








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (1): 100-112.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0509
Previous Articles Next Articles
WANG Jun-fang( ), HUANG Qiu-bin, ZHANG Piao-dan, ZHANG Peng-pai(
), HUANG Qiu-bin, ZHANG Piao-dan, ZHANG Peng-pai( )
)
Received:2023-05-30
Online:2024-01-26
Published:2024-02-06
Contact:
ZHANG Peng-pai
E-mail:jfw188@163.com;bio_apai@163.com
WANG Jun-fang, HUANG Qiu-bin, ZHANG Piao-dan, ZHANG Peng-pai. Structure and Biosynthesis of Surfactin as well as Its Role in Biological Control[J]. Biotechnology Bulletin, 2024, 40(1): 100-112.

Fig. 2 Principle of non-ribosomal peptide(NRP)biosynthesis (A)Crystal structure of non-ribosomal peptide synthetases(NRPSs)related domain.(B)The principle of action of NRPSs.(C)Catalytic reaction of NRPSs structure domain. The TE enzyme domain is responsible for the cyclization and release of lipopeptides. In the figure, I is the linear product obtained through hydrolysis method, while II is the cyclization method to obtain cyclic products. The specific method depends on the recognition characteristics of the NRPSs
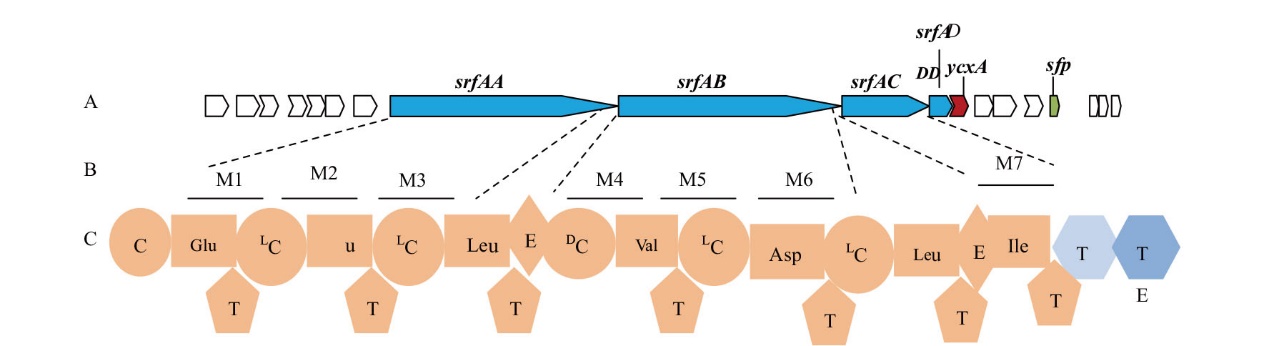
Fig. 3 Composition of gene cluster of surfactin biosynthesis A: Surfactin biosynthesis gene cluster, and the structural genes of NRPS for biosynthesis of surfactin are sfAA, srfAB, srfAC, and srfAD. B: Classic module composition of SrfAA-SrfAD. C: Domain architecture of SrfAA-SrfAD
| [1] |
Li Y, Zou AH, Ye RQ, et al. Effects of molecular structure on surfactin micellization activity[J]. Acta Phys Chim Sin, 2011, 27(5): 1128-1134.
doi: 10.3866/PKU.WHXB20110436 URL |
| [2] |
Kundu D, Hazra C, Chatterjee A, et al. Surfactin-functionalized poly(methyl methacrylate)as an eco-friendly nano-adsorbent: from size-controlled scalable fabrication to adsorptive removal of inorganic and organic pollutants[J]. RSC Adv, 2016, 6(84): 80438-80454.
doi: 10.1039/C6RA10804K URL |
| [3] |
Kim SD, Park SK, Cho JY, et al. Surfactin C inhibits platelet aggregation[J]. J Pharm Pharmacol, 2010, 58(6): 867-870.
doi: 10.1211/jpp.58.6.0018 URL |
| [4] | Jacques P. Surfactin and other lipopeptides from Bacillus spp.[M]// Biosurfactants. Berlin, Heidelberg: Springer, 2011: 57-91. |
| [5] | Théatre A, Hoste ACR, Rigolet A, et al. Bacillus sp.: a remarkable source of bioactive lipopeptides[M]//Biosurfactants for the Biobased Economy. Cham: Springer International Publishing, 2021: 123-179. |
| [6] |
Arima K, Kakinuma A, Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation[J]. Biochem Biophys Res Commun, 1968, 31(3): 488-494.
doi: 10.1016/0006-291X(68)90503-2 URL |
| [7] |
Kakinuma A, Hori M, Isono M, et al. Determination of amino acid sequence in surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis[J]. Agric Biol Chem, 1969, 33(6): 971-972.
doi: 10.1080/00021369.1969.10859408 URL |
| [8] |
Kakinuma A, Sugino H, Isono M, et al. Determination of fatty acid in surfactin and elucidation of the total structure of surfactin[J]. Agric Biol Chem, 1969, 33(6): 973-976.
doi: 10.1080/00021369.1969.10859409 URL |
| [9] |
Maget-Dana R, Ptak M. Interfacial properties of surfactin[J]. J Colloid Interface Sci, 1992, 153(1): 285-291.
doi: 10.1016/0021-9797(92)90319-H URL |
| [10] |
Liu JF, Mbadinga SM, Yang SZ, et al. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation[J]. Int J Mol Sci, 2015, 16(3): 4814-4837.
doi: 10.3390/ijms16034814 URL |
| [11] |
Augustyn AR, Pott RWM, Tadie M. The interactions of the biosurfactant surfactin in coal flotation[J]. Colloids Surf A, 2021, 627: 127122.
doi: 10.1016/j.colsurfa.2021.127122 URL |
| [12] |
Deleu M, Bouffioux O, Razafindralambo H, et al. Interaction of surfactin with membranes: a computational approach[J]. Langmuir, 2003, 19(8): 3377-3385.
doi: 10.1021/la026543z URL |
| [13] |
Grau A, Gómez Fernández JC, Peypoux F, et al. A study on the interactions of surfactin with phospholipid vesicles[J]. Biochim Biophys Acta, 1999, 1418(2): 307-319.
pmid: 10320682 |
| [14] |
Chen XY, Zhao HY, Meng FQ, et al. Ameliorated effects of a lipopeptide surfactin on insulin resistance in vitro and in vivo[J]. Food Sci Nutr, 2022, 10(7): 2455-2469.
doi: 10.1002/fsn3.v10.7 URL |
| [15] |
Kotoky R, Pandey P. Rhizosphere mediated biodegradation of benzo(a)pyrene by surfactin producing soil bacilli applied through Melia azadirachta rhizosphere[J]. Int J Phytoremediation, 2020, 22(4): 363-372.
doi: 10.1080/15226514.2019.1663486 URL |
| [16] |
Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production[J]. Plant Physiol, 2004, 134(1): 307-319.
doi: 10.1104/pp.103.028712 URL |
| [17] |
Zhao F, Zhu HB, Cui QF, et al. Anaerobic production of surfactin by a new Bacillus subtilis isolate and the in situ emulsification and viscosity reduction effect towards enhanced oil recovery applications[J]. J Petrol Sci Eng, 2021, 201: 108508.
doi: 10.1016/j.petrol.2021.108508 URL |
| [18] |
Tank JG, Pandya RV. Anti-proliferative activity of surfactins on human cancer cells and their potential use in therapeutics[J]. Peptides, 2022, 155: 170836.
doi: 10.1016/j.peptides.2022.170836 URL |
| [19] |
Meena KR, Sharma A, Kanwar SS. Antitumoral and antimicrobial activity of surfactin extracted from Bacillus subtilis KLP2015[J]. Int J Pept Res Ther, 2020, 26(1): 423-433.
doi: 10.1007/s10989-019-09848-w |
| [20] |
Lim JH, Park BK, Kim MS, et al. The anti-thrombotic activity of surfactins[J]. J Vet Sci, 2005, 6(4): 353-355.
doi: 10.4142/jvs.2005.6.4.353 URL |
| [21] |
Wang Y, Tian JH, Shi FF, et al. Protective effect of surfactin on copper sulfate-induced inflammation, oxidative stress, and hepatic injury in zebrafish[J]. Microbiol Immunol, 2021, 65(10): 410-421.
doi: 10.1111/mim.v65.10 URL |
| [22] |
Mnif I, Segovia R, Bouallegue A, et al. Identification of different lipopeptides isoforms produced by Bacillus mojavensis BI2 and evaluation of their surface activities for potential environmental application[J]. J Polym Environ, 2023, 31(6): 2668-2685.
doi: 10.1007/s10924-022-02752-3 |
| [23] |
Ishikawa F, Konno S, Uchida C, et al. Chemoproteomics profiling of surfactin-producing nonribosomal peptide synthetases in living bacterial cells[J]. Cell Chem Biol, 2022, 29(1): 145-156.e8.
doi: 10.1016/j.chembiol.2021.05.014 URL |
| [24] |
Fortinez CM, Bloudoff K, Harrigan C, et al. Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module[J]. Nat Commun, 2022, 13(1): 548.
doi: 10.1038/s41467-022-28221-y pmid: 35087027 |
| [25] |
Finking R, Mofid MR, Marahiel MA. Mutational analysis of peptidyl carrier protein and acyl carrier protein synthase unveils residues involved in protein-protein recognition[J]. Biochemistry, 2004, 43(28): 8946-8956.
pmid: 15248752 |
| [26] |
Peypoux F, Bonmatin JM, Labbé H, et al. Isolation and characterization of a new variant of surfactin, the[Val7]surfactin[J]. Eur J Biochem, 1991, 202(1): 101-106.
pmid: 1935967 |
| [27] |
Dowling DP, Kung Y, Croft AK, et al. Structural elements of an NRPS cyclization domain and its inter module docking domain[J]. Proc Natl Acad Sci USA, 2016, 113(44): 12432-12437.
pmid: 27791103 |
| [28] |
Schneider A, Marahiel MA. Genetic evidence for a role of thioesterase domains, integrated in or associated with peptide synthetases, in non-ribosomal peptide biosynthesis in Bacillus subtilis[J]. Arch Microbiol, 1998, 169(5): 404-410.
pmid: 9560421 |
| [29] |
Sieber SA, Marahiel MA. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics[J]. Chem Rev, 2005, 105(2): 715-738.
pmid: 15700962 |
| [30] |
Tseng CC, Bruner SD, Kohli RM, et al. Characterization of the surfactin synthetase C-terminal thioesterase domain as a cyclic depsipeptide synthase[J]. Biochemistry, 2002, 41(45): 13350-13359.
pmid: 12416979 |
| [31] |
Bruner SD, Weber T, Kohli RM, et al. Structural basis for the cyclization of the lipopeptide antibiotic surfactin by the thioesterase domain SrfTE[J]. Structure, 2002, 10(3): 301-310.
pmid: 12005429 |
| [32] |
Linne U, Schwarzer D, Schroeder GN, et al. Mutational analysis of a type II thioesterase associated with nonribosomal peptide synthesis[J]. Eur J Biochem, 2004, 271(8): 1536-1545.
pmid: 15066179 |
| [33] |
Schwarzer D, Mootz HD, Linne U, et al. Regeneration of misprimednonribosomal peptide synthetases by type II thioesterases[J]. PNAS, 2002, 99(22): 14083-14088.
pmid: 12384573 |
| [34] |
Chiocchini C, Linne U, Stachelhaus T. In vivo biocombinatorial synthesis of lipopeptides by COM domain-mediated reprogramming of the surfactin biosynthetic complex[J]. Chem Biol, 2006, 13(8): 899-908.
doi: 10.1016/j.chembiol.2006.06.015 URL |
| [35] |
Nakano MM, Zuber P. The primary role of comA in establishment of the competent state in Bacillus subtilis is to activate expression of srfA[J]. J Bacteriol, 1991, 173(22): 7269-7274.
pmid: 1938921 |
| [36] |
Nakano MM, Xia LA, Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis[J]. J Bacteriol, 1991, 173(17): 5487-5493.
pmid: 1715856 |
| [37] |
Ogura M, Fujita Y. Bacillus subtilis rapD, a direct target of transcription repression by RghR, negatively regulates srfA expression[J]. FEMS Microbiol Lett, 2007, 268(1): 73-80.
doi: 10.1111/fml.2007.268.issue-1 URL |
| [38] |
Liang Z, Qiao JQ, Li PP, et al. A novel Rap-Phr system in Bacillus velezensis NAU-B3 regulates surfactin production and sporulation via interaction with ComA[J]. Appl Microbiol Biotechnol, 2020, 104(23): 10059-10074.
doi: 10.1007/s00253-020-10942-z pmid: 33043389 |
| [39] |
Nakano MM, Corbell N, Besson J, et al. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis[J]. Mol Gen Genet, 1992, 232(2): 313-321.
doi: 10.1007/BF00280011 |
| [40] |
Tsuge K, Ohata Y, Shoda M. Gene yerP, involved in surfactin self-resistance in Bacillus subtilis[J]. Antimicrob Agents Chemother, 2001, 45(12): 3566-3573.
pmid: 11709341 |
| [41] |
Li X, Yang H, Zhang DL, et al. Overexpression of specific proton motive force-dependent transporters facilitate the export of surfactin in Bacillus subtilis[J]. J Ind Microbiol Biotechnol, 2015, 42(1): 93-103.
doi: 10.1007/s10295-014-1527-z URL |
| [42] | 王镜岩. 生物化学[M]. 第3版. 北京: 高等教育出版社, 2002. |
| Wang JY. Biochemistry[M]. 3rd ed. Beijing: Higher Education Press, 2002. | |
| [43] |
Youssef NH, Wofford N, McInerney MJ. Importance of the long-chain fatty acid beta-hydroxylating cytochrome P450 enzyme YbdT for lipopeptide biosynthesis in Bacillus subtilis strain OKB105[J]. Int J Mol Sci, 2011, 12(3): 1767-1786.
doi: 10.3390/ijms12031767 pmid: 21673922 |
| [44] |
Hlavica P, Lehnerer M. Oxidative biotransformation of fatty acids by cytochromes P450: predicted key structural elements orchestrating substrate specificity, regioselectivity and catalytic efficiency[J]. Curr Drug Metab, 2010, 11(1): 85-104.
pmid: 20302567 |
| [45] |
Steller S, Sokoll A, Wilde C, et al. Initiation of surfactin biosynthesis and the role of the SrfD-thioesterase protein[J]. Biochemistry, 2004, 43(35): 11331-11343.
pmid: 15366943 |
| [46] | Dae KS, Cho J, Park HJ, et al. A comparison of the anti-inflammatory activity of surfactin A, B, C, and D from Bacillus subtilis[J]. J Microbiol Biotechnol, 2006, 16: 1656-1659. |
| [47] |
Bonmatin JM, Labbé H, Grangemard I, et al. Production, isolation and characterization of[Leu4]- and[Ile4]surfactins from Bacillus subtilis[J]. Lett Pept Sci, 1995, 2(1): 41-47.
doi: 10.1007/BF00122922 URL |
| [48] |
Park G, Nam J, Kim J, et al. Structure and mechanism of surfactin peptide from Bacillus velezensis antagonistic to fungi plant pathogens[J]. Bull Korean Chem Soc, 2019, 40(7): 704-709.
doi: 10.1002/bkcs.2019.40.issue-7 URL |
| [49] |
Vater J, Stein T, Vollenbroich D, et al. The modular organization of multifunctional peptide synthetases[J]. J Protein Chem, 1997, 16(5): 557-564.
doi: 10.1023/a:1026386100259 pmid: 9246644 |
| [50] |
Grady EN, MacDonald J, Ho MT, et al. Characterization and complete genome analysis of the surfactin-producing, plant-protecting bacterium Bacillus velezensis 9D-6[J]. BMC Microbiol, 2019, 19(1): 5.
doi: 10.1186/s12866-018-1380-8 |
| [51] |
Nerurkar AS. Structural and molecular characteristics of lichenysin and its relationship with surface activity[J]. Adv Exp Med Biol, 2010, 672: 304-315.
pmid: 20545292 |
| [52] |
Naruse N, Tenmyo O, Kobaru S, et al. Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity[J]. J Antibiot, 1990, 43(3): 267-280.
pmid: 2157695 |
| [53] |
Kecskeméti A, Bartal A, Bóka B, et al. High-frequency occurrence of surfactin monomethyl isoforms in the ferment broth of a Bacillus subtilis strain revealed by ion trap mass spectrometry[J]. Molecules, 2018, 23(9): 2224.
doi: 10.3390/molecules23092224 URL |
| [54] |
Théatre A, Cano-Prieto C, Bartolini M, et al. The surfactin-like lipopeptides from Bacillus spp.: natural biodiversity and synthetic biology for a broader application range[J]. Front Bioeng Biotechnol, 2021, 9: 623701.
doi: 10.3389/fbioe.2021.623701 URL |
| [55] |
Liu XY, Yang SZ, Mu BZ. Production and characterization of a C15-surfactin-O-methyl ester by a lipopeptide producing strain Bacillus subtilis HSO121[J]. Process Biochem, 2009, 44(10): 1144-1151.
doi: 10.1016/j.procbio.2009.06.014 URL |
| [56] |
Li YM, Yang SZ, Mu BZ. Structural characterization of lipopeptide methyl esters produced by Bacillus licheniformis HSN 221[J]. Chem Biodivers, 2010, 7(8): 2065-2075.
doi: 10.1002/cbdv.v7:8 URL |
| [57] | 刘皓, 杨欢, 李雪, 等. 脂肽-糖脂混合生物表面活性剂产生菌筛选和优化培养[J]. 生物工程学报, 2013, 29(12): 1870-1874. |
| Liu H, Yang H, Li X, et al. Identification of Bacillus subtilis THY-7 and high titer optimization for the blend-biosurfactant of lipopeptide and glycolipid[J]. Chin J Biotechnol, 2013, 29(12): 1870-1874.. | |
| [58] |
Wang CY, Cao YX, Wang YP, et al. Enhancing surfactin production by using systematic CRISPRi repression to screen amino acid biosynthesis genes in Bacillus subtilis[J]. Microb Cell Fact, 2019, 18(1): 90.
doi: 10.1186/s12934-019-1139-4 |
| [59] | 周泽宇, 张婉茹, 张柔萱, 等. 代谢工程改造Bacillus amyloliquefaciens提高Surfactin产量[J]. 南开大学学报: 自然科学版, 2018, 51(5): 18-26. |
| Zhou ZY, Zhang WR, Zhang RX, et al. Metabolic engineering of Bacillus amyloliquefaciens to improve surfactin production[J]. Acta Sci Nat Univ Nankaiensis, 2018, 51(5): 18-26. | |
| [60] |
Klausmann P, Hennemann K, Hoffmann M, et al. Bacillus subtilis high cell density fermentation using a sporulation-deficient strain for the production of surfactin[J]. Appl Microbiol Biotechnol, 2021, 105(10): 4141-4151.
doi: 10.1007/s00253-021-11330-x pmid: 33991199 |
| [61] |
Hu FX, Cai WJ, Lin JZ, et al. Genetic engineering of the precursor supply pathway for the overproduction of the nC14-surfactin isoform with promising MEOR applications[J]. Microb Cell Fact, 2021, 20(1): 96.
doi: 10.1186/s12934-021-01585-4 |
| [62] |
Jiang J, Gao L, Bie XM, et al. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme[J]. BMC Microbiol, 2016, 16: 31.
doi: 10.1186/s12866-016-0645-3 pmid: 26957318 |
| [63] |
Eppelmann K, Stachelhaus T, Marahiel MA. Exploitation of the selectivity-conferring code of nonribosomal peptide synthetases for the rational design of novel peptide antibiotics[J]. Biochemistry, 2002, 41(30): 9718-9726.
pmid: 12135394 |
| [64] |
Mootz HD, Kessler N, Linne U, et al. Decreasing the ring size of a cyclic nonribosomal peptide antibiotic by in-frame module deletion in the biosynthetic genes[J]. J Am Chem Soc, 2002, 124(37): 10980-10981.
pmid: 12224936 |
| [65] | 高圣风, 王锋, 刘爱勤, 等. Bacillus subtilis VD18R19脂肽类产物鉴定及其对胡椒花叶病的田间生防效果[J]. 热带农业科学, 2019, 39(10): 89-94. |
| Gao SF, Wang F, Liu AQ, et al. Identification and field application of the lipopeptides produced by Bacillus subtilis VD18R19 against black pepper mosaic disease[J]. Chin J Trop Agric, 2019, 39(10): 89-94. | |
| [66] |
高毓晗, 李世东, 郭荣君. sfp基因转化增强了Bacillus subtilis 168的定殖能力和对黄瓜茎内枯萎病菌的抑制作用[J]. 中国生物防治学报, 2016, 32(1): 76-85.
doi: 10.16409/j.cnki.2095-039x.2015.06.012 |
| Gao YH, Li SD, Guo RJ. Transformation of sfp gene into Bacillus subtilis 168 promotes its colonization on cucumber roots and suppression of Fusarium oxysporum f. sp. cucumerinum in cucumber stems[J]. Chin J Biol Contr, 2016, 32(1): 76-85. | |
| [67] | 周维, 田丹丹, 杨扬, 等. 解淀粉芽孢杆菌G9R-3脂肽类化合物抑制香蕉枯萎病菌机理及防效评价[J]. 西南农业学报, 2019, 32(8): 1810-1816. |
| Zhou W, Tian DD, Yang Y, et al. Antifungal mechanism and control effects on Fusarium oxysporum f. sp. cubense race 4 of lipopeptides produced by Bacillus amyloliquefaciens G9R-3[J]. Southwest China J Agric Sci, 2019, 32(8): 1810-1816. | |
| [68] |
Sarwar A, Hassan MN, Imran M, et al. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease[J]. Microbiol Res, 2018, 209: 1-13.
doi: 10.1016/j.micres.2018.01.006 URL |
| [69] | Laird M, Piccoli D, Weselowski B, et al. Surfactin-producing Bacillus velezensis 1B-23 and Bacillus sp. 1D-12 protect tomato against bacterial canker caused by Clavibactermichiganensis subsp. michiganensis[J]. J Plant Pathol, 2020, 102(2): 451-458. |
| [70] |
Fan HY, Zhang ZW, Li Y, et al. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization[J]. Front Microbiol, 2017, 8: 1973.
doi: 10.3389/fmicb.2017.01973 URL |
| [71] |
Li Y, Héloir MC, Zhang X, et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation[J]. Mol Plant Pathol, 2019, 20(8): 1037-1050.
doi: 10.1111/mpp.2019.20.issue-8 URL |
| [72] | 任鹏举, 谢永丽, 张岩, 等. 枯草芽孢杆菌OKB105产生的surfactin防治烟草花叶病毒病及其机理研究[J]. 中国生物防治学报, 2014, 30(2): 216-221. |
| Ren PJ, Xie YL, Zhang Y, et al. Effect and mechanism of controlling TMV disease on tobacco by surfactin produced by Bacillus subtilis OKB105[J]. Chin J Biol Contr, 2014, 30(2): 216-221. | |
| [73] |
Ali SAM, Sayyed RZ, Mir MI, et al. Induction of systemic resistance in maize and antibiofilm activity of surfactin from Bacillus velezensis MS20[J]. Front Microbiol, 2022, 13: 879739.
doi: 10.3389/fmicb.2022.879739 URL |
| [74] |
Le Mire G, Siah A, Brisset MN, et al. Surfactin protects wheat against Zymoseptoriatritici and activates both salicylic acid- and jasmonic acid-dependent defense responses[J]. Agriculture, 2018, 8(1): 11.
doi: 10.3390/agriculture8010011 URL |
| [75] |
Rodríguez J, Tonelli ML, Figueredo MS, et al. The lipopeptide surfactin triggers induced systemic resistance and priming state responses in Arachis hypogaea L.[J]. Eur J Plant Pathol, 2018, 152(3): 845-851.
doi: 10.1007/s10658-018-1524-6 |
| [76] |
Cawoy H, Mariutto M, Henry G, et al. Plant defense stimulation by natural isolates of bacillus depends on efficient surfactin production[J]. Mol Plant Microbe Interact, 2014, 27(2): 87-100.
doi: 10.1094/MPMI-09-13-0262-R URL |
| [77] |
Krishnan N, Velramar B, Velu RK. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusariummoniliforme and its impact in seed germination and mycotoxicosis[J]. Pestic Biochem Physiol, 2019, 155: 101-107.
doi: 10.1016/j.pestbp.2019.01.010 URL |
| [78] |
Wang YY, Zhang CY, Liang J, et al. Surfactin and fengycin B extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms[J]. Appl Microbiol Biotechnol, 2020, 104(17): 7467-7481.
doi: 10.1007/s00253-020-10773-y |
| [79] |
Henry G, Deleu M, Jourdan E, et al. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses[J]. Cell Microbiol, 2011, 13(11): 1824-1837.
doi: 10.1111/j.1462-5822.2011.01664.x pmid: 21838773 |
| [80] | Carrillo C, Teruel JA, Aranda FJ, et al. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin[J]. Biochim Biophys Acta, 2003, 1611(1/2): 91-97. |
| [81] |
Heerklotz H, Seelig J. Leakage and lysis of lipid membranes induced by the lipopeptide surfactin[J]. Eur Biophys J, 2007, 36(4/5): 305-314.
doi: 10.1007/s00249-006-0091-5 URL |
| [82] |
Deravel J, Lemière S, Coutte F, et al. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew[J]. Appl Microbiol Biotechnol, 2014, 98(14): 6255-6264.
doi: 10.1007/s00253-014-5663-1 pmid: 24723290 |
| [83] | de Andrade CJ, Barros FF, de Andrade LM, et al. Ultrafiltration based purification strategies for surfactin produced by Bacillus subtilis LB5A using cassava wastewater as substrate[J]. J Chem Technol Biotechnol, 2016, 91(12): 3018-3027. |
| [84] |
Marin CP, Kaschuk JJ, Frollini E, et al. Potential use of the liquor from sisal pulp hydrolysis as substrate for surfactin production[J]. Ind Crops Prod, 2015, 66: 239-245.
doi: 10.1016/j.indcrop.2015.01.001 URL |
| [85] |
Zanotto AW, Valério A, de Andrade CJ, et al. New sustainable alternatives to reduce the production costs for surfactin 50 years after the discovery[J]. Appl Microbiol Biotechnol, 2019, 103(21/22): 8647-8656.
doi: 10.1007/s00253-019-10123-7 |
| [86] |
de Oliveira Schmidt VK, Vicente R, et al. Cassava wastewater valorization for the production of biosurfactants: surfactin, rhamnolipids, and mannosileritritol lipids[J]. World J Microbiol Biotechnol, 2022, 39(2): 65.
doi: 10.1007/s11274-022-03510-2 |
| [87] |
Kisil OV, Trefilov VS, Sadykova VS, et al. Surfactin: its biological activity and possibility of application in agriculture[J]. Appl Biochem Microbiol, 2023, 59(1): 1-13.
doi: 10.1134/S0003683823010027 |
| [1] | CHEN Zhi-min, LI Cui, WEI Ji-tian, LI Xin-ran, LIU Yi, GUO Qiang. Research Progress in the Regulation of Chlorogenic Acid Biosynthesis and Its Application [J]. Biotechnology Bulletin, 2024, 40(1): 57-71. |
| [2] | LI Liang, XU Shan-shan, JIANG Yan-jun. Research Progress in the Production of Ergothioneine by Biosynthesis [J]. Biotechnology Bulletin, 2024, 40(1): 86-99. |
| [3] | CHU Rui, LI Zhao-xuan, ZHANG Xue-qing, YANG Dong-ya, CAO Hang-hang, ZHANG Xue-yan. Screening and Identification of Antagonistic Bacillus spp. Against Cucumber Fusarium wilt and Its Biocontrol Effect [J]. Biotechnology Bulletin, 2023, 39(8): 262-271. |
| [4] | YE Yun-fang, TIAN Qing-yin, SHI Ting-ting, WANG Liang, YUE Yuan-zheng, YANG Xiu-lian, WANG Liang-gui. Research Progress in the Biosynthesis and Regulation of β-ionone in Plants [J]. Biotechnology Bulletin, 2023, 39(8): 91-105. |
| [5] | ZHANG Man, ZHANG Ye-zhuo, HE Qi-zou-hong, E Yi-lan, LI Ye. Advances in Plant Cell Wall Structure and Imaging Technology [J]. Biotechnology Bulletin, 2023, 39(7): 113-122. |
| [6] | WANG Ling, ZHUO Shen, FU Xue-sen, LIU Zi-xuan, LIU Xiao-rong, WANG Zhi-hui, ZHOU Ri-bao, LIU Xiang-dan. Advances in the Biosynthetic Pathways and Related Genes of Lotus Alkaloids [J]. Biotechnology Bulletin, 2023, 39(7): 56-66. |
| [7] | CUI Xue-qiang, HUANG Chang-yan, DENG Jie-ling, LI Xian-min, LI Xiu-ling, ZHANG Zi-bin. SNP Markers Development and Genetic Relationship Analysis of Dendrobium Germplasms Using SLAF-seq Technology [J]. Biotechnology Bulletin, 2023, 39(6): 141-148. |
| [8] | LI Tuo, LI Long-ping, QU Lei. Research Progress in the Structure of Tailed Bacteriophage and Its Receptors [J]. Biotechnology Bulletin, 2023, 39(6): 88-101. |
| [9] | JIANG Qing-chun, DU Jie, WANG Jia-cheng, YU Zhi-he, WANG Yun, LIU Zhong-yu. Expression and Function Analysis of Transcription Factor PcMYB2 from Polygonum cuspidatum [J]. Biotechnology Bulletin, 2023, 39(5): 217-223. |
| [10] | ZHOU Ding-ding, LI Hui-hu, TANG Xing-yong, YU Fa-xin, KONG Dan-yu, LIU Yi. Research Progress in the Biosynthesis and Regulation of Glycyrrhizic Acid and Liquiritin [J]. Biotechnology Bulletin, 2023, 39(5): 44-53. |
| [11] | YU Hui-li, LI Ai-tao. Application of Cytochrome P450 in the Biosynthesis of Flavors and Fragrances [J]. Biotechnology Bulletin, 2023, 39(4): 24-37. |
| [12] | ZHANG Le-le, WANG Guan, LIU Feng, HU Han-qiao, REN Lei. Isolation, Identification and Biocontrol Mechanism of an Antagonistic Bacterium Against Anthracnose on Mango Caused by Colletotrichum gloeosporioides [J]. Biotechnology Bulletin, 2023, 39(4): 277-287. |
| [13] | YANG Jun-zhao, ZHANG Xin-rui, ZHAO Guo-zhu, ZHENG Fei. Structure and Function Analysis of Novel GH5 Multi-domain Cellulase [J]. Biotechnology Bulletin, 2023, 39(4): 71-80. |
| [14] | YI Xi, LIAO Hong-dong, ZHENG Jing-yuan. Research Progress in Plant Endophytic Fungi for Root-knot Nematode Control [J]. Biotechnology Bulletin, 2023, 39(3): 43-51. |
| [15] | WANG Wei-chen, ZHAO Jin, HUANG Wei-yi, GUO Xin-zhu, LI Wan-ying, ZHANG Zhuo. Research Progress in Metabolites Produced by Bacillus Against Three Common Plant Pathogenic Fungi [J]. Biotechnology Bulletin, 2023, 39(3): 59-68. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||