








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (12): 261-275.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0501
Previous Articles Next Articles
LIAO Qing-nan1( ), ZHOU Long-jian1,2,3, YANG Zhi-you1,2,3, FENG Yun-kai1, HUANG Yi-jun1, HU Xue-qiong1,3, ZHANG Yi1,2,3(
), ZHOU Long-jian1,2,3, YANG Zhi-you1,2,3, FENG Yun-kai1, HUANG Yi-jun1, HU Xue-qiong1,3, ZHANG Yi1,2,3( ), LIU Ya-yue1,2,3(
), LIU Ya-yue1,2,3( )
)
Received:2023-05-26
Online:2023-12-26
Published:2024-01-11
Contact:
ZHANG Yi, LIU Ya-yue
E-mail:17854225126@163.com;hubeizhangyi@163.com;yayue_liu@163.com
LIAO Qing-nan, ZHOU Long-jian, YANG Zhi-you, FENG Yun-kai, HUANG Yi-jun, HU Xue-qiong, ZHANG Yi, LIU Ya-yue. Studies on Anti-inflammatory Activity and Chemical Diversity of Secondary Metabolites from Symbiotic Fungi in Stony Corals[J]. Biotechnology Bulletin, 2023, 39(12): 261-275.
| 菌株编号Strain No. | 菌属Bacterial genus | 珊瑚来源Coral source |
|---|---|---|
| C1-5 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚Porites pukoensis |
| C3-1cc | - | 牡丹珊瑚Pavona Lamarck |
| C3-3 | 土曲霉Aspergillus terreus | 牡丹珊瑚P. Lamarck |
| C3-4 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C3-5 | 肉座菌属Hypocreaceae sp. | 牡丹珊瑚P. Lamarck |
| C3-7 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C3-8 | 青霉属Penicillium citrinum | 牡丹珊瑚P. Lamarck |
| C3-9 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C14-2B | - | 丛生盔形珊瑚Galaxea fascicularis |
| C14-2 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-5 | 镰刀霉属Fusarium sp. | 丛生盔形珊瑚G. fascicularis |
| C14-7 | 座囊霉菌属Dothideales sp. | 丛生盔形珊瑚G. fascicularis |
| C14-8 | Curreya austroafricana | 丛生盔形珊瑚G. fascicularis |
| C14-9 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-10 | 透孢黑团壳Massarina sp. | 丛生盔形珊瑚G. fascicularis |
| C14-12 | 赤散囊菌Eurotium rubrum | 丛生盔形珊瑚G. fascicularis |
| C14-13 | 节孢霉属Arthrinium sp. | 丛生盔形珊瑚G. fascicularis |
| C14-17 | 葡萄穗霉属Stachybotrys sp. | 丛生盔形珊瑚G. fascicularis |
| C14-18 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-19 | 葡萄穗霉属Stachybotrys sp. | 丛生盔形珊瑚G. fascicularis |
| C21-1 | 青霉属P. citrinum | 普哥滨珊瑚Porites pukoensis |
| C21-2 | 土曲霉A. terreus | 普哥滨珊瑚P. pukoensis |
| C21-5 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚P. pukoensis |
| C21-6 | 青霉属P. citrinum | 普哥滨珊瑚P. pukoensis |
| C21-8 | 枝顶孢属Acremonium persicinum | 普哥滨珊瑚P. pukoensis |
| C21-11 | 土曲霉A. terreus | 普哥滨珊瑚P. pukoensis |
| C21-13 | 赤霉属Gibberella sp. | 普哥滨珊瑚P. pukoensis |
| C21-17 | 蕉孢壳属Eutypella sp. | 普哥滨珊瑚P. pukoensis |
| C21-18 | - | 普哥滨珊瑚P. pukoensis |
| C21-19 | 弯孢属Curvularia sp. | 普哥滨珊瑚P. pukoensis |
| C21-20 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚P. pukoensis |
Table 1 31 coral symbiotic fungal genera and coral sources
| 菌株编号Strain No. | 菌属Bacterial genus | 珊瑚来源Coral source |
|---|---|---|
| C1-5 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚Porites pukoensis |
| C3-1cc | - | 牡丹珊瑚Pavona Lamarck |
| C3-3 | 土曲霉Aspergillus terreus | 牡丹珊瑚P. Lamarck |
| C3-4 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C3-5 | 肉座菌属Hypocreaceae sp. | 牡丹珊瑚P. Lamarck |
| C3-7 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C3-8 | 青霉属Penicillium citrinum | 牡丹珊瑚P. Lamarck |
| C3-9 | 土曲霉A. terreus | 牡丹珊瑚P. Lamarck |
| C14-2B | - | 丛生盔形珊瑚Galaxea fascicularis |
| C14-2 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-5 | 镰刀霉属Fusarium sp. | 丛生盔形珊瑚G. fascicularis |
| C14-7 | 座囊霉菌属Dothideales sp. | 丛生盔形珊瑚G. fascicularis |
| C14-8 | Curreya austroafricana | 丛生盔形珊瑚G. fascicularis |
| C14-9 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-10 | 透孢黑团壳Massarina sp. | 丛生盔形珊瑚G. fascicularis |
| C14-12 | 赤散囊菌Eurotium rubrum | 丛生盔形珊瑚G. fascicularis |
| C14-13 | 节孢霉属Arthrinium sp. | 丛生盔形珊瑚G. fascicularis |
| C14-17 | 葡萄穗霉属Stachybotrys sp. | 丛生盔形珊瑚G. fascicularis |
| C14-18 | 土曲霉A. terreus | 丛生盔形珊瑚G. fascicularis |
| C14-19 | 葡萄穗霉属Stachybotrys sp. | 丛生盔形珊瑚G. fascicularis |
| C21-1 | 青霉属P. citrinum | 普哥滨珊瑚Porites pukoensis |
| C21-2 | 土曲霉A. terreus | 普哥滨珊瑚P. pukoensis |
| C21-5 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚P. pukoensis |
| C21-6 | 青霉属P. citrinum | 普哥滨珊瑚P. pukoensis |
| C21-8 | 枝顶孢属Acremonium persicinum | 普哥滨珊瑚P. pukoensis |
| C21-11 | 土曲霉A. terreus | 普哥滨珊瑚P. pukoensis |
| C21-13 | 赤霉属Gibberella sp. | 普哥滨珊瑚P. pukoensis |
| C21-17 | 蕉孢壳属Eutypella sp. | 普哥滨珊瑚P. pukoensis |
| C21-18 | - | 普哥滨珊瑚P. pukoensis |
| C21-19 | 弯孢属Curvularia sp. | 普哥滨珊瑚P. pukoensis |
| C21-20 | 旋孢腔菌属Cochliobolus sp. | 普哥滨珊瑚P. pukoensis |
| 粗提物编号/浓度 Crude extract No./ Concentration/(µg·mL-1) | 盐度为0.3% Salinity of 0.3% | 盐度为3% Salinity of 3% | 盐度为10% Salinity of 10% | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | ||||
| C1-5 | - | - | - | - | - | - | - | - | *** | **** | - | - | **** | **** | **** | |||
| C3-1cc | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | - | |||
| C3-3 | - | - | - | - | - | - | - | - | - | - | - | - | ** | - | - | |||
| C3-4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C3-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | * | |||
| C3-7 | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | - | |||
| C3-8 | - | - | - | - | - | - | - | - | * | - | - | - | - | - | *** | |||
| C3-9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-2B | - | - | - | - | - | - | - | - | - | - | - | - | - | *** | - | |||
| C14-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-7 | - | - | - | - | - | - | - | - | **** | **** | ** | - | - | * | - | |||
| C14-8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | * | |||
| C14-9 | - | - | - | - | - | - | - | - | **** | **** | - | - | - | - | ** | |||
| C14-10 | - | - | - | - | - | - | - | - | **** | **** | - | - | - | **** | **** | |||
| C14-12 | - | - | - | - | - | - | * | ** | *** | **** | / | / | / | / | / | |||
| C14-13 | - | - | ** | **** | **** | - | - | - | - | - | - | - | - | - | - | |||
| C14-16 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-17 | - | - | - | - | - | - | - | - | - | - | - | - | - | *** | **** | |||
| C14-18 | - | - | **** | **** | **** | - | - | - | - | - | - | - | - | - | - | |||
| C14-19 | - | - | - | - | - | - | * | - | - | - | - | - | **** | **** | - | |||
| C21-1 | - | - | - | - | - | - | - | - | * | **** | - | *** | **** | - | - | |||
| C21-2 | - | - | - | - | - | - | - | - | - | - | - | **** | - | - | - | |||
| C21-6 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-11 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-13 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-17 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-18 | - | - | - | - | - | - | * | *** | - | - | - | - | ** | *** | **** | |||
| C21-19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | |||
| C21-20 | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | **** | |||
Table 2 Anti-inflammatory activities of crude extracts extracted from different strains under PDB medium conditions
| 粗提物编号/浓度 Crude extract No./ Concentration/(µg·mL-1) | 盐度为0.3% Salinity of 0.3% | 盐度为3% Salinity of 3% | 盐度为10% Salinity of 10% | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | ||||
| C1-5 | - | - | - | - | - | - | - | - | *** | **** | - | - | **** | **** | **** | |||
| C3-1cc | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | - | |||
| C3-3 | - | - | - | - | - | - | - | - | - | - | - | - | ** | - | - | |||
| C3-4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C3-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | * | |||
| C3-7 | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | - | |||
| C3-8 | - | - | - | - | - | - | - | - | * | - | - | - | - | - | *** | |||
| C3-9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-2B | - | - | - | - | - | - | - | - | - | - | - | - | - | *** | - | |||
| C14-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-7 | - | - | - | - | - | - | - | - | **** | **** | ** | - | - | * | - | |||
| C14-8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | * | |||
| C14-9 | - | - | - | - | - | - | - | - | **** | **** | - | - | - | - | ** | |||
| C14-10 | - | - | - | - | - | - | - | - | **** | **** | - | - | - | **** | **** | |||
| C14-12 | - | - | - | - | - | - | * | ** | *** | **** | / | / | / | / | / | |||
| C14-13 | - | - | ** | **** | **** | - | - | - | - | - | - | - | - | - | - | |||
| C14-16 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-17 | - | - | - | - | - | - | - | - | - | - | - | - | - | *** | **** | |||
| C14-18 | - | - | **** | **** | **** | - | - | - | - | - | - | - | - | - | - | |||
| C14-19 | - | - | - | - | - | - | * | - | - | - | - | - | **** | **** | - | |||
| C21-1 | - | - | - | - | - | - | - | - | * | **** | - | *** | **** | - | - | |||
| C21-2 | - | - | - | - | - | - | - | - | - | - | - | **** | - | - | - | |||
| C21-6 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-11 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-13 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-17 | - | - | - | - | - | - | - | - | - | - | / | / | / | / | / | |||
| C21-18 | - | - | - | - | - | - | * | *** | - | - | - | - | ** | *** | **** | |||
| C21-19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | |||
| C21-20 | - | - | - | - | - | - | - | - | - | - | - | - | - | **** | **** | |||
| 粗提物编号/浓度 Crude extract No./ Concentration/(µg·mL-1) | 盐度为0.3% Salinity of 0.3% | 盐度为3% Salinity of 3% | 盐度为10% Salinity of 10% | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | ||||
| C1-5 | - | - | ** | **** | **** | - | - | ** | - | - | - | - | * | *** | * | |||
| C3-1cc | - | - | - | *** | - | - | - | - | ** | - | - | - | - | **** | - | |||
| C3-3 | - | - | - | **** | **** | - | - | - | **** | **** | - | - | ** | **** | **** | |||
| C3-4 | - | - | - | - | - | - | ** | - | - | - | - | ** | - | - | - | |||
| C3-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C3-7 | - | - | * | **** | **** | * | - | - | - | - | - | - | - | - | - | |||
| C3-8 | - | - | - | - | - | - | **** | **** | **** | - | - | *** | **** | **** | - | |||
| C3-9 | - | **** | **** | - | - | * | *** | **** | **** | - | - | - | - | *** | **** | |||
| C14-2 | - | - | - | - | - | - | - | *** | **** | - | / | / | / | / | / | |||
| C14-2B | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-5 | - | **** | **** | **** | - | - | - | - | - | - | - | - | ** | - | **** | |||
| C14-7 | / | / | / | / | / | - | - | - | - | - | - | - | - | - | - | |||
| C14-8 | - | - | - | - | - | - | * | **** | **** | **** | - | - | - | **** | - | |||
| C14-9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-10 | - | - | - | - | - | - | - | *** | **** | - | - | - | - | - | - | |||
| C14-12 | - | - | * | **** | ** | * | - | - | - | - | - | - | - | - | - | |||
| C14-13 | - | - | - | * | - | - | - | - | - | - | - | - | **** | **** | **** | |||
| C14-16 | - | * | - | - | - | - | - | - | - | - | * | ** | **** | **** | - | |||
| C14-17 | - | *** | - | - | - | - | - | - | **** | - | - | * | *** | **** | ** | |||
| C14-18 | - | * | ** | - | - | - | ** | - | - | - | - | - | - | - | - | |||
| C14-19 | - | * | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-1 | - | - | *** | - | - | - | - | - | - | - | - | - | - | **** | **** | |||
| C21-2 | - | - | **** | - | - | - | - | - | - | - | - | * | **** | **** | - | |||
| C21-6 | - | - | - | - | - | * | - | - | - | - | - | - | - | - | *** | |||
| C21-8 | ** | - | - | - | - | - | - | - | *** | **** | - | - | - | - | - | |||
| C21-11 | - | **** | - | - | - | - | * | - | - | - | - | - | - | **** | - | |||
| C21-13 | - | - | - | - | - | ** | - | - | - | - | - | - | *** | - | - | |||
| C21-17 | - | - | * | - | - | - | - | **** | **** | - | * | - | - | **** | - | |||
| C21-18 | - | - | - | - | - | - | - | * | **** | - | - | - | - | - | **** | |||
| C21-19 | - | - | - | ** | - | - | - | - | - | - | - | - | - | **** | ** | |||
| C21-20 | - | - | - | - | - | - | - | - | - | - | - | - | - | * | **** | |||
Table 3 Anti-inflammatory activity of crude extracts obtained from different strains under brown rice medium conditions
| 粗提物编号/浓度 Crude extract No./ Concentration/(µg·mL-1) | 盐度为0.3% Salinity of 0.3% | 盐度为3% Salinity of 3% | 盐度为10% Salinity of 10% | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | 1 | 10 | 20 | 50 | 100 | ||||
| C1-5 | - | - | ** | **** | **** | - | - | ** | - | - | - | - | * | *** | * | |||
| C3-1cc | - | - | - | *** | - | - | - | - | ** | - | - | - | - | **** | - | |||
| C3-3 | - | - | - | **** | **** | - | - | - | **** | **** | - | - | ** | **** | **** | |||
| C3-4 | - | - | - | - | - | - | ** | - | - | - | - | ** | - | - | - | |||
| C3-5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C3-7 | - | - | * | **** | **** | * | - | - | - | - | - | - | - | - | - | |||
| C3-8 | - | - | - | - | - | - | **** | **** | **** | - | - | *** | **** | **** | - | |||
| C3-9 | - | **** | **** | - | - | * | *** | **** | **** | - | - | - | - | *** | **** | |||
| C14-2 | - | - | - | - | - | - | - | *** | **** | - | / | / | / | / | / | |||
| C14-2B | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-5 | - | **** | **** | **** | - | - | - | - | - | - | - | - | ** | - | **** | |||
| C14-7 | / | / | / | / | / | - | - | - | - | - | - | - | - | - | - | |||
| C14-8 | - | - | - | - | - | - | * | **** | **** | **** | - | - | - | **** | - | |||
| C14-9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C14-10 | - | - | - | - | - | - | - | *** | **** | - | - | - | - | - | - | |||
| C14-12 | - | - | * | **** | ** | * | - | - | - | - | - | - | - | - | - | |||
| C14-13 | - | - | - | * | - | - | - | - | - | - | - | - | **** | **** | **** | |||
| C14-16 | - | * | - | - | - | - | - | - | - | - | * | ** | **** | **** | - | |||
| C14-17 | - | *** | - | - | - | - | - | - | **** | - | - | * | *** | **** | ** | |||
| C14-18 | - | * | ** | - | - | - | ** | - | - | - | - | - | - | - | - | |||
| C14-19 | - | * | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| C21-1 | - | - | *** | - | - | - | - | - | - | - | - | - | - | **** | **** | |||
| C21-2 | - | - | **** | - | - | - | - | - | - | - | - | * | **** | **** | - | |||
| C21-6 | - | - | - | - | - | * | - | - | - | - | - | - | - | - | *** | |||
| C21-8 | ** | - | - | - | - | - | - | - | *** | **** | - | - | - | - | - | |||
| C21-11 | - | **** | - | - | - | - | * | - | - | - | - | - | - | **** | - | |||
| C21-13 | - | - | - | - | - | ** | - | - | - | - | - | - | *** | - | - | |||
| C21-17 | - | - | * | - | - | - | - | **** | **** | - | * | - | - | **** | - | |||
| C21-18 | - | - | - | - | - | - | - | * | **** | - | - | - | - | - | **** | |||
| C21-19 | - | - | - | ** | - | - | - | - | - | - | - | - | - | **** | ** | |||
| C21-20 | - | - | - | - | - | - | - | - | - | - | - | - | - | * | **** | |||
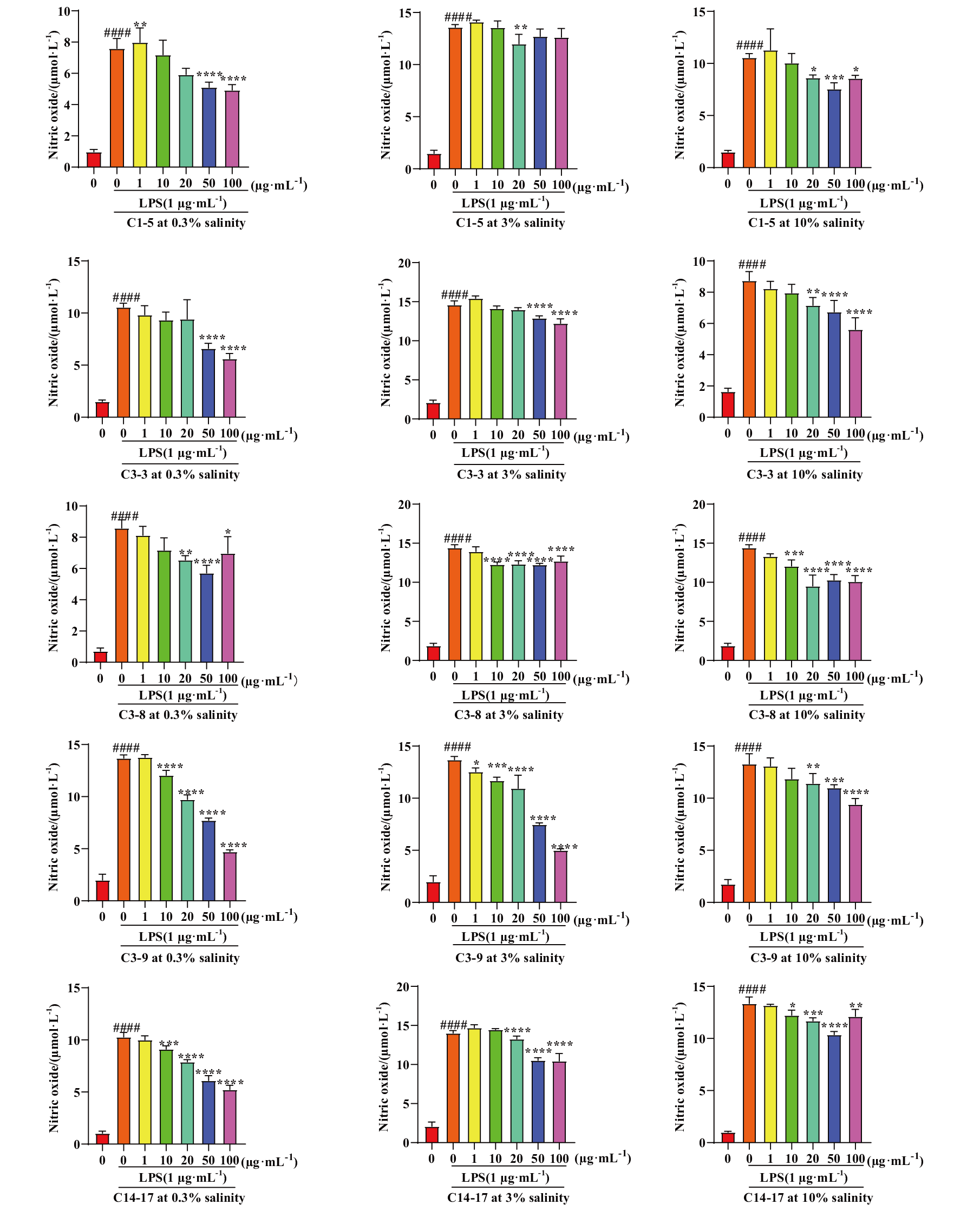
Fig. 1 Effects of crude extracts from five strains of symbiotic fungi on NO production in BV-2 cells under brown rice medium conditions “#” indicates a significant stimulatory effect of LPS on cells compared to the control group, ####: P<0.000 1; “*” indicates that the crude extract of the strain is significantly different at that concentration, *: P<0.05, ****: P<0.000 1. The same below

Fig. 4 TLC fingerprinting and iron trichloride-potassium ferricyanide color development of the crude extracts from five strains A, B, C, D and E indicate strains C1-5, C3-3, C3-8, C3-9 and C14-17, respectively. 1, 2 and 3 are the 254 nm UV image, 365 nm fluorescence image and iron trichloride-potassium ferricyanide color development image of each crude extract, respectively; and 1-9 below indicate 0.3% salinity PDB medium ethyl acetate extract, 0.3% salinity PDB medium methanolic extract, 3% salinity PDB medium ethyl acetate extract, 3% salinity PDB medium methanolic extract, 10% salinity PDB medium ethyl acetate extract, 10% salinity PDB medium methanolic extract, 0.3% salinity brown rice medium methanolic extract, 3% salinity brown rice medium methanolic extract and 10% salinity brown rice medium methanolic,respectively
| 峰编号 Peak No. | 化合物名称 Compound name | 准分子离子 Quasi-molecular ion | 特征碎片离子 Characteristic fragment ions | 分子式 Molecular formula | 保留时间 Retention time /min | 生物来源 Biological origin | 峰面积比(3%盐度比10%盐度) Peak area ratio(3% salinity vs 10% salinity) |
|---|---|---|---|---|---|---|---|
| 1 | Cordycepin | m/z 274[M+Na]+ | m/z 136 | C10H13N5O3 | 3.143 | Plant, Fungi | 17.610 |
| 2 | Visnagin | m/z 231[M+H]+ | m/z 216, 160, 176 | C13H10O4 | 3.146 | Plant | 13.837 |
| 3 | Thymol | m/z 173[M+Na]+ | m/z 89, 110, 149 | C10H14O | 3.216 | Plant | 12.373 |
| 4 | 6-Methoxyflavone | m/z 275[M+Na]+ | m/z 238, 210 | C16H12O3 | 3.888 | Plant | 11.589 |
| 5 | Periplogenin | m/z 391[M+H]+ | m/z 43, 55 | C23H34O5 | 4.439 | Plant | 11.486 |
| 6 | Spathulenol | m/z 243[M+Na]+ | m/z 202, 205, 43 | C15H24O | 5.196 | Essential oil | 11.444 |
| 7 | Ectoine | m/z 143[M+H]+ | m/z 143, 97, 101 | C6H10N2O2 | 5.201 | Fungi | 11.330 |
| 8 | Gallocatechin | m/z 307[M+H]+ | m/z 125, 137, 109 | C15H14O7 | 5.991 | Plant | 11.165 |
Table 4 Database mining of strain C3-9 base peak chromatograms with differential primary peaks in yield at 3% and 10% salinity and the multiplicity of changes in their peak areas
| 峰编号 Peak No. | 化合物名称 Compound name | 准分子离子 Quasi-molecular ion | 特征碎片离子 Characteristic fragment ions | 分子式 Molecular formula | 保留时间 Retention time /min | 生物来源 Biological origin | 峰面积比(3%盐度比10%盐度) Peak area ratio(3% salinity vs 10% salinity) |
|---|---|---|---|---|---|---|---|
| 1 | Cordycepin | m/z 274[M+Na]+ | m/z 136 | C10H13N5O3 | 3.143 | Plant, Fungi | 17.610 |
| 2 | Visnagin | m/z 231[M+H]+ | m/z 216, 160, 176 | C13H10O4 | 3.146 | Plant | 13.837 |
| 3 | Thymol | m/z 173[M+Na]+ | m/z 89, 110, 149 | C10H14O | 3.216 | Plant | 12.373 |
| 4 | 6-Methoxyflavone | m/z 275[M+Na]+ | m/z 238, 210 | C16H12O3 | 3.888 | Plant | 11.589 |
| 5 | Periplogenin | m/z 391[M+H]+ | m/z 43, 55 | C23H34O5 | 4.439 | Plant | 11.486 |
| 6 | Spathulenol | m/z 243[M+Na]+ | m/z 202, 205, 43 | C15H24O | 5.196 | Essential oil | 11.444 |
| 7 | Ectoine | m/z 143[M+H]+ | m/z 143, 97, 101 | C6H10N2O2 | 5.201 | Fungi | 11.330 |
| 8 | Gallocatechin | m/z 307[M+H]+ | m/z 125, 137, 109 | C15H14O7 | 5.991 | Plant | 11.165 |

Fig. 6 Local magnification of metabolites of strain C3-9 in 0.3% and 10% salinity brown rice medium based on secondary mass spectrometric correlation of molecular network Each node indicates the average mass with the same molecular ionic mass, and different colored cross sections in the nodes indicate different samples, indicating brown rice medium at 3% salinity and brown rice medium at 10% salinity, respectively
| [1] |
Recio MC, Andujar I, Rios JL. Anti-inflammatory agents from plants: progress and potential[J]. Curr Med Chem, 2012, 19(14): 2088-2103.
pmid: 22414101 |
| [2] |
Rubió L, Motilva MJ, Romero MP. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles[J]. Crit Rev Food Sci Nutr, 2013, 53(9): 943-953.
doi: 10.1080/10408398.2011.574802 pmid: 23768186 |
| [3] |
Huggett MJ, Apprill A. Coral microbiome database: integration of sequences reveals high diversity and relatedness of coral-associated microbes[J]. Environ Microbiol Rep, 2019, 11(3): 372-385.
doi: 10.1111/emi4.2019.11.issue-3 URL |
| [4] |
Sang VT, Dat TTH, Vinh LB, et al. Coral and coral-associated microorganisms: a prolific source of potential bioactive natural products[J]. Mar Drugs, 2019, 17(8): 468.
doi: 10.3390/md17080468 URL |
| [5] |
Raimundo I, Silva SG, Costa R, et al. Bioactive secondary metabolites from octocoral-associated microbes-new chances for blue growth[J]. Mar Drugs, 2018, 16(12): 485.
doi: 10.3390/md16120485 URL |
| [6] | 李金凤, 姚励功, 曾艳波, 等. 西沙乳白肉芝软珊瑚中的化学成分及其抗炎活性研究[J]. 药学学报, 2022, 57(3): 741-749. |
| Li JF, Yao LG, Zeng YB, et al. Chemical constituents of soft coral Sarcophyton glaucum collected from Xisha and their anti-inflammatory activity[J]. Acta Pharm Sin, 2022, 57(3): 741-749. | |
| [7] | 李旺盛, 高天润, 章海燕, 等. 西瑁岛软珊瑚Dendronephthya sp. 的化学成分和生物活性研究[J]. 中国海洋药物, 2022, 41(2): 7-13. |
| Li WS, Gao TR, Zhang HY, et al. Chemical constituents and bioactivities of soft coral Dendronephthya sp. from Ximao Island[J]. Chin J Mar Drugs, 2022, 41(2): 7-13. | |
| [8] |
Liu MT, Zhou Q, Wang JP, et al. Anti-inflammatory butenolide derivatives from the coral-derived fungus Aspergillus terreus and structure revisions of aspernolides D and G, butyrolactone VI and 4’, 8’’-diacetoxy butyrolactone VI[J]. RSC Adv, 2018, 8(23): 13040-13047.
doi: 10.1039/C8RA01840E URL |
| [9] |
Liu ZM, Qiu P, Liu HJ, et al. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: in vitro approaches and molecular-modeling[J]. Bioorg Chem, 2019, 88: 102973.
doi: 10.1016/j.bioorg.2019.102973 URL |
| [10] |
Shen HJ, Liu XW, Jiang MH, et al. Anti-inflammatory cembrane-type diterpenoids and prostaglandins from soft coral Lobophytum sarcophytoides[J]. Mar Drugs, 2019, 17(8): 481.
doi: 10.3390/md17080481 URL |
| [11] | 朱美林, 王皓天. OSMAC方法在微生物次级代谢产物研究中的应用[J]. 科学技术创新, 2020(26): 82-83. |
| Zhu ML, Wang HT. Application of OSMAC method in the study of microbial secondary metabolites[J]. Sci Technol Innov, 2020(26): 82-83. | |
| [12] | 周德勇, 胡少伟, 邱俊娜, 等. 天然产物中具有抗炎活性的酚类成分[C]// 中华中医药学会第十二届中药化学学术年会论文集. 2017: 196-200. |
| Zhou DY, Hu SW, Qiu JN, et al. Phenolic components of natural products with anti-inflammatory activity[C]// Proceedings of the 12th Annual Conference of Chinese Medicine Chemistry, China Association of Traditional Chinese Medicine. 2017: 196-200. | |
| [13] | 孟明民, 徐鹏舒, 李如雯, 等. 利用颜色反应鉴别儿茶酚及其保护类型[J/OL]. 热带生物学报, 2023. https://kns.cnki.net/kcms/detail/46.1078.S.20230409.1106.002.html. |
| Meng MM, Xu PS, Li RW, et al. Identification of catechols and their protective types using color reaction[J/OL]. Journal of Tropical Biology, 2023. https://kns.cnki.net/kcms/detail/46.1078.S.20230409.1106.002.html. | |
| [14] | Phelan VV. Feature-based molecular networking for metabolite annotation[M]// Computational Methods and Data Analysis for Metabolomics. New York: Humana, 2020: 227-243. |
| [15] | 许佳怡, 宋双. OSMAC策略在海洋天然产物研究中的应用综述[J]. 中山大学研究生学刊: 自然科学与医学版, 2016(2): 39-47. |
| Xu JY, Song S. OSMAC strategy in the research of marine-derived nature compounds[J]. Graduate Journal of Sun Yat-sen University: Natural Science & Medicine, 2016(2): 39-47. | |
| [16] | 刘俊慧. 基于OSMAC策略的海洋微生物次级代谢产物研究[D]. 大连: 大连理工大学, 2015. |
| Liu JH. The study of secondary metabolites from marine microorganism based on the strategy of OSMAC[D]. Dalian: Dalian University of Technology, 2015. | |
| [17] | 谢绵测, 李先国, 张大海. OSMAC策略及其在烟曲霉菌次级代谢产物研究中的应用[J]. 天然产物研究与开发, 2015, 27(9): 1668-1673. |
| Xie MC, Li XG, Zhang DH. A mini review of OSMAC approach and its application in Aspergillus fumigatus secondary metabolites[J]. Nat Prod Res Dev, 2015, 27(9): 1668-1673. | |
| [18] | 彭艾, 左满, 王乾军, 等. OSMAC策略指导下深海真菌Stachybotrys sp. 3A00409次级代谢产物的研究[J]. 山东化工, 2022, 51(3): 19-21, 24. |
| Peng A, Zuo M, Wang QJ, et al. Studies on secondary metabolites of the deep-sea fungus Stachybotrys sp. 3A00409 under the guidance of OSMAC strategy[J]. Shandong Chem Ind, 2022, 51(3): 19-21, 24. | |
| [19] |
Si YY, Tang MX, Lin S, et al. Cytotoxic cytochalasans from Aspergillus flavipes PJ03-11 by OSMAC method[J]. Tetrahedron Lett, 2018, 59(18): 1767-1771.
doi: 10.1016/j.tetlet.2018.03.077 URL |
| [20] |
da Silva Lima G, da Rocha AM, dos Santos GF, et al. Metabolic response of Aspergillus sydowii to OSMAC modulation produces acetylcholinesterase inhibitors[J]. Phytochem Lett, 2018, 24: 39-45.
doi: 10.1016/j.phytol.2018.01.007 URL |
| [21] | 赵意平. 十二碳二元酸生产菌培养基碳源与氮源的优化[J]. 化学工业与工程技术, 2014, 35(3): 66-69. |
| Zhao YP. Optimization ofcarbon sources and nitrogen sources for medium of bacterium producing dodecanedioic acid[J]. J Chem Ind Eng, 2014, 35(3): 66-69. | |
| [22] |
Cheng TF, Zhang YH, Ye J, et al. Investigation of the chemical compounds in Pheretima aspergillum(E. Perrier)using a combination of mass spectral molecular networking and unsupervised substructure annotation topic modeling together with in silico fragmentation prediction[J]. J Pharm Biomed Anal, 2020, 184: 113197.
doi: 10.1016/j.jpba.2020.113197 URL |
| [23] |
de Oliveira GG, Carnevale Neto F, Demarque DP, et al. Dereplication of flavonoid glycoconjugates from Adenocalymma imperatoris-maximilianii by untargeted tandem mass spectrometry-based molecular networking[J]. Planta Med, 2017, 83(7): 636-646.
doi: 10.1055/s-0042-118712 pmid: 27806406 |
| [24] |
Rivera-Mondragón A, Tuenter E, Ortiz O, et al. UPLC-MS/MS-based molecular networking and NMR structural determination for the untargeted phytochemical characterization of the fruit of Crescentia cujete(Bignoniaceae)[J]. Phytochemistry, 2020, 177: 112438.
doi: 10.1016/j.phytochem.2020.112438 pmid: 32619738 |
| [25] |
马小翔, 刘亚月, 聂影影, 等. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-1398 |
| Ma XX, Liu YY, Nie YY, et al. LC-MS/MS based molecular network analysis of the effects of chemical regulation on the secondary metabolites and biological activities of a fungal strain Aspergillus terreus C23-3[J]. Biotechnol Bull, 2021, 37(8): 95-110. | |
| [26] |
Wang XH, Serrano R, González-Menéndez V, et al. A molecular networking based discovery of diketopiperazine heterodimers and aspergillicins from Aspergillus caelatus[J]. J Nat Prod, 2022, 85(1): 25-33.
doi: 10.1021/acs.jnatprod.1c00526 URL |
| [27] |
Freire VF, Gubiani JR, Spencer TM, et al. Feature-based molecular networking discovery of bromopyrrole alkaloids from the marine sponge Agelas dispar[J]. J Nat Prod, 2022, 85(5): 1340-1350.
doi: 10.1021/acs.jnatprod.2c00094 pmid: 35427139 |
| [28] | Nothias LF, Petras D, Schmid R, et al. Feature-based molecular networking in the GNPS analysis environment[J]. Nat Methods, 2020, 17(9): 905-908. |
| [29] |
Tan L, Song X, Ren YL, et al. Anti-inflammatory effects of cordycepin: a review[J]. Phytother Res, 2021, 35(3): 1284-1297.
doi: 10.1002/ptr.v35.3 URL |
| [30] |
Lee JK, Jung JS, Park SH, et al. Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells[J]. Arch Pharm Res, 2010, 33(11): 1843-1850.
doi: 10.1007/s12272-010-1117-1 URL |
| [31] |
Wang LM, Yang H, Yan HJ, et al. Thymol protects against Aspergillus fumigatus keratitis by inhibiting the LOX-1/IL-1β signaling pathway[J]. Curr Med Sci, 2022, 42(3): 620-628.
doi: 10.1007/s11596-022-2512-9 |
| [32] |
Chen WF, Shih YH, Liu HC, et al. 6-methoxyflavone suppresses neuroinflammation in lipopolysaccharide- stimulated microglia through the inhibition of TLR4/MyD88/p38 MAPK/NF-κB dependent pathways and the activation of HO-1/NQO-1 signaling[J]. Phytomedicine, 2022, 99: 154025.
doi: 10.1016/j.phymed.2022.154025 URL |
| [33] |
Zhang WJ, Song ZB, Bao YL, et al. Periplogenin induces necroptotic cell death through oxidative stress in HaCaT cells and ameliorates skin lesions in the TPA- and IMQ-induced psoriasis-like mouse models[J]. Biochem Pharmacol, 2016, 105: 66-79.
doi: 10.1016/j.bcp.2016.02.001 URL |
| [34] |
do Nascimento KF, Moreira FMF, Alencar Santos J, et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol[J]. J Ethnopharmacol, 2018, 210: 351-358.
doi: S0378-8741(17)31747-6 pmid: 28844678 |
| [35] | Bilstein A, Heinrich A, Rybachuk A, et al. Ectoine in the treatment of irritations and inflammations of the eye surface[J]. Biomed Res Int, 2021: 8885032. |
| [36] |
Siebert DA, Paganelli CJ, Queiroz GS, et al. Anti-inflammatory activity of the epicuticular wax and its isolated compounds catechin and gallocatechin from Eugenia brasiliensis Lam.(Myrtaceae)leaves[J]. Nat Prod Res, 2021, 35(22): 4720-4723.
doi: 10.1080/14786419.2019.1710707 URL |
| [1] | ZHANG Sheng-liang, CHU Xiao-xiao, ZHAO You-xing, KONG Fan-dong, HUANG Xiao-long. Isolation,Identification,and Antibacterial Activity of Fungi Associated with Marine Organisms [J]. Biotechnology Bulletin, 2019, 35(3): 59-64. |
| [2] | LUO Guo-cong, HUANG Yun-yi, CHAI Hui-zi, LEI Xiao-ling, NIE Fang-hong. Morphological Identification and Antibacterial Activity on Metabolites of Fungi Isolated from Ascidean [J]. Biotechnology Bulletin, 2018, 34(9): 244-248. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||