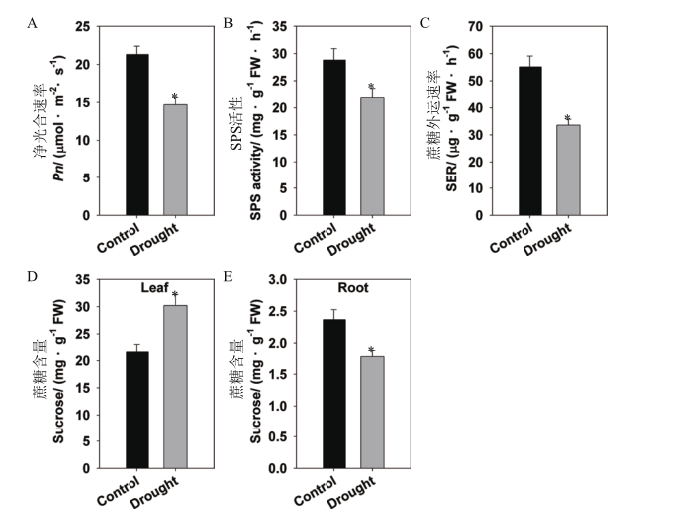
Fig. 1 Effects of drought stress on sugar metabolism in rice Rice seedlings were growing in normal IRRI solution for 6 weeks,then treated with 15% PEG for 10 d. A:Net photosynthetic rate(Pn). B:Sucrose phosphate synthase(SPS)activity. C:Rate of sucrose export(SER)from the leaf. D-E:Sucrose contents of the leaf(D)and root(E). The values are means ± SE of 5 replicates. Significant differences between normal and 15% PEG treatment are indicated with asterisks(P < 0.05). FW:Fresh weight
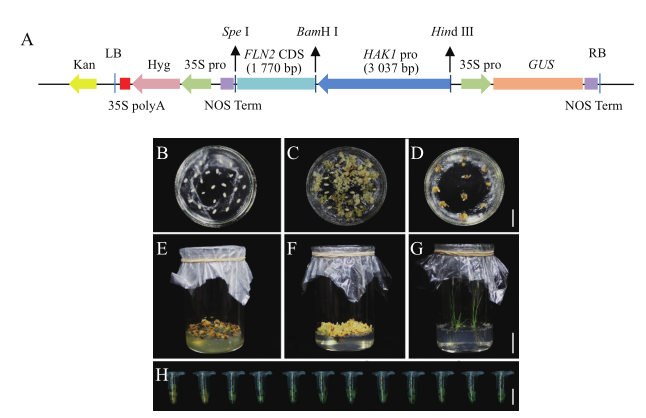
Fig. 2 Construction of expression vector and process of genetic transformation in rice A:Construction map of expression vector. B-G:Callus induction(B,C),sele-ction of transformed calli(D),shoot regeneration from resistant calli(E,F),and hardening of transgenic plants(G),bar in B-D=2 cm,bar in E-G=3 cm. H:Iden-tification of positive transgenic lines by GUS staining in T0 generation,bar=1 cm
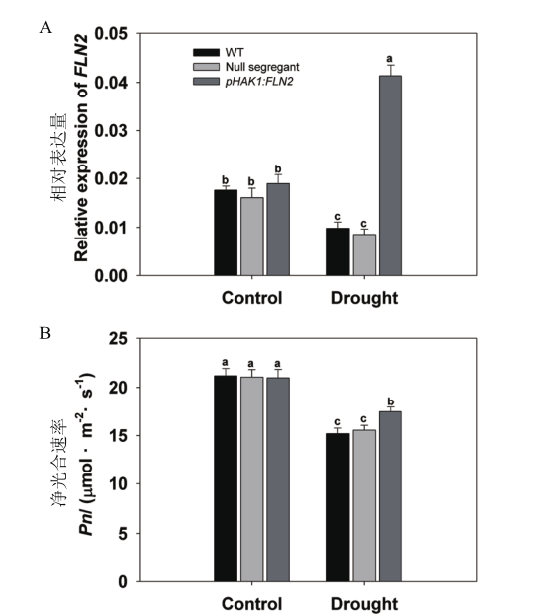
Fig. 3 Differential analysis of FLN2 expression and net photosynthetic rate between T1 generation of pHAK1:FLN2 transgenic lines and WT in response to drought stress Rice seedlings were growing in normal IRRI solution for 2 weeks,then treated with 15% PEG for 7 d. A:RT-qPCR analysis of endogenous OsFLN2 in the shoots of seedlings. UBQ5 was chosen as the reference sequence. B:Net photosynthetic rate. Results for the five null segregants were combined together as a second control(null segregant),while five transgenic lines were combined together as pHAK1:FLN2. The values are means±SE. Significant differences at P < 0.05 are indicated with different letters
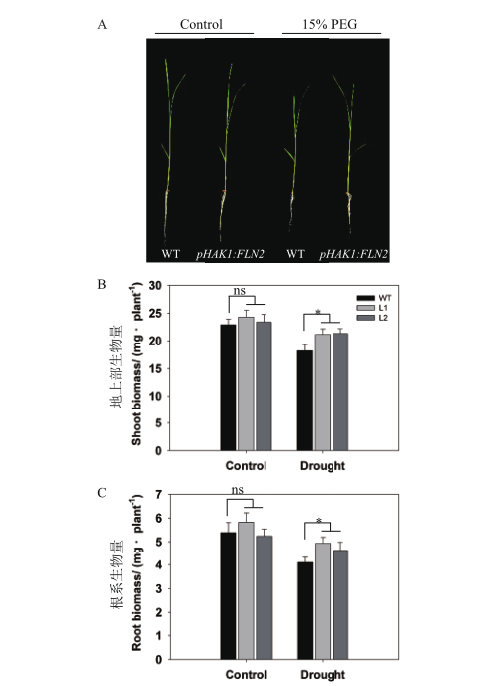
Fig. 4 Seedling growth of pHAK1:FLN2 transgenic lines compared with WT in response to drought stress A:Growth performance of the seedlings under normal and 15% PEG treatment,bar = 5 cm. B-C:Shoot(B)and root(C)biomass(dry weight). The values are means ± SE of 5 replicates. Significant differences between WT and transgenic lines are indicated with asterisks(P < 0.05)and ns indicates non-significant differences at that level of significance. The same below
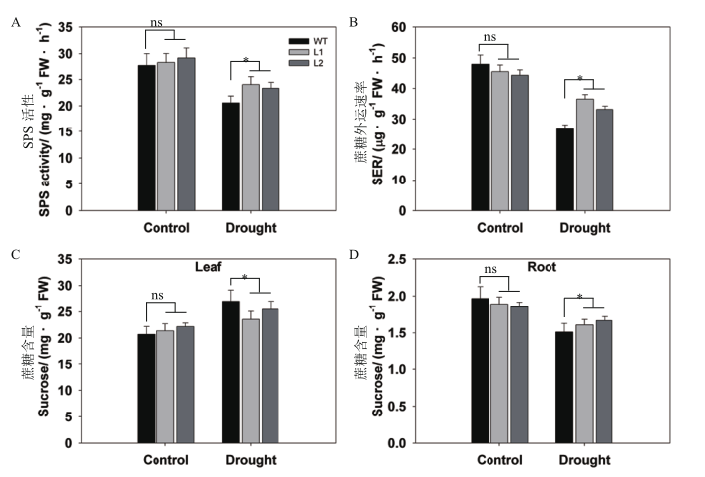
Fig. 5 Differential analysis of sugar metabolism between pHAK1:FLN2 transgenic lines and WT in response to drought stress A:Sucrose phosphate synthase(SPS)activity. B:Rate of sucrose export(SER)from the leaf. C-D:Sucrose contents of the leaf(C)and root(D).The values are means±SE of 5 replicates

Fig. 6 Differential analysis of root system architecture be-tween pHAK1:FLN2 transgenic lines and WT in response to drought stress A:Total root length. B:Root surface area. The values are means ± SE of 5 replicates
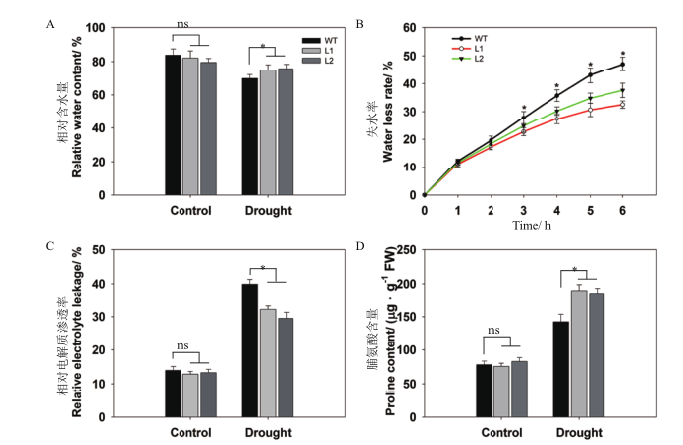
Fig. 7 Differential analysis of water-content ability and lipid peroxidation between pHAK1:FLN2 trans-genic lines and WT in response to drought stress A:Relative water content. B:Water loss rate. C:Relative electrolyte leakage. D:Proline content. The values are means±SE of 5 replicates
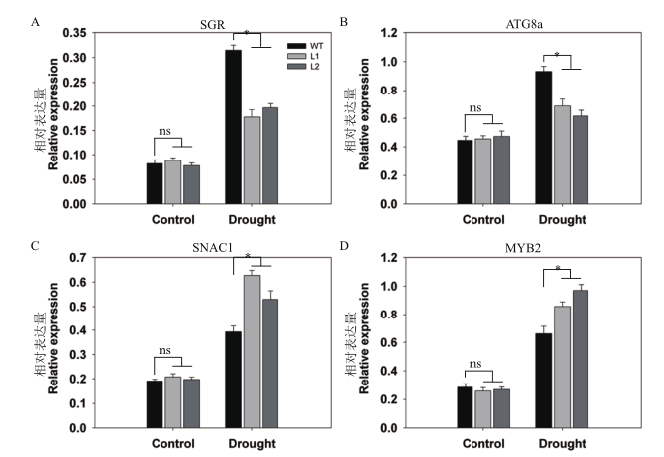
Fig. 8 Differential analysis of expressions of senescence-associated genes and stress-responsive genes between pHAK1:FLN2 transgenic lines and WT in response to drought stress The genes assayed are(A)SGR,(B)ATG8a,(C)SNAC1 and(D)MYB2. UBQ5 is chosen as the reference gene. The values are means±SE of 3 replicates
| [1] |
Khan MIR, Palakolanu SR, Chopra P, et al. Improving drought tolerance in rice:Ensuring food security through multi-dimensional approaches[J]. Physiol Plant, 2021, 172(2):645-668.
doi: 10.1111/ppl.13223 URL |
| [2] |
Ganie SA, Ahammed GJ. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice[J]. Plant Cell Rep, 2021, 40(3):437-459.
doi: 10.1007/s00299-020-02649-2 URL |
| [3] | 张磊, 许泉. 水稻干旱胁迫相关转录因子研究进展[J]. 江西农业学报, 2014, 26(10):5-11. |
| Zhang L, Xu Q. Research progress in transcription factors related to drought tolerance of rice[J]. Acta Agric Jiangxi, 2014, 26(10):5-11. | |
| [4] | Sahebi M, Hanafi MM, Rafii MY, et al. Improvement of drought tolerance in rice(Oryza sativa L.):genetics, genomic tools, and the WRKY gene family[J]. Biomed Res Int, 2018, 2018:3158474. |
| [5] |
Mathan J, Singh A, Ranjan A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice[J]. Physiol Plant, 2021, 171(4):620-637.
doi: 10.1111/ppl.13210 URL |
| [6] |
Verslues PE, Agarwal M, Katiyar-Agarwal S, et al. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status[J]. Plant J, 2006, 45(4):523-539.
pmid: 16441347 |
| [7] |
Zhang C, Li X, He Y, et al. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance[J]. Plant Physiol Biochem, 2017, 115:328-342.
doi: 10.1016/j.plaphy.2017.03.019 URL |
| [8] |
Joshi R, Sahoo KK, Singh AK, et al. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions[J]. J Exp Bot, 2020, 71(2):653-668.
doi: 10.1093/jxb/erz462 URL |
| [9] |
Cui LH, Byun MY, Oh HG, et al. Poaceae type II galactinol synthase 2 from Antarctic flowering plant Deschampsia antarctica and rice improves cold and drought tolerance by accumulation of raffinose family oligosaccharides in transgenic rice plants[J]. Plant Cell Physiol, 2020, 61(1):88-104.
doi: 10.1093/pcp/pcz180 URL |
| [10] |
Chen G, Hu J, Lian J, et al. Functional characterization of OsHAK1 promoter in response to osmotic/drought stress by deletion analysis in transgenic rice[J]. Plant Growth Regul, 2019, 88(3):241-251.
doi: 10.1007/s10725-019-00504-3 URL |
| [11] | Chen G, Hu J, Dong LL, et al. The tolerance of salinity in rice requires the presence of a functional copy of FLN2[J]. Biomolecules, 2019, 10(1):E17. |
| [12] |
Chen G, Feng H, Hu Q, et al. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development[J]. Plant Biotechnol J, 2015, 13(6):833-848.
doi: 10.1111/pbi.12320 URL |
| [13] |
Chen G, Liu C, Gao Z, et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a Japonica rice cultivar[J]. Front Plant Sci, 2017, 8:2216.
doi: 10.3389/fpls.2017.02216 URL |
| [14] |
Chen G, Zhang Y, Ruan B, et al. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism[J]. Plant Sci, 2018, 274:261-270.
doi: 10.1016/j.plantsci.2018.05.034 URL |
| [15] |
Chen G, Hu Q, Luo L, et al. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges[J]. Plant Cell Environ, 2015, 38(12):2747-2765.
doi: 10.1111/pce.12585 URL |
| [16] |
Chen G, Wu C, He L, et al. Knocking out the gene RLS1 induces hypersensitivity to oxidative stress and premature leaf senescence in rice[J]. Int J Mol Sci, 2018, 19(10):2853.
doi: 10.3390/ijms19102853 URL |
| [17] |
Chen G, Liu CL, Gao ZY, et al. Driving the expression of RAA1 with a drought-responsive promoter enhances root growth in rice, its accumulation of potassium and its tolerance to moisture stress[J]. Environ Exp Bot, 2018, 147:147-156.
doi: 10.1016/j.envexpbot.2017.12.008 URL |
| [18] |
Chen G, Liu C, Gao Z, et al. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice[J]. Front Plant Sci, 2017, 8:1885.
doi: 10.3389/fpls.2017.01885 URL |
| [19] |
Gupta AK, Kaur N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants[J]. J Biosci, 2005, 30(5):761-776.
doi: 10.1007/BF02703574 URL |
| [20] |
Lemoine R, La Camera S, Atanassova R, et al. Source-to-sink transport of sugar and regulation by environmental factors[J]. Front Plant Sci, 2013, 4:272.
doi: 10.3389/fpls.2013.00272 pmid: 23898339 |
| [21] | Cui Y, Wang M, Zhou H, et al. OsSGL, a novel DUF1645 domain-containing protein, confers enhanced drought tolerance in transgenic rice and Arabidopsis[J]. Front Plant Sci, 2016, 7:2001. |
| [22] |
Jung H, Chung PJ, Park SH, et al. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance[J]. Plant Biotechnol J, 2017, 15(10):1295-1308.
doi: 10.1111/pbi.12716 URL |
| [23] |
Lee DK, Jung H, Jang G, et al. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance[J]. Plant Physiol, 2016, 172(1):575-588.
doi: 10.1104/pp.16.00379 URL |
| [24] |
Aliche EB, Theeuwen TPJM, Oortwijn M, et al. Carbon partitioning mechanisms in POTATO under drought stress[J]. Plant Physiol Biochem, 2020, 146:211-219.
doi: 10.1016/j.plaphy.2019.11.019 URL |
| [25] | Jiang YJ, Qiu YP, Hu YR, et al. Heterologous expression of AtWRKY57 confers drought tolerance in Oryza sativa[J]. Front Plant Sci, 2016, 7:145. |
| [26] |
Song SY, Chen Y, Zhao MG, et al. A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses[J]. Environ Exp Bot, 2012, 80:1-9.
doi: 10.1016/j.envexpbot.2012.02.001 URL |
| [27] |
Székely G, Abrahám E, Cséplo A, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis[J]. Plant J, 2008, 53(1):11-28.
doi: 10.1111/j.1365-313X.2007.03318.x URL |
| [28] |
Wang Z, Wang Y, Hong X, et al. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1(UAP1)induces early leaf senescence and defence responses in rice[J]. J Exp Bot, 2015, 66(3):973-987.
doi: 10.1093/jxb/eru456 URL |
| [29] |
Xia K, Liu T, Ouyang J, et al. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice(Oryza sativa L.)[J]. DNA Res, 2011, 18(5):363-377.
doi: 10.1093/dnares/dsr024 URL |
| [30] |
Zhou QY, Yu Q, Wang ZQ, et al. Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice[J]. Plant Cell Environ, 2013, 36(8):1476-1489.
doi: 10.1111/pce.12078 URL |
| [31] |
Shen J, Lv B, Luo L, et al. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice[J]. Sci Rep, 2017, 7:40641.
doi: 10.1038/srep40641 URL |
| [32] |
Hu H, Dai M, Yao J, et al. Overexpressing a NAM, ATAF, and CUC(NAC)transcription factor enhances drought resistance and salt tolerance in rice[J]. PNAS, 2006, 103(35):12987-12992.
doi: 10.1073/pnas.0604882103 URL |
| [33] |
Yang A, Dai X, Zhang WH. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice[J]. J Exp Bot, 2012, 63(7):2541-2556.
doi: 10.1093/jxb/err431 pmid: 22301384 |
| [1] | WANG Zi-ying, LONG Chen-jie, FAN Zhao-yu, ZHANG Lei. Screening of OsCRK5-interacted Proteins in Rice Using Yeast Two-hybrid System [J]. Biotechnology Bulletin, 2023, 39(9): 117-125. |
| [2] | WU Yuan-ming, LIN Jia-yi, LIU Yu-xi, LI Dan-ting, ZHANG Zong-qiong, ZHENG Xiao-ming, PANG Hong-bo. Identification of Rice Plant Height-associated QTL Using BSA-seq and RNA-seq [J]. Biotechnology Bulletin, 2023, 39(8): 173-184. |
| [3] | YAO Sha-sha, WANG Jing-jing, WANG Jun-jie, LIANG Wei-hong. Molecular Mechanisms of Rice Grain Size Regulation Related to Plant Hormone Signaling Pathways [J]. Biotechnology Bulletin, 2023, 39(8): 80-90. |
| [4] | LI Yu, LI Su-zhen, CHEN Ru-mei, LU Hai-qiang. Advances in the Regulation of Iron Homeostasis by bHLH Transcription Factors in Plant [J]. Biotechnology Bulletin, 2023, 39(7): 26-36. |
| [5] | LIANG Cheng-gang, WANG Yan, LI Tian, OHSUGI Ryu, AOKI Naohiro. Effect of SP1 on Panicle Architecture by Regulating Carbohydrate Remobilization [J]. Biotechnology Bulletin, 2023, 39(5): 152-159. |
| [6] | LIU Kui, LI Xing-fen, YANG Pei-xin, ZHONG Zhao-chen, CAO Yi-bo, ZHANG Ling-yun. Functional Study and Validation of Transcriptional Coactivator PwMBF1c in Picea wilsonii [J]. Biotechnology Bulletin, 2023, 39(5): 205-216. |
| [7] | ZHOU Ding-ding, LI Hui-hu, TANG Xing-yong, YU Fa-xin, KONG Dan-yu, LIU Yi. Research Progress in the Biosynthesis and Regulation of Glycyrrhizic Acid and Liquiritin [J]. Biotechnology Bulletin, 2023, 39(5): 44-53. |
| [8] | YANG Mao, LIN Yu-feng, DAI Yang-shuo, PAN Su-jun, PENG Wei-ye, YAN Ming-xiong, LI Wei, WANG Bing, DAI Liang-ying. OsDIS1 Negatively Regulates Rice Drought Tolerance Through Antioxidant Pathways [J]. Biotechnology Bulletin, 2023, 39(2): 88-95. |
| [9] | JIANG Min-xuan, LI Kang, LUO Liang, LIU Jian-xiang, LU Hai-ping. Advances on the Expressions of Foreign Proteins in Plants [J]. Biotechnology Bulletin, 2023, 39(11): 110-122. |
| [10] | JIANG Nan, SHI Yang, ZHAO Zhi-hui, LI Bin, ZHAO Yi-hui, YANG Jun-biao, YAN Jia-ming, JIN Yu-fan, CHEN Ji, HUANG Jin. Expression and Functional Analysis of OsPT1 Gene in Rice Under Cadmium Stress [J]. Biotechnology Bulletin, 2023, 39(1): 166-174. |
| [11] | CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. Identification and Gene Functional Analysis of Salinity-hypersensitive Mutant ss2 in Rice [J]. Biotechnology Bulletin, 2022, 38(9): 158-166. |
| [12] | GAO Xiao-rong, DING Yao, LV Jun. Effects of Pseudomonas sp. PR3,a Pyrene-degrading Bacterium with Plant Growth-promoting Properties,on Rice Growth Under Pyrene Stress [J]. Biotechnology Bulletin, 2022, 38(9): 226-236. |
| [13] | HUANG Jing, ZHU Liang, XUE Peng-bo, FU Qiang. Research on Mechanism and QTL Mapping Associated with Cadmium Accumulation in Rice Leaves and Grains [J]. Biotechnology Bulletin, 2022, 38(8): 118-126. |
| [14] | LI Bai, CAI Zhi-jun, WANG Lei, CHEN Jie, CAO Kui-rong, LI Jun, CHONG Gao-jun. Development and Application of the Combinatorial Marker for the Rice Blast Resistance Gene Pigm [J]. Biotechnology Bulletin, 2022, 38(7): 153-159. |
| [15] | SHI Jia, ZHU Xiu-mei, XUE Meng-yu, YU Chao, WEI Yi-ming, YANG Feng-huan, CHEN Hua-min. Optimization and Application of the Chromatin Immunoprecipitation Based on Rice Protoplast [J]. Biotechnology Bulletin, 2022, 38(7): 62-69. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||