








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (5): 103-111.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1106
Previous Articles Next Articles
WANG Ling-ling1( ), MA Ai-hong1, SONG Qian-jin2, WANG Xiao-long2, CAO Xiao-zhen2, LIU Zhi-feng2, CHEN Ya-fei2, LI Ji-dong1(
), MA Ai-hong1, SONG Qian-jin2, WANG Xiao-long2, CAO Xiao-zhen2, LIU Zhi-feng2, CHEN Ya-fei2, LI Ji-dong1( )
)
Received:2023-11-23
Online:2024-05-26
Published:2024-06-13
Contact:
LI Ji-dong
E-mail:1752448665@qq.com;lijidongi@foxmail.com
WANG Ling-ling, MA Ai-hong, SONG Qian-jin, WANG Xiao-long, CAO Xiao-zhen, LIU Zhi-feng, CHEN Ya-fei, LI Ji-dong. Establishment and Application of Dual RPA-LFD Detection Method for Capripox Virus and Orf Virus[J]. Biotechnology Bulletin, 2024, 40(5): 103-111.
| 引物 Primer | 上游和下游引物 Upstream and downstream primers | 引物序列 Primer sequence(5'-3') | 目标长度 Target length/bp |
|---|---|---|---|
| CaPV-PCR | F1 | GGTCGCGAAATTTCAGATGTAG | 503 |
| R1 | TCATATCCCCCTGTGTACGAAT | ||
| ORFV-PCR | F1 | TCCACTATCAAGAACCTCGGGC | 709 |
| R1 | TTATTGGCTTGCAGAACTCCGA | ||
| CaPV-RPA | F1 | TTATATGGGAAAAGGTAGAAAAATCAGGAGG | 287 |
| R1 | CTATTATGAGAAGTTTCACGTAATTGGAAAATG | ||
| CaPV-RPA | F2 | CAAAACACTTTAGTTTATGGAAATCGTATG | 184 |
| R2 | CTATTATGAGAAGTTTCACGTAATTGGAAA | ||
| CaPV-RPA | F3 | CTTATATGGGAAAAGGTAGAAAAATCAGGAGG | 289 |
| R3 | Biotin-TTATGAGAAGTTTCACGTAATTGGAAAATGTC | ||
| ORFV-RPA | F1 | CACCAACAAGCACCTGGCCTGGGACCTCATGAAC | 170 |
| R1 | Biotin-GCGAGTCAGAGAAGAATACGCCGCCCCCGGAGTG | ||
| ORFV-RPA | F2 | CCACCAACAAGCACCTGGCCTGGGACCTCATGAAC | 198 |
| R2 | TGCGGTAGAAGCCTAAGAAGCGCTCCGGCGAGTCA | ||
| ORFV-RPA | F3 | GCΑCCGCΑTΑGΑGΑΑCGCCΑΑGΑΑCΑGCΑTC | 176 |
| R3 | CCGCGTTCTTCCACTCGGTGATGATCACG | ||
| CaPV-HP | Probe | Digoxin-CTΑTTGCΑΑΑΑCΑCTTTΑGTTTΑTGGΑΑΑT/idsp/GTΑTGCCGΑTGCGGΑT-C3-spacer | 47 |
| ORFV-HP | Probe | FΑM-GCTCTGCTGCGCCGTCGTCΑCGCCCΑCGGC/idsp/ ΑCGΑΑCTTCCΑCCTCΑ-C3-spacer | 47 |
Table 1 Primers and probe sequences
| 引物 Primer | 上游和下游引物 Upstream and downstream primers | 引物序列 Primer sequence(5'-3') | 目标长度 Target length/bp |
|---|---|---|---|
| CaPV-PCR | F1 | GGTCGCGAAATTTCAGATGTAG | 503 |
| R1 | TCATATCCCCCTGTGTACGAAT | ||
| ORFV-PCR | F1 | TCCACTATCAAGAACCTCGGGC | 709 |
| R1 | TTATTGGCTTGCAGAACTCCGA | ||
| CaPV-RPA | F1 | TTATATGGGAAAAGGTAGAAAAATCAGGAGG | 287 |
| R1 | CTATTATGAGAAGTTTCACGTAATTGGAAAATG | ||
| CaPV-RPA | F2 | CAAAACACTTTAGTTTATGGAAATCGTATG | 184 |
| R2 | CTATTATGAGAAGTTTCACGTAATTGGAAA | ||
| CaPV-RPA | F3 | CTTATATGGGAAAAGGTAGAAAAATCAGGAGG | 289 |
| R3 | Biotin-TTATGAGAAGTTTCACGTAATTGGAAAATGTC | ||
| ORFV-RPA | F1 | CACCAACAAGCACCTGGCCTGGGACCTCATGAAC | 170 |
| R1 | Biotin-GCGAGTCAGAGAAGAATACGCCGCCCCCGGAGTG | ||
| ORFV-RPA | F2 | CCACCAACAAGCACCTGGCCTGGGACCTCATGAAC | 198 |
| R2 | TGCGGTAGAAGCCTAAGAAGCGCTCCGGCGAGTCA | ||
| ORFV-RPA | F3 | GCΑCCGCΑTΑGΑGΑΑCGCCΑΑGΑΑCΑGCΑTC | 176 |
| R3 | CCGCGTTCTTCCACTCGGTGATGATCACG | ||
| CaPV-HP | Probe | Digoxin-CTΑTTGCΑΑΑΑCΑCTTTΑGTTTΑTGGΑΑΑT/idsp/GTΑTGCCGΑTGCGGΑT-C3-spacer | 47 |
| ORFV-HP | Probe | FΑM-GCTCTGCTGCGCCGTCGTCΑCGCCCΑCGGC/idsp/ ΑCGΑΑCTTCCΑCCTCΑ-C3-spacer | 47 |
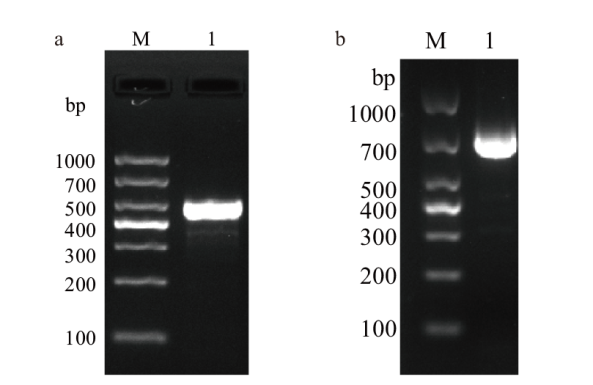
Fig. 1 Results of recombinant plasmid construction a: Results of CaPV P32 gene(M: DNA marker DL1000; 1: P32 gene). b: Results of ORFV 011 gene(M: DNA marker DL1000; 1: 011 gene)
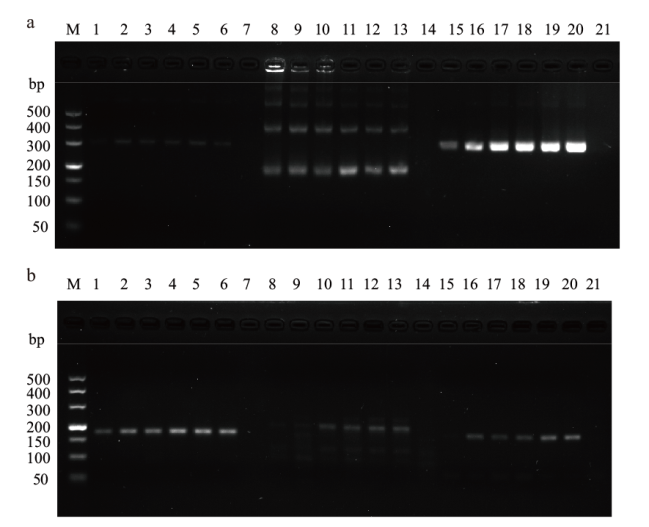
Fig. 2 Primer screening of CaPV and ORFV a: Primer screening of CaPV(M: DNA marker DL500; 1-6: CaPV-RPA-F1/R1; 8-13: CaPV-RPA-F2/R2; 15-20: CaPV-RPA-F3/R3; 7, 14, 21: negative control).b: Primer screening of ORFV(M: DNA marker DL500; 1-6: ORFV-RPA-F1/R1; 8-13: ORFV-RPA-F2/R2; 15-20: ORFV-RPA-F3/R3; 7, 14, 21: negative control)

Fig. 3 Results of reaction condition optimization a: Optimization of reaction time(1-7: 5, 7, 9, 11, 13, 15 min, negative control). b: Optimization of reaction temperature(1-9: 25, 30, 35, 37, 39, 42, 47, 52℃, negative control). c: Optimization of primer ratio(1-5: CaPV-RPA-F3/R3: ORFV-RPA-F1/R1: 0.8∶1.6; 1∶1.4; 1.2∶1.2; 1.4∶1; 1.6∶0.8). d: Optimization of probe ratio(1-5: CaPV-HP: ORFV-HP: 0.1∶0.9; 0.3∶0.7; 0.5∶0.5; 0.7∶0.3; 0.9∶0.1). C: Quality control line; T1: CaPV; T2: ORFV. The same below
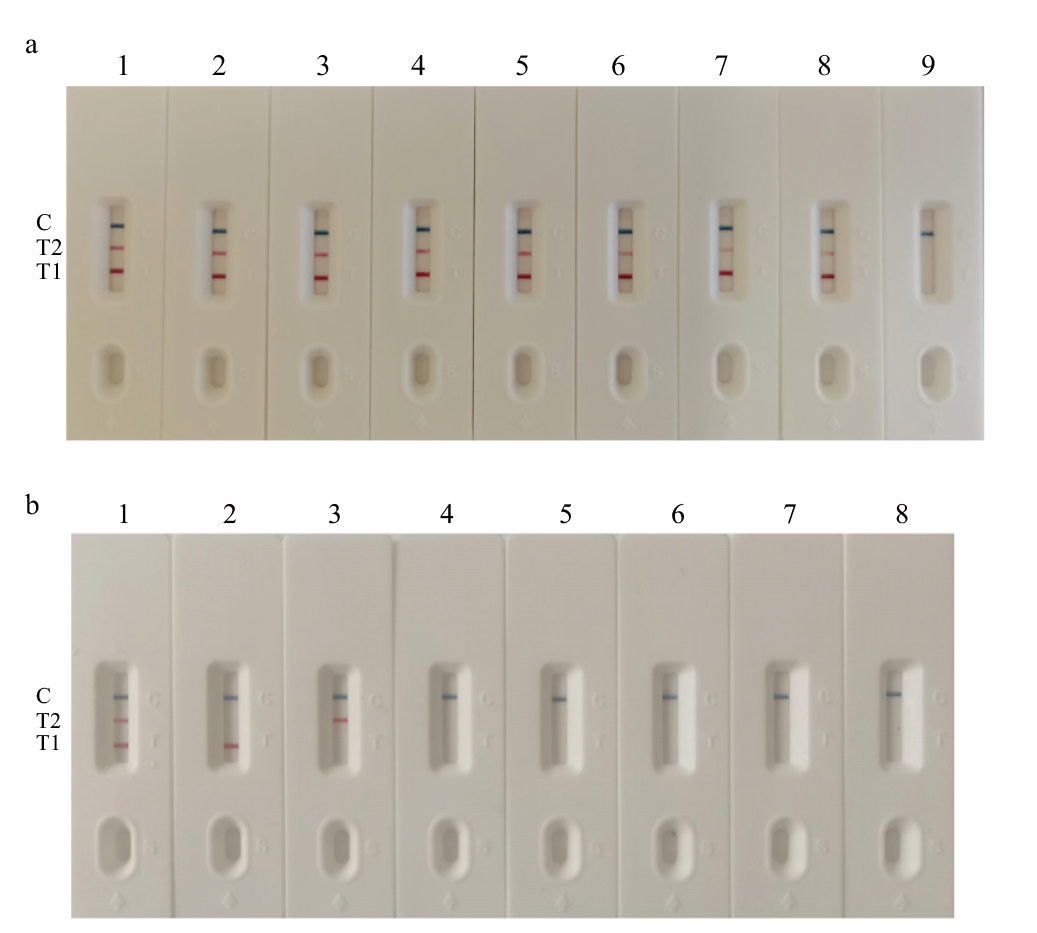
Fig. 4 Sensitivity(a)and specificity(b)test results a: 1-9(CaPV/ORFV): 4.65/3.94×107 copies/μL; 4.65/3.94×106 copies/μL; 4.65/3.94×105 copies/μL; 4.65/3.94×104 copies/μL; 4.65/3.94×103 copies/μL; 4.65/3.94×102 copies/μL; 4.65/3.94×101 copies/μL; 4.65/3.94×100; negative control. b: 1-8: CaPV ORFV; CaPV; ORFV; PPRV; IBRV; Sau; SPN; negative control

Fig. 5 Experiment for repeatability a: Repeated experiment within the group(1-3: CaPV/ORFV: 4.65/3.94×105 copies/μL; 5-7: CaPV/ORFV: 4.65/3.94×106 copies/μL; 9-11: CaPV/ORFV: 4.65/3.94×107 copies/μL; 4, 8, 12: negative control). b: Repeated experiments between groups(1: CaPV/ORFV: 4.65/3.94×107 copies/μL; 2: CaPV/ORFV: 4.65/3.94×106 copies/μL; 3: CaPV/ORFV: 4.65/3.94×105 copies/μL; 4: negative control)
| 检测方法 Detection method | CaPV阳性病例数 Number of CaPV positive cases | ORFV阳性病例数 Number of ORFV positive cases | CaPV、ORFV阳性病例数 Number of CaPV and ORFV positive cases |
|---|---|---|---|
| PCR | 5 | 3 | 3 |
| RPA-LFD | 5 | 3 | 3 |
Table 2 Analysis of PCR and RPA-LFD results of 55 clinical samples
| 检测方法 Detection method | CaPV阳性病例数 Number of CaPV positive cases | ORFV阳性病例数 Number of ORFV positive cases | CaPV、ORFV阳性病例数 Number of CaPV and ORFV positive cases |
|---|---|---|---|
| PCR | 5 | 3 | 3 |
| RPA-LFD | 5 | 3 | 3 |
| [1] | 刘芳. 一起疑似羊痘病毒感染的实验室诊断及病毒分离鉴定[D]. 长春: 吉林大学, 2020. |
| Liu F. Laboratory diagnosis and virus isolation and identification of a suspected sheep pox virus infection[D]. Changchun: Jilin University, 2020. | |
| [2] | Aregahagn S, Tadesse B, Tegegne B, et al. Spatiotemporal distributions of sheep and goat pox disease outbreaks in the period 2013-2019 in eastern Amhara region, Ethiopia[J]. Vet Med Int, 2021, 2021: 6629510. |
| [3] | 刘宇馨. 中国及周边地区绵羊痘和山羊痘的时空流行特征及环境风险因素研究[D]. 哈尔滨: 东北农业大学, 2022. |
| Liu YX. Temporal and spatial epidemic characteristics and environmental risk factors of sheep pox and goat pox in China and its surrounding areas[D]. Harbin: Northeast Agricultural University, 2022. | |
| [4] | Hamdi J, Bamouh Z, Jazouli M, et al. Experimental infection of indigenous North African goats with goatpox virus[J]. Acta Vet Scand, 2021, 63(1): 9. |
| [5] | Hamdi J, Munyanduki H, Tadlaoui KO, et al. Capripoxvirus infections in ruminants: a review[J]. Microorganisms, 2021, 9(5): 902. |
| [6] | Muhsen M, Protschka M, Schneider LE, et al. Orf virus(ORFV)infection in a three-dimensional human skin model: characteristic cellular alterations and interference with keratinocyte differentiation[J]. PLoS One, 2019, 14(1): e0210504. |
| [7] | 张成. 羊传染性脓疱病毒感染新西兰兔模型的建立[D]. 阿拉尔: 塔里木大学, 2022. |
| Zhang C. Establishment of A New Zealand rabbit model infected with infectious goat impetigo virus[D]. Ala'er: Tarim University, 2022. | |
| [8] | 王改丽. ORFV感染宿主细胞的mRNA表达谱变化及其诱导细胞自噬机制的研究[D]. 长春: 吉林大学, 2015. |
| Wang GL. The changes of mRNA expression profile in host cells infected with ORFV and the mechanism of ORFV-induced cell autophagy[D]. Changchun: Jilin University, 2015. | |
| [9] | 姚晓婷. 羊口疮病毒的遗传演化及其在与山羊细胞互作中的双向转录组研究[D]. 杨凌: 西北农林科技大学, 2022. |
| Yao XT. Genetic evolution of sheep mouth sore virus and its two-way transcriptome study in interaction with goat cells[D]. Yangling: Northwest A & F University, 2022. | |
| [10] | Bukar AM, Jesse FFA, Abdullah CAC, et al. Immunomodulatory strategies for parapoxvirus: current status and future approaches for the development of vaccines against orf virus infection[J]. Vaccines, 2021, 9(11): 1341. |
| [11] | 何师师. 羊传染性脓疱病毒ORF120基因缺失株的免疫效果评价[D]. 长春: 吉林大学, 2023. |
| He SS. Evaluation of immune effect of ORF120 gene deletion strain of infectious pustular virus in sheep[D]. Changchun: Jilin University, 2023. | |
| [12] |
刘昂, 程逸文, 安琪, 等. 羊源多杀性巴氏杆菌重组酶聚合酶扩增诊断方法的建立[J]. 中国畜牧兽医, 2021, 48(10): 3752-3760.
doi: 10.16431/j.cnki.1671-7236.2021.10.027 |
| Liu A, Cheng YW, An Q, et al. Establishment of a rapid diagnostic method of RPA for Pasteurella multocida from goat[J]. China Anim Husb Vet Med, 2021, 48(10): 3752-3760. | |
| [13] | Tan MY, Liao C, Liang LN, et al. Recent advances in recombinase polymerase amplification: principle, advantages, disadvantages and applications[J]. Front Cell Infect Microbiol, 2022, 12: 1019071. |
| [14] | Wang F, Ge DB, Wang L, et al. Rapid and sensitive recombinase polymerase amplification combined with lateral flow strips for detecting Candida albicans[J]. Anal Biochem, 2021, 633: 114428. |
| [15] | Liu LB, Wang JF, Nie FP, et al. Development of the isothermal recombinase polymerase amplification assays for rapid detection of the genus Capripoxvirus[J]. J Virol Methods, 2023, 320: 114788. |
| [16] | 周峰, 王志宇, 张梅, 等. 绵羊痘病毒和山羊痘病毒通用RPA检测方法的建立与优化[J]. 中国兽医科学, 2022, 52(7): 830-836. |
| Zhou F, Wang ZY, Zhang M, et al. Establishment and optimization of a RPA diganostic method for sheep pox virus and goat pox virus[J]. Chin Vet Sci, 2022, 52(7): 830-836. | |
| [17] | Onchan W, Ritbamrung O, Changtor P, et al. Sensitive and rapid detection of Babesia species in dogs by recombinase polymerase amplification with lateral flow dipstick(RPA-LFD)[J]. Sci Rep, 2022, 12(1): 20560. |
| [18] | 张珊珊, 李楠, 郝镯, 等. 布鲁氏菌RPA-LFD快速检测方法的建立与应用[J]. 中国兽医科学, 2021, 51(10): 1215-1220. |
| Zhang SS, Li N, Hao Z, et al. Development and evaluation of a rapid visualization detection method for Brucella based on RPA-LFD[J]. Chin Vet Sci, 2021, 51(10): 1215-1220. | |
| [19] | Haegeman A, De Vleeschauwer A, De Leeuw I, et al. Overview of diagnostic tools for capripox virus infections[J]. Prev Vet Med, 2020, 181: 104704. |
| [20] | 岳怀宁, 李艳蕊, 张亚楠, 等. 非洲猪瘟病毒可视化重组酶辅助扩增检测方法的建立与初步应用[J]. 中国兽医杂志, 2023, 59(5): 19-25. |
| Yue HN, Li YR, Zhang YN, et al. Establishment and preliminary application of visual recombinase aided amplification method for detection of African swine fever virus[J]. Chin J Vet Med, 2023, 59(5): 19-25. | |
| [21] | 王姝然, 范厚勇, 徐嘉楠, 等. 弗氏柠檬酸杆菌RPA-LFD快速检测方法的建立及应用[J]. 大连海洋大学学报, 2024, 39(2):241-249. |
| Wang SR, Fan HY, Xu JN, et al. Establishment and application of rapid detection method for Citrobacter Frei RPA-LFD[J]. J Dalian Ocean Univ, 2024, 39(2):241-249. | |
| [22] | Pang F, Long QQ. Recent advances in diagnostic approaches for orf virus[J]. Appl Microbiol Biotechnol, 2023, 107(5/6): 1515-1523. |
| [23] |
Murray L, Edwards L, Tuppurainen ESM, et al. Detection of capripoxvirus DNA using a novel loop-mediated isothermal amplification assay[J]. BMC Vet Res, 2013, 9: 90.
doi: 10.1186/1746-6148-9-90 pmid: 23634704 |
| [24] |
Das A, Babiuk S, McIntosh MT. Development of a loop-mediated isothermal amplification assay for rapid detection of capripoxviruses[J]. J Clin Microbiol, 2012, 50(5): 1613-1620.
doi: 10.1128/JCM.06796-11 pmid: 22357504 |
| [25] |
Yang Y, Qin XD, Wang GX, et al. Development of a fluorescent probe-based recombinase polymerase amplification assay for rapid detection of orf virus[J]. Virol J, 2015, 12: 206.
doi: 10.1186/s12985-015-0440-z pmid: 26631157 |
| [26] | 朱宇翔, 秦嘉超, 季英华, 等. 菜豆黄花叶病毒RPA-LFD技术快速检测方法的建立与应用[J]. 江苏农业科学, 2023, 51(14): 70-75. |
| Zhu YX, Qin JC, Ji YH, et al. Establishment and application of rapid detection method of bean yellow mosaic virus RPA-LFD technology[J]. Jiangsu Agric Sci, 2023, 51(14): 70-75. | |
| [27] | 陈思楠, 王金凤, 张倩, 等. 猪圆环病毒2型可视化RPA快速检测方法的建立[J]. 中国兽医科学, 2022, 52(3): 304-309. |
| Chen SN, Wang JF, Zhang Q, et al. Development of the visual RPA assay for rapid detection of porcine circovirus 2[J]. Chin Vet Sci, 2022, 52(3): 304-309. | |
| [28] | Zheng M, Liu Q, Jin NY, et al. A duplex PCR assay for simultaneous detection and differentiation of capripox virus and orf virus[J]. Mol Cell Probes, 2007, 21(4): 276-281. |
| [29] |
Venkatesan G, Balamurugan V, Bhanuprakash V. TaqMan based real-time duplex PCR for simultaneous detection and quantitation of capripox and orf virus genomes in clinical samples[J]. J Virol Methods, 2014, 201: 44-50.
doi: 10.1016/j.jviromet.2014.02.007 pmid: 24552953 |
| [30] |
Liao L, Shi W, Ma C, et al. Duplex detection of Vibrio cholerae and Vibrio parahaemolyticus by real-time recombinase polymerase amplification[J]. Biomed Environ Sci, 2022, 35(12): 1161-1165.
doi: 10.3967/bes2022.148 pmid: 36597298 |
| [1] | CHEN Hui-qin ,WANG Xiao-ping ,LUO Shu-hong ,WANG Li-jie ,CHEN Qian-qian, HAO Wen-bo. Expression and Purification of orf Virus Protein ORFV035 and Preparation of Polyclonal Antibody [J]. Biotechnology Bulletin, 2017, 33(7): 150-154. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||