








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (10): 321-333.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0314
Previous Articles Next Articles
HUANG Chu-lan1( ), ZENG Rui2, CHEN Pei-rong2, ZHAO Ya-rong2(
), ZENG Rui2, CHEN Pei-rong2, ZHAO Ya-rong2( ), WANG Xu2, YAO Dong-sheng1(
), WANG Xu2, YAO Dong-sheng1( )
)
Received:2025-03-26
Online:2025-10-26
Published:2025-10-28
Contact:
ZHAO Ya-rong, YAO Dong-sheng
E-mail:1515817278@qq.com;zyr520zyr@163.com;dsyao2001@263.net
HUANG Chu-lan, ZENG Rui, CHEN Pei-rong, ZHAO Ya-rong, WANG Xu, YAO Dong-sheng. Proteomic Analysis Reveals the Role of AflaILVB/G/Ⅰ Gene in Aflatoxin Biosynthesis Based on 4D Label-free Technology[J]. Biotechnology Bulletin, 2025, 41(10): 321-333.
菌株名称 Name of strain | 基因型 Genotype | 来源 Source |
|---|---|---|
| A. flavus NRRL 3357 TJES20.1 | Δku70,ΔargB:: AfpyrG (Wild-type) | 江苏师范大学杨教授惠赠 |
| ΔAflaILVB/G/I(AFLA_000930) | Δku70,ΔargB:: AfpyrG,ΔAflaILVB/G/I:: argB | 本研究构建 |
Table 1 Aspergillus strains used in this study
菌株名称 Name of strain | 基因型 Genotype | 来源 Source |
|---|---|---|
| A. flavus NRRL 3357 TJES20.1 | Δku70,ΔargB:: AfpyrG (Wild-type) | 江苏师范大学杨教授惠赠 |
| ΔAflaILVB/G/I(AFLA_000930) | Δku70,ΔargB:: AfpyrG,ΔAflaILVB/G/I:: argB | 本研究构建 |
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| Actin-QF | ACGGTGTCGTCACAAACTGG |
| Actin-QR | CGGTTGGACTTAGGGTTGATAG |
| aflP-QF | ACGAAGCCACTGGTAGAGGAGATG |
| aflP-QR | GTGAATGACGGCAGGCAGGT |
| aflO-QF | GATTGGGATGTGGTCATGCGATT |
| aflO-QR | GCCTGGGTCCGAAGAATGC |
| aflK-QF | CTCGCACTTTGGCATGTACG |
| aflK-QR | AATCCTCCCGCCTCAATCAC |
| aflD-QF | GTGGTGGTTGCCAATGCG |
| aflD-QR | CTGAAACAGTAGGACGGGAGC |
| hypC-QF | GCATGGTGCCTTACACATGG |
| hypC-QR | CCTACCAACCTCACGCTCTC |
Table 2 Primer sequences for fluorescence quantification
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| Actin-QF | ACGGTGTCGTCACAAACTGG |
| Actin-QR | CGGTTGGACTTAGGGTTGATAG |
| aflP-QF | ACGAAGCCACTGGTAGAGGAGATG |
| aflP-QR | GTGAATGACGGCAGGCAGGT |
| aflO-QF | GATTGGGATGTGGTCATGCGATT |
| aflO-QR | GCCTGGGTCCGAAGAATGC |
| aflK-QF | CTCGCACTTTGGCATGTACG |
| aflK-QR | AATCCTCCCGCCTCAATCAC |
| aflD-QF | GTGGTGGTTGCCAATGCG |
| aflD-QR | CTGAAACAGTAGGACGGGAGC |
| hypC-QF | GCATGGTGCCTTACACATGG |
| hypC-QR | CCTACCAACCTCACGCTCTC |

Fig. 1 Quality control of mass spectrometry dataA: Total protein identification by mass spectrometry. B: Charge and length distribution of specific peptides. C: Peptide number distribution of quantified proteins. D: Relative molecular weight distribution of quantified proteins
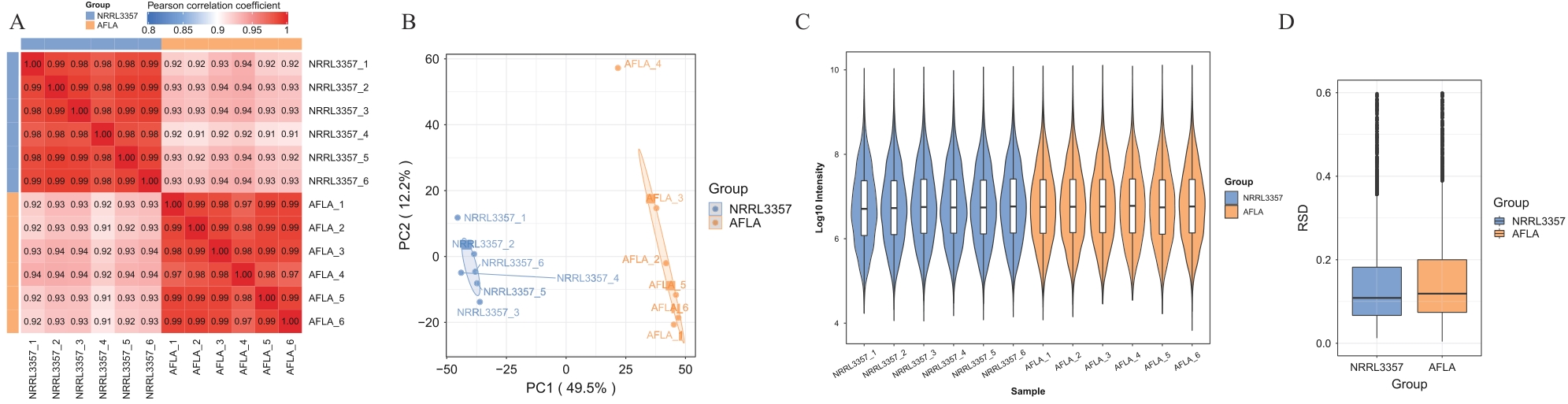
Fig. 2 Sample reproducibility analysisA: PCC analysis heatmap. B: PCA Principal component analysis. C:RSD Analysis violin plot. D: RSD analysis boxplot

Fig. 4 GO functional enrichment analysis of differentially expressed proteinsA: GO enrichment bubble plot of upregulated differentially expressed proteins. B: GO enrichment bubble plot of downregulated differentially expressed proteins

Fig. 5 KEGG functional enrichment analysis of differentially expressed proteinsA: KEGG enrichment bar plot of upregulated differentially expressed proteins. B: KEGG enrichment bar plot of downregulated differentially expressed protein
蛋白质编码 Protein accession | 蛋白质描述 Protein description | 基因名称 Gene name | P值 P value | 调控类型Regulated type |
|---|---|---|---|---|
| B8MYP9 | Branched-chain amino acid aminotransferase | AFLA_081880 | 2.204 49E-08 | Down |
| B8N7W8 | Dihydroxy acid dehydratase Ilv3, putative | AFLA_105610 | 4.045 78E-08 | Up |
| B8N9W8 | Acetohydroxy-acid synthase small subunit | AFLA_112640 | 4.260 63E-08 | Up |
| B8N9L9 | Threonine dehydratase | AFLA_111650 | 9.742 79E-08 | Up |
| B8NEC1 | Ketol-acid reductoisomerase, mitochondrial | AFLA_060300 | 1.855 62E-06 | Up |
| B8N1U6 | Pyruvate decarboxylase | AFLA_032500 | 2.141 48E-06 | Up |
| B8N9Q5 | L-serine dehydratase, putative | AFLA_112010 | 7.377 42E-05 | Down |
| B8NEE4 | Pyridoxal-phosphate dependent enzyme, putative | AFLA_060530 | 0.000 130 39 | Down |
| B8NB44 | Branched-chain-amino-acid aminotransferase | AFLA_044190 | 0.000 135 251 | Up |
Table 3 Differentially expressed proteins involved in the branched-chain amino acid biosynthesis pathway
蛋白质编码 Protein accession | 蛋白质描述 Protein description | 基因名称 Gene name | P值 P value | 调控类型Regulated type |
|---|---|---|---|---|
| B8MYP9 | Branched-chain amino acid aminotransferase | AFLA_081880 | 2.204 49E-08 | Down |
| B8N7W8 | Dihydroxy acid dehydratase Ilv3, putative | AFLA_105610 | 4.045 78E-08 | Up |
| B8N9W8 | Acetohydroxy-acid synthase small subunit | AFLA_112640 | 4.260 63E-08 | Up |
| B8N9L9 | Threonine dehydratase | AFLA_111650 | 9.742 79E-08 | Up |
| B8NEC1 | Ketol-acid reductoisomerase, mitochondrial | AFLA_060300 | 1.855 62E-06 | Up |
| B8N1U6 | Pyruvate decarboxylase | AFLA_032500 | 2.141 48E-06 | Up |
| B8N9Q5 | L-serine dehydratase, putative | AFLA_112010 | 7.377 42E-05 | Down |
| B8NEE4 | Pyridoxal-phosphate dependent enzyme, putative | AFLA_060530 | 0.000 130 39 | Down |
| B8NB44 | Branched-chain-amino-acid aminotransferase | AFLA_044190 | 0.000 135 251 | Up |
蛋白质编码 Protein accession | 蛋白质描述 Protein description | 基因名称 Gene name | P值 P value | 调控类型Regulated type |
|---|---|---|---|---|
| P55790 | Sterigmatocystin 8-O-methyltransferase | omtA/aflP/AFLA_139210 | 1.205 77E-13 | Down |
| Q9P900 | Demethylsterigmatocystin 6-O-methyltransferase | omtB/aflO/AFLA_139220 | 1.503 66E-13 | Down |
| B8NHY3 | AflK/VERB synthase | vbs/aflK/AFLA_139190 | 1.187 86E-12 | Down |
| B8NHZ4 | AflM/dehydrogenase/ketoreductase | ver-1/aflM/AFLA_139300 | 6.337 76E-12 | Down |
| B8NI02 | AflD/reductase | nor-1/aflD/AFLA_139390 | 1.591 52E-11 | Down |
| B8NHY1 | AflW/monooxygenase | moxY/aflW/AFLA_139170 | 2.287 05E-11 | Down |
| B8NHX9 | AflY | hypA/aflY/AFLA_139150 | 3.178 44E-11 | Down |
| B8N9Y6 | Toxin biosynthesis ketoreductase | AFLA_112820 | 3.624 58E-11 | Up |
| B8NHY0 | AflX/monooxygenase/oxidase | ordB/aflX/AFLA_139160 | 2.447 17E-09 | Down |
| B8NI03 | Noranthrone monooxygenase | hypC/AFLA_139400 | 6.130 27E-09 | Down |
| B8NT69 | O-methyltransferase | AFLA_053520 | 3.688 78E-08 | Down |
| B8NHY9 | AflL/desaturase/ P450 monooxygenase | verb/aflL/AFLA_139250 | 5.666 24E-08 | Down |
| B8NHZ6 | AflJ/esterase | estA/aflJ/AFLA_139320 | 8.454 44E-08 | Down |
| B8NHZ2 | AflN/monooxygenase | ver-A/aflN/AFLA_139280 | 9.288 83E-08 | Down |
| B8NI04 | AflC/polyketide synthase | pskA/aflC/AFLA_139410 | 1.609 73E-05 | Down |
| B8N0Z3 | N-alkane-inducible cytochrome P450 | AFLA_027680 | 0.000 725 794 | Up |
| B8N628 | O-methyltransferase family protein | AFLA_016120 | 0.017 023 299 | Down |
Table 4 Differentially expressed proteins involved in the aflatoxin biosynthesis pathway
蛋白质编码 Protein accession | 蛋白质描述 Protein description | 基因名称 Gene name | P值 P value | 调控类型Regulated type |
|---|---|---|---|---|
| P55790 | Sterigmatocystin 8-O-methyltransferase | omtA/aflP/AFLA_139210 | 1.205 77E-13 | Down |
| Q9P900 | Demethylsterigmatocystin 6-O-methyltransferase | omtB/aflO/AFLA_139220 | 1.503 66E-13 | Down |
| B8NHY3 | AflK/VERB synthase | vbs/aflK/AFLA_139190 | 1.187 86E-12 | Down |
| B8NHZ4 | AflM/dehydrogenase/ketoreductase | ver-1/aflM/AFLA_139300 | 6.337 76E-12 | Down |
| B8NI02 | AflD/reductase | nor-1/aflD/AFLA_139390 | 1.591 52E-11 | Down |
| B8NHY1 | AflW/monooxygenase | moxY/aflW/AFLA_139170 | 2.287 05E-11 | Down |
| B8NHX9 | AflY | hypA/aflY/AFLA_139150 | 3.178 44E-11 | Down |
| B8N9Y6 | Toxin biosynthesis ketoreductase | AFLA_112820 | 3.624 58E-11 | Up |
| B8NHY0 | AflX/monooxygenase/oxidase | ordB/aflX/AFLA_139160 | 2.447 17E-09 | Down |
| B8NI03 | Noranthrone monooxygenase | hypC/AFLA_139400 | 6.130 27E-09 | Down |
| B8NT69 | O-methyltransferase | AFLA_053520 | 3.688 78E-08 | Down |
| B8NHY9 | AflL/desaturase/ P450 monooxygenase | verb/aflL/AFLA_139250 | 5.666 24E-08 | Down |
| B8NHZ6 | AflJ/esterase | estA/aflJ/AFLA_139320 | 8.454 44E-08 | Down |
| B8NHZ2 | AflN/monooxygenase | ver-A/aflN/AFLA_139280 | 9.288 83E-08 | Down |
| B8NI04 | AflC/polyketide synthase | pskA/aflC/AFLA_139410 | 1.609 73E-05 | Down |
| B8N0Z3 | N-alkane-inducible cytochrome P450 | AFLA_027680 | 0.000 725 794 | Up |
| B8N628 | O-methyltransferase family protein | AFLA_016120 | 0.017 023 299 | Down |

Fig. 8 Protein-protein interaction network analysis of differentially expressed proteinsEllipses and squares indicate differentially expressed proteins in the branched-chain amino acid synthesis pathway that interact with proteins, where ellipses interact with the AFLA_000930 protein; triangles indicate differentially expressed proteins in the aflatoxin synthesis pathway that interact with proteins; diamonds indicate connecting node proteins. Red indicates upregulated differentially expressed proteins; blue indicates downregulated differentially expressed proteins

Fig. 10 Analysis of intracellular metabolic reprogramming× indicates metabolic pathways affected by AHAS functional deficiency; red indicates upregulated metabolic pathways; blue indicates downregulated metabolic pathways
| [1] | 王晓燕, 梁柳柯, 魏闪, 等. AflbasR基因对储粮霉菌黄曲霉生长发育和毒素合成的影响 [J]. 河南工业大学学报: 自然科学版, 2024, 45(6): 45-53. |
| Wang XY, Liang LK, Wei S, et al. Effects of AflbasR gene on the growth, development and aflatoxin biosynthesis of the grain storage fungus Aspergillus flavus [J]. J Henan Univ Technol Nat Sci | |
| Ed, 2024, 45(6): 45-53. | |
| [2] | Pickova D, Ostry V, Malir F. A recent overview of producers and important dietary sources of aflatoxins [J]. Toxins, 2021, 13(3): 186. |
| [3] | 尚艳娥, 杨卫民. CAC、欧盟、美国与中国粮食中真菌毒素限量标准的差异分析 [J]. 食品科学技术学报, 2019, 37(1): 10-15. |
| Shang YE, Yang WM. Variation analysis of cereals mycotoxin limit standards of CAC, EU, USA, and China [J]. J Food Sci Technol, 2019, 37(1): 10-15. | |
| [4] | International Agency for Research on Cancer (IARC), World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 56: Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins [M]. New York: Cancer causes & control, 1994, 5(1): 89-90. |
| [5] | Xue MY, Qu Z, Moretti A, et al. Aspergillus mycotoxins: the major food contaminants [J]. Adv Sci, 2025, 12(9): e2412757. |
| [6] | Mitchell NJ, Bowers E, Hurburgh C, et al. Potential economic losses to the US corn industry from aflatoxin contamination [J]. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 2016, 33(3): 540-550. |
| [7] | Cheng XB, Vella A, Stasiewicz MJ. Classification of aflatoxin contaminated single corn kernels by ultraviolet to near infrared spectroscopy [J]. Food Control, 2019, 98: 253-261. |
| [8] | Chhaya RS, O’Brien J, Nag R, et al. Prevalence and concentration of mycotoxins in bovine feed and feed components: a global systematic review and meta-analysis [J]. Sci Total Environ, 2024, 929: 172323. |
| [9] | Gruber-Dorninger C, Müller A, Rosen R. Multi-mycotoxin contamination of aquaculture feed: a global survey [J]. Toxins, 2025, 17(3): 116. |
| [10] | 雷元培, 周建川,郑文革, 等. 2019-2020年中国饲料原料和饲料中霉菌毒素污染调查报告 [J]. 饲料工业, 2022, 43(20): 59-64. |
| Lei YP, Zhou JC, Zheng WG, et al. Investigation report on mycotoxin contamination in feed ingredients and feeds in China from 2019 to 2020 [J]. Feed Industry, 2022, 43(20): 59-64. | |
| [11] | Zentai A, Jóźwiak Á, Süth M, et al. Carry-over of aflatoxin B1 from feed to cow milk-a review [J]. Toxins, 2023, 15(3): 195. |
| [12] | Marchese S, Polo A, Ariano A, et al. Aflatoxin B1 and M1: biological properties and their involvement in cancer development [J]. Toxins, 2018, 10(6): 214. |
| [13] | De Baere S, Ochieng PE, Kemboi DC, et al. Development of high-throughput sample preparation procedures for the quantitative determination of aflatoxins in biological matrices of chickens and cattle using UHPLC-MS/MS [J]. Toxins, 2023, 15(1): 37. |
| [14] | Khalil HMA, Eid WAM, El-Nablaway M, et al. Date seeds powder alleviate the aflatoxin B1 provoked heart toxicity in male offspring rat [J]. Sci Rep, 2024, 14(1): 30480. |
| [15] | Cao HH, Molina S, Sumner S, et al. An untargeted metabolomic analysis of acute AFB1 treatment in liver, breast, and lung cells [J]. PLoS One, 2025, 20(1): e0313159. |
| [16] | Herzallah. Aflatoxin b1 residues in eggs and flesh of laying hens fed aflatoxin b1 contaminated diet [J]. Am J Agric Biol Sci, 2013, 8(2): 156-161. |
| [17] | He XN, Zeng ZZ, Jiang WD, et al. Aflatoxin B1 decreased flesh flavor and inhibited muscle development in grass carp (Ctenopharyngodon idella) [J]. Anim Nutr, 2024, 18: 27-38. |
| [18] | Liu H, Xie RT, Huang WB, et al. Effects of dietary aflatoxin B1 on hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) growth, intestinal health, and muscle quality [J]. Aquac Nutr, 2024, 2024: 3920254. |
| [19] | Pożarska A, Karpiesiuk K, Kozera W, et al. AFB1 toxicity in human food and animal feed consumption: a review of experimental treatments and preventive measures [J]. Int J Mol Sci, 2024, 25(10): 5305. |
| [20] | Benkerroum N, Ismail A. Human breast milk contamination with aflatoxins, impact on children’s health, and possible control means: a review [J]. Int J Environ Res Public Health, 2022, 19(24): 16792. |
| [21] | Liang YF, Long ZX, Zhang YJ, et al. The chemical mechanisms of the enzymes in the branched-chain amino acids biosynthetic pathway and their applications [J]. Biochimie, 2021, 184: 72-87. |
| [22] | Lonhienne T, Low YS, Garcia MD, et al. Structures of fungal and plant acetohydroxyacid synthases [J]. Nature, 2020, 586(7828): 317-321. |
| [23] | Liu YD, Li YY, Wang XY. Acetohydroxyacid synthases: evolution, structure, and function [J]. Appl Microbiol Biotechnol, 2016, 100(20): 8633-8649. |
| [24] | Zhang YY, Li Y, Liu X, et al. Molecular architecture of the acetohydroxyacid synthase holoenzyme [J]. Biochem J, 2020, 477(13): 2439-2449. |
| [25] | Chong NF, Van de Wouw AP, Idnurm A. The ilv2 gene, encoding acetolactate synthase for branched chain amino acid biosynthesis, is required for plant pathogenicity by Leptosphaeria maculans [J]. Mol Biol Rep, 2024, 51(1): 682. |
| [26] | Low YS, Garcia MD, Lonhienne T, et al. Triazolopyrimidine herbicides are potent inhibitors of Aspergillus fumigatus acetohydroxyacid synthase and potential antifungal drug leads [J]. Sci Rep, 2021, 11(1): 21055. |
| [27] | Agnew-Francis KA, Tang YC, Lin X, et al. Herbicides that target acetohydroxyacid synthase are potent inhibitors of the growth of drug-resistant Candida auris [J]. ACS Infect Dis, 2020, 6(11): 2901-2912. |
| [28] | Shao SN, Li B, Sun Q, et al. Acetolactate synthases regulatory subunit and catalytic subunit genes VdILVs are involved in BCAA biosynthesis, microscletotial and conidial formation and virulence in Verticillium dahlia e [J]. Fungal Genet Biol, 2022, 159: 103667. |
| [29] | Du Y, Zhang HF, Hong L, et al. Acetolactate synthases MoIlv2 and MoIlv6 are required for infection-related morphogenesis in Magnaporthe oryzae [J]. Mol Plant Pathol, 2013, 14(9): 870-884. |
| [30] | Liu X, Han Q, Xu JH, et al. Acetohydroxyacid synthase FgIlv2 and FgIlv6 are involved in BCAA biosynthesis, mycelial and conidial morphogenesis, and full virulence in Fusarium graminearum [J]. Sci Rep, 2015, 5: 16315. |
| [31] | Zhao YR, Huang CL, Zeng R, et al. AflaILVB/G/I and AflaILVD are involved in mycelial production, aflatoxin biosynthesis, and fungal virulence in Aspergillus flavus [J]. Front Cell Infect Microbiol, 2024, 14: 1372779. |
| [32] | Cimbalo A, Frangiamone M, Font G, et al. The importance of transcriptomics and proteomics for studying molecular mechanisms of mycotoxin exposure: a review [J]. Food Chem Toxicol, 2022, 169: 113396. |
| [33] | Lv YY, Lv A, Zhai HC, et al. Insight into the global regulation of laeA in Aspergillus flavus based on proteomic profiling [J]. Int J Food Microbiol, 2018, 284: 11-21. |
| [34] | Zhang F, Zhong H, Han XY, et al. Proteomic profile of Aspergillus flavus in response to water activity [J]. Fungal Biol, 2015, 119(2/3): 114-124. |
| [35] | Bai YH, Wang S, Zhong H, et al. Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature [J]. Sci Rep, 2015, 5: 14582. |
| [36] | Tiwari S, Thakur R, Goel G, et al. Nano-LC-Q-TOF analysis of proteome revealed germination of Aspergillus flavus conidia is accompanied by MAPK signalling and cell wall modulation [J]. Mycopathologia, 2016, 181(11-12): 769-786. |
| [37] | Khan R, Ghazali FM, Mahyudin NA, et al. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: a review [J]. J Fungi, 2021, 7(8): 606. |
| [38] | McCourt JA, Duggleby RG. Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids [J]. Amino Acids, 2006, 31(2): 173-210. |
| [39] | Yamamoto K, Tsuchisaka A, Yukawa H. Branched-chain amino acids [M]//Amino Acid Fermentation. Tokyo: Springer Japan, 2016: 103-128. |
| [40] | Nie CX, He T, Zhang WJ, et al. Branched chain amino acids: beyond nutrition metabolism [J]. Int J Mol Sci, 2018, 19(4): 954. |
| [41] | Neinast M, Murashige D, Arany Z. Branched chain amino acids [J]. Annu Rev Physiol, 2019, 81: 139-164. |
| [42] | Sivanand S, Vander Heiden MG. Emerging roles for branched-chain amino acid metabolism in cancer [J]. Cancer Cell, 2020, 37(2): 147-156. |
| [43] | Dimou A, Tsimihodimos V, Bairaktari E. The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain α- keto acid dehydrogenase (BCKD), in human pathophysiology [J]. Int J Mol Sci, 2022, 23(7): 4022. |
| [44] | Huang ZC, Wang Q, Khan IA, et al. The methylcitrate cycle and its crosstalk with the glyoxylate cycle and tricarboxylic acid cycle in pathogenic fungi [J]. Molecules, 2023, 28(18): 6667. |
| [45] | Akram M. Citric acid cycle and role of its intermediates in metabolism [J]. Cell Biochem Biophys, 2014, 68(3): 475-478. |
| [46] | Huang YM, Huber GA, Wang N, et al. Brownian dynamic study of an enzyme metabolon in the TCA cycle: Substrate kinetics and channeling [J]. Protein Sci, 2018, 27(2): 463-471. |
| [47] | Chang LC, Chiang SK, Chen SE, et al. Targeting 2-oxoglutarate dehydrogenase for cancer treatment [J]. Am J Cancer Res, 2022, 12(4): 1436-1455. |
| [48] | Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement [J]. Redox Biol, 2020, 37: 101674. |
| [49] | MacLean A, Legendre F, Appanna VD. The tricarboxylic acid (TCA) cycle: a malleable metabolic network to counter cellular stress [J]. Crit Rev Biochem Mol Biol, 2023, 58(1): 81-97. |
| [50] | Liu XJ, Si WZ, He L, et al. The existence of a nonclassical TCA cycle in the nucleus that wires the metabolic-epigenetic circuitry [J]. Signal Transduct Target Ther, 2021, 6(1): 375. |
| [51] | Doan MT, Teitell MA. Krebs and an alternative TCA cycle! [J]. Cell Res, 2022, 32(6): 509-510. |
| [52] | Arnold PK, Jackson BT, Paras KI, et al. A non-canonical tricarboxylic acid cycle underlies cellular identity [J]. Nature, 2022, 603(7901): 477-481. |
| [53] | Yan SJ, Liang YT, Zhang JD, et al. Aspergillus flavus grown in peptone as the carbon source exhibits spore density- and peptone concentration-dependent aflatoxin biosynthesis [J]. BMC Microbiol, 2012, 12: 106. |
| [1] | JIANG Tian-wei, MA Pei-jie, LI Ya-jiao, CHEN Cai-jun, LIU Xiao-xia, WANG Xiao-li. Metabolic Response Analysis of Brachypodium distachyon to Photoperiods [J]. Biotechnology Bulletin, 2025, 41(7): 237-247. |
| [2] | YANG Wei, GUAN Hai-feng, REN Xin-hui, PENG Jin-ju, CHEN Zhi-bao. Alleviating Effect of Astaxanthin on Liver Injury Induced by Aflatoxin B 1 and Its Mechanism [J]. Biotechnology Bulletin, 2025, 41(10): 334-342. |
| [3] | YANG Dong, TANG Ying. Enzymatic Characterization and Degradation Sites of AFB1 Degradation by the Extracellular Enzyme of Bacillus subtilis Strain WTX1 [J]. Biotechnology Bulletin, 2023, 39(4): 93-102. |
| [4] | TANG Ying, HUANG Jia, DENG Zhan-rui, YANG Xiao-nan. Product Analysis of Degrading Aflatoxin B1 by a Strain Bacillus subtilis [J]. Biotechnology Bulletin, 2021, 37(12): 82-90. |
| [5] | GENG Long-po, WANG Xin-wang, HUANG Lu-hua, DENG Ji-li, WANG Shi-hua, ZHANG Feng. Expression Analysis of Ribosomal Protein Genes in Aspergillus flavus [J]. Biotechnology Bulletin, 2018, 34(4): 194-200. |
| [6] | REN Ya-lin, LI Yun, WU Jing. Combined Hepatotoxicity Assessment of Mycotoxins AFB1 and Zearalenone on Hepatocellular Carcinoma Cells HepG2 in vitro and Its Mechanisms [J]. Biotechnology Bulletin, 2018, 34(11): 160-167. |
| [7] | ZENG Kun, DU Dao-lin, XUE Yong-lai. Research Advances on Rapid Detection Methods for Aflatoxin Based on Biological Binders [J]. Biotechnology Bulletin, 2016, 32(8): 47-55. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||