








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (2): 233-244.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0874
Previous Articles Next Articles
LI Hao( ), WU Guo-qiang(
), WU Guo-qiang( ), WEI Ming, HAN Yue-xin
), WEI Ming, HAN Yue-xin
Received:2023-09-11
Online:2024-02-26
Published:2024-03-13
Contact:
WU Guo-qiang
E-mail:1044624636@qq.com;gqwu@lut.edu.cn
LI Hao, WU Guo-qiang, WEI Ming, HAN Yue-xin. Genome-wide Identification of the BvBADH Gene Family in Sugar Beet(Beta vulgaris)and Their Expression Analysis Under High Salt Stress[J]. Biotechnology Bulletin, 2024, 40(2): 233-244.
| 基因Gene | 正向引物序列Forward primer sequence(5'-3') | 反向引物序列Reverse primer sequence(5'-3') |
|---|---|---|
| BvBADH1 | GCAGTGCAACATCCAGACTC | CCCCCTCACTCTTTGCTGTT |
| BvBADH2 | GTCCTGTTGTCAGCAAGGGA | GCATGGACGTGGAGACATCA |
| BvBADH3 | CCAAATTGGGGGAAGTTTGTGT | TGGATCACCAACAACCCAAGA |
| BvBADH4 | ACGGGGAATTTGTGGATGCT | TCATCACCCTTCCCCTTTCA |
| BvBADH5 | ATTGAGCATGGCAAGCGAGA | TGCCATTCGCTCGCTCTATC |
| BvBADH6 | GCCGATTTAGCACCACTTCT | GCAGAAGTGGTGCTAAATCGG |
| BvBADH7 | GCATCTGATTATGGGCTGGC | CCACTCATTTTGTACCCGCC |
| BvBADH8 | TCGTCGAGCCAATAACACCC | CCACCAAATGGAATGCCTGC |
| BvBADH9 | GGCCAGTTCAGAGGTGAGGA | GAACTTGGACCCAAGCCTTTT |
| BvACTIN | ACTGGTATTGTGCTTGACTC | ATGAGATAATCAGTGAGATC |
Table 1 Sequences of primers used for RT-qPCR in this study
| 基因Gene | 正向引物序列Forward primer sequence(5'-3') | 反向引物序列Reverse primer sequence(5'-3') |
|---|---|---|
| BvBADH1 | GCAGTGCAACATCCAGACTC | CCCCCTCACTCTTTGCTGTT |
| BvBADH2 | GTCCTGTTGTCAGCAAGGGA | GCATGGACGTGGAGACATCA |
| BvBADH3 | CCAAATTGGGGGAAGTTTGTGT | TGGATCACCAACAACCCAAGA |
| BvBADH4 | ACGGGGAATTTGTGGATGCT | TCATCACCCTTCCCCTTTCA |
| BvBADH5 | ATTGAGCATGGCAAGCGAGA | TGCCATTCGCTCGCTCTATC |
| BvBADH6 | GCCGATTTAGCACCACTTCT | GCAGAAGTGGTGCTAAATCGG |
| BvBADH7 | GCATCTGATTATGGGCTGGC | CCACTCATTTTGTACCCGCC |
| BvBADH8 | TCGTCGAGCCAATAACACCC | CCACCAAATGGAATGCCTGC |
| BvBADH9 | GGCCAGTTCAGAGGTGAGGA | GAACTTGGACCCAAGCCTTTT |
| BvACTIN | ACTGGTATTGTGCTTGACTC | ATGAGATAATCAGTGAGATC |
| 基因名称 Gene name | 基因登录号 Gene ID | CDS /bp | 相对分子量 Mw/kD | 亲水性平均值 GRAVY | 等电点 pI | 脂肪指数 Aliphatic index | 氨基酸数目 Protein length/aa | 不稳定指数 Instability index |
|---|---|---|---|---|---|---|---|---|
| BvBADH1 | Bv5_ 116250_aodi | 1 500 | 54.72 | -0.077 | 5.45 | 88.78 | 500 | 32.48 |
| BvBADH2 | Bv5_ 116230_ntjn | 1 512 | 54.78 | -0.062 | 5.37 | 88.51 | 503 | 31.62 |
| BvBADH3 | Bv8_ 195870_rfyd | 1 509 | 54.69 | -0.081 | 5.24 | 87.45 | 502 | 28.42 |
| BvBADH4 | Bv8_ 195860_yiyp | 1 506 | 54.25 | -0.079 | 5.60 | 89.00 | 501 | 22.07 |
| BvBADH5 | Bv8_ 195850_giqy | 1 509 | 54.34 | -0.039 | 6.01 | 88.05 | 502 | 25.97 |
| BvBADH6 | Bv5_ 116750_zqhy | 1 605 | 58.11 | -0.111 | 6.65 | 85.90 | 534 | 31.08 |
| BvBADH7 | Bv6_ 149040_dgrz | 1 605 | 57.84 | -0.076 | 6.53 | 89.55 | 534 | 32.99 |
| BvBADH8 | Bv6_ 149060_fmfu | 1 608 | 58.71 | -0.109 | 6.54 | 85.74 | 535 | 32.04 |
| BvBADH9 | Bv2_047750_ktxe | 1 602 | 57.11 | 0.066 | 6.98 | 95.72 | 533 | 38.12 |
Table 2 Identification of BvBADH gene family members in sugar beet
| 基因名称 Gene name | 基因登录号 Gene ID | CDS /bp | 相对分子量 Mw/kD | 亲水性平均值 GRAVY | 等电点 pI | 脂肪指数 Aliphatic index | 氨基酸数目 Protein length/aa | 不稳定指数 Instability index |
|---|---|---|---|---|---|---|---|---|
| BvBADH1 | Bv5_ 116250_aodi | 1 500 | 54.72 | -0.077 | 5.45 | 88.78 | 500 | 32.48 |
| BvBADH2 | Bv5_ 116230_ntjn | 1 512 | 54.78 | -0.062 | 5.37 | 88.51 | 503 | 31.62 |
| BvBADH3 | Bv8_ 195870_rfyd | 1 509 | 54.69 | -0.081 | 5.24 | 87.45 | 502 | 28.42 |
| BvBADH4 | Bv8_ 195860_yiyp | 1 506 | 54.25 | -0.079 | 5.60 | 89.00 | 501 | 22.07 |
| BvBADH5 | Bv8_ 195850_giqy | 1 509 | 54.34 | -0.039 | 6.01 | 88.05 | 502 | 25.97 |
| BvBADH6 | Bv5_ 116750_zqhy | 1 605 | 58.11 | -0.111 | 6.65 | 85.90 | 534 | 31.08 |
| BvBADH7 | Bv6_ 149040_dgrz | 1 605 | 57.84 | -0.076 | 6.53 | 89.55 | 534 | 32.99 |
| BvBADH8 | Bv6_ 149060_fmfu | 1 608 | 58.71 | -0.109 | 6.54 | 85.74 | 535 | 32.04 |
| BvBADH9 | Bv2_047750_ktxe | 1 602 | 57.11 | 0.066 | 6.98 | 95.72 | 533 | 38.12 |
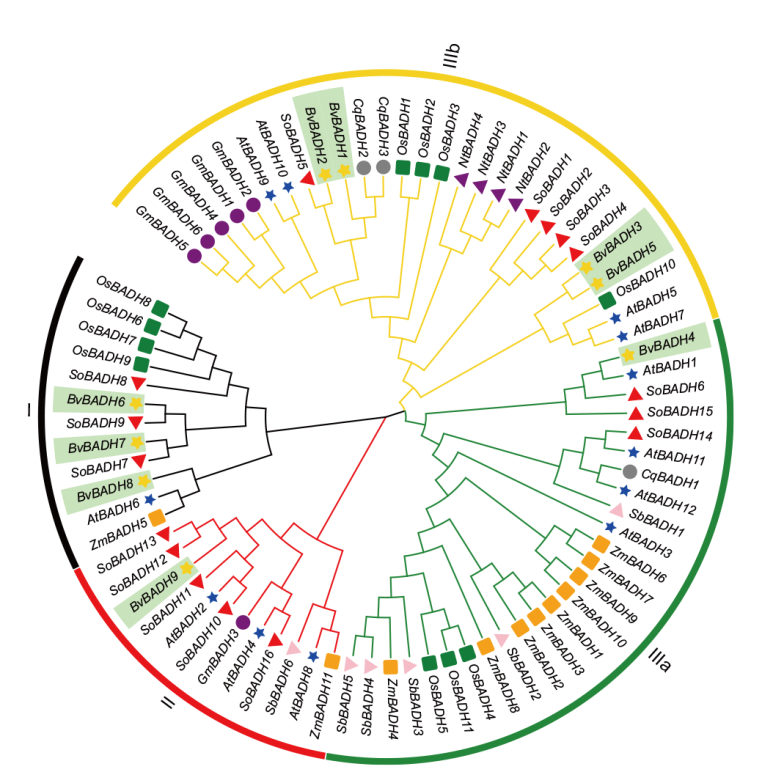
Fig. 2 Phylogenetic tree of BADH gene family in plants BADHs is divided into three clusters. Cluster I(black), II(red)and III. Cluster III is further divided into Cluster III a(green)and III b(gold). The dark green marks in the figure represent the BvBADH family members
| 顺式作用元件Cis-acting element | 功能Function | 序列Sequence | BvBADH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| ABRE | Abscisic acid-responsive element | ACGTG | 3 | 2 | 5 | 3 | 3 | 2 | 0 | 4 | 3 | |
| TCA-element | Involved in salicylic acid responsiveness | CCATCTTTTT | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | |
| TGA-element | Auxin-responsive element | AACGAC | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |
| ARE | Anaerobic-responsive element | AAACCA | 4 | 3 | 1 | 2 | 4 | 4 | 5 | 2 | 6 | |
| LTR | Low-temperature responsiveness | CCGAAA | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | |
| Box 4 | Involved in light responsiveness | ATTAAT | 3 | 3 | 2 | 1 | 0 | 1 | 0 | 3 | 1 | |
| GT1-motif | Involved in light responsiveness | GGTTAAT | 2 | 0 | 4 | 3 | 4 | 0 | 2 | 2 | 1 | |
| TCT-motif | Involved in light responsiveness | TCTTAC | 6 | 0 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |
| AE-box | Modul for light response | AGAAACTT | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | |
| O2-site | Zein metabolism regulation | GATGACATGA | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | |
| G-box | Involved in light responsiveness | TACGTG | 1 | 2 | 7 | 0 | 4 | 4 | 3 | 0 | 0 | |
Table 3 Analysis of cis-acting regulatory elements in the promoter of BvBADHs genes
| 顺式作用元件Cis-acting element | 功能Function | 序列Sequence | BvBADH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| ABRE | Abscisic acid-responsive element | ACGTG | 3 | 2 | 5 | 3 | 3 | 2 | 0 | 4 | 3 | |
| TCA-element | Involved in salicylic acid responsiveness | CCATCTTTTT | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | |
| TGA-element | Auxin-responsive element | AACGAC | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |
| ARE | Anaerobic-responsive element | AAACCA | 4 | 3 | 1 | 2 | 4 | 4 | 5 | 2 | 6 | |
| LTR | Low-temperature responsiveness | CCGAAA | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | |
| Box 4 | Involved in light responsiveness | ATTAAT | 3 | 3 | 2 | 1 | 0 | 1 | 0 | 3 | 1 | |
| GT1-motif | Involved in light responsiveness | GGTTAAT | 2 | 0 | 4 | 3 | 4 | 0 | 2 | 2 | 1 | |
| TCT-motif | Involved in light responsiveness | TCTTAC | 6 | 0 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |
| AE-box | Modul for light response | AGAAACTT | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | |
| O2-site | Zein metabolism regulation | GATGACATGA | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | |
| G-box | Involved in light responsiveness | TACGTG | 1 | 2 | 7 | 0 | 4 | 4 | 3 | 0 | 0 | |
| 蛋白名称 Protein name | α螺旋 α-helix | 伸展主链 Extended strand | β转角 β-turn | 无规卷曲 Random coil |
|---|---|---|---|---|
| BvBADH1 | 43.40 | 16.40 | 7.80 | 32.40 |
| BvBADH2 | 42.94 | 15.90 | 7.55 | 33.60 |
| BvBADH3 | 40.84 | 17.53 | 8.17 | 33.47 |
| BvBADH4 | 40.32 | 17.56 | 7.98 | 34.13 |
| BvBADH5 | 39.44 | 17.73 | 8.37 | 34.46 |
| BvBADH6 | 43.07 | 15.54 | 7.49 | 33.90 |
| BvBADH7 | 41.76 | 15.54 | 7.87 | 34.83 |
| BvBADH8 | 42.80 | 16.07 | 7.85 | 33.27 |
| BvBADH9 | 48.22 | 15.01 | 8.07 | 28.71 |
Table 4 Secondary structure composition of BvBADHs proteins %
| 蛋白名称 Protein name | α螺旋 α-helix | 伸展主链 Extended strand | β转角 β-turn | 无规卷曲 Random coil |
|---|---|---|---|---|
| BvBADH1 | 43.40 | 16.40 | 7.80 | 32.40 |
| BvBADH2 | 42.94 | 15.90 | 7.55 | 33.60 |
| BvBADH3 | 40.84 | 17.53 | 8.17 | 33.47 |
| BvBADH4 | 40.32 | 17.56 | 7.98 | 34.13 |
| BvBADH5 | 39.44 | 17.73 | 8.37 | 34.46 |
| BvBADH6 | 43.07 | 15.54 | 7.49 | 33.90 |
| BvBADH7 | 41.76 | 15.54 | 7.87 | 34.83 |
| BvBADH8 | 42.80 | 16.07 | 7.85 | 33.27 |
| BvBADH9 | 48.22 | 15.01 | 8.07 | 28.71 |

Fig. 8 Relative expressions of BvBADHs in sugar beet leaves under various concentrations of NaCl The vertical bar represents the standard error(SE), n=3. The lowercase letter at the top of each bar indicates a significant deviation at the P<0.05 level
| [1] |
Zhang HM, Zhu JH, Gong ZZ, et al. Abiotic stress responses in plants[J]. Nat Rev Genet, 2022, 23(2): 104-119.
doi: 10.1038/s41576-021-00413-0 |
| [2] |
Singh A. Soil salinization management for sustainable development: a review[J]. J Environ Manage, 2021, 277: 111383.
doi: 10.1016/j.jenvman.2020.111383 URL |
| [3] |
Singh A. Alternative management options for irrigation-induced salinization and waterlogging under different climatic conditions[J]. Ecol Indic, 2018, 90: 184-192.
doi: 10.1016/j.ecolind.2018.03.014 URL |
| [4] |
Hazen SP, Wu YJ, Kreps JA. Gene expression profiling of plant responses to abiotic stress[J]. Funct Integr Genomics, 2003, 3(3): 105-111.
doi: 10.1007/s10142-003-0088-4 URL |
| [5] |
Kumar S, Dhingra A, Daniell H. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance[J]. Plant Physiol, 2004, 136(1): 2843-2854.
doi: 10.1104/pp.104.045187 pmid: 15347789 |
| [6] |
Zhou SF, Chen XY, Zhang XG, et al. Improved salt tolerance in tobacco plants by co-transformation of a betaine synthesis gene BADH and a vacuolar Na+/H+ antiporter gene SeNHX1[J]. Biotechnol Lett, 2008, 30(2): 369-376.
doi: 10.1007/s10529-007-9548-6 URL |
| [7] |
Chen THH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes[J]. Curr Opin Plant Biol, 2002, 5(3): 250-257.
doi: 10.1016/s1369-5266(02)00255-8 pmid: 11960744 |
| [8] |
Shahbazi M, Tohidfar M, Aliniaeifard S, et al. Transgenic tobacco co-expressing flavodoxin and betaine aldehyde dehydrogenase confers cadmium tolerance through boosting antioxidant capacity[J]. Protoplasma, 2022, 259(4): 965-979.
doi: 10.1007/s00709-021-01714-1 |
| [9] | 刘振林, 戴思兰. 植物甜菜碱醛脱氢酶基因研究进展[J]. 西北农林科技大学学报: 自然科学版, 2004, 32(3): 104-112. |
| Liu ZL, Dai SL. Studies on betaine aldehyde dehydrogenase(BADH)gene in plants[J]. J Northwest Sci Tech Univ Agric For, 2004, 32(3): 104-112. | |
| [10] |
Abid M, Gu SC, Zhang YJ, et al. Comparative transcriptome and metabolome analysis reveal key regulatory defense networks and genes involved in enhanced salt tolerance of Actinidia(kiwifruit)[J]. Hortic Res, 2022, 9: uhac189.
doi: 10.1093/hr/uhac189 URL |
| [11] |
Mitsuya S, Kuwahara J, Ozaki K, et al. Isolation and characterization of a novel peroxisomal choline monooxygenase in barley[J]. Planta, 2011, 234(6): 1215-1226.
doi: 10.1007/s00425-011-1478-9 pmid: 21769646 |
| [12] |
Yang XH, Liang Z, Wen XG, et al. Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants[J]. Plant Mol Biol, 2008, 66(1/2): 73-86.
doi: 10.1007/s11103-007-9253-9 URL |
| [13] |
Omari AF. Metabolic engineering of osmoprotectants to elucidate the mechanism(s)of salt stress tolerance in crop plants[J]. Planta, 2021, 253(1): 24.
doi: 10.1007/s00425-020-03550-8 |
| [14] |
Nakamura T, Nomura M, Mori H, et al. An isozyme of betaine aldehyde dehydrogenase in barley[J]. Plant Cell Physiol, 2001, 42(10): 1088-1092.
pmid: 11673624 |
| [15] |
Weretilnyk EA, Hanson AD. Betaine aldehyde dehydrogenase from spinach leaves: purification, in vitro translation of the mRNA, and regulation by salinity[J]. Arch Biochem Biophys, 1989, 271(1): 56-63.
pmid: 2712575 |
| [16] |
Ishitani M, Nakamura T, Han SY, et al. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid[J]. Plant Mol Biol, 1995, 27(2): 307-315.
doi: 10.1007/BF00020185 pmid: 7888620 |
| [17] |
Wood AJ, Saneoka H, Rhodes D, et al. Betaine aldehyde dehydrogenase in sorghum(molecular cloning and expression of two related genes)[J]. Plant Physiol, 1996, 110(4): 1301-1308.
doi: 10.1104/pp.110.4.1301 pmid: 8934627 |
| [18] |
Nakamura T, Yokota S, Muramoto Y, et al. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes[J]. Plant J, 1997, 11(5): 1115-1120.
doi: 10.1046/j.1365-313x.1997.11051115.x pmid: 9193078 |
| [19] |
Legaria J, Rajsbaum R, Muñoz-Clares RA, et al. Molecular characterization of two genes encoding betaine aldehyde dehydrogenase from amaranth. Expression in leaves under short-term exposure to osmotic stress or abscisic acid[J]. Gene, 1998, 218(1/2): 69-76.
doi: 10.1016/S0378-1119(98)00381-3 URL |
| [20] |
Hibino T, Meng YL, Kawamitsu Y, et al. Molecular cloning and functional characterization of two kinds of betaine-aldehyde dehydrogenase in betaine-accumulating mangrove Avicennia marina(Forsk.)Vierh[J]. Plant Mol Biol, 2001, 45(3): 353-363.
pmid: 11292080 |
| [21] | Yin XJ, Zhao YX, Luo D, et al. Isolating the promoter of a stress-induced gene encoding betaine aldehyde dehydrogenase from the halophyte Atriplex centralasiatica Iljin[J]. Biochim Biophys Acta, 2002, 1577(3): 452-456. |
| [22] |
Li QL, Gao XR, Yu XH, et al. Molecular cloning and characterization of betaine aldehyde dehydrogenase gene from Suaeda liaotun-gensis and its use in improved tolerance to salinity in transgenic tobacco[J]. Biotechnol Lett, 2003, 25(17): 1431-1436.
doi: 10.1023/A:1025003628446 URL |
| [23] |
Rezaei Qusheh Bolagh F, Solouki A, Tohidfar M, et al. Agrobacte-rium-mediated transformation of Persian walnut using BADH gene for salt and drought tolerance[J]. J Hortic Sci Biotechnol, 2021, 96(2): 162-171.
doi: 10.1080/14620316.2020.1812446 URL |
| [24] |
Di H, Tian Y, Zu HY, et al. Enhanced salinity tolerance in transgenic maize plants expressing a BADH gene from Atriplex micran-tha[J]. Euphytica, 2015, 206(3): 775-783.
doi: 10.1007/s10681-015-1515-z URL |
| [25] |
Ali A, Ali Q, Iqbal MS, et al. Salt tolerance of potato genetically engineered with the Atriplex canescens BADH gene[J]. Biol Plant, 2020, 64: 271-279.
doi: 10.32615/bp.2019.080 URL |
| [26] |
Liu ZH, Zhang HM, Li GL, et al. Enhancement of salt tolerance in alfalfa transformed with the gene encoding for betaine aldehyde dehydrogenase[J]. Euphytica, 2011, 178(3): 363-372.
doi: 10.1007/s10681-010-0316-7 URL |
| [27] |
Chaum S, Supaibulwatana K, Kirdmanee C. Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and salt-sensitive lines of indica rice(Oryza sativa L. ssp. indica)in response to salt stress[J]. J Agron Crop Sci, 2007, 193(3): 157-166.
doi: 10.1111/jac.2007.193.issue-3 URL |
| [28] |
Li PF, Cai J, Luo X, et al. Transformation of wheat Triticum aes-tivum with the HvBADH1 transgene from hulless barley improves salinity-stress tolerance[J]. Acta Physiol Plant, 2019, 41(9): 155.
doi: 10.1007/s11738-019-2940-8 |
| [29] | Wang FW, Wang ML, Guo C, et al. Cloning and characterization of a novel betaine aldehyde dehydrogenase gene from Suaeda cornic-ulata[J]. Genet Mol Res, 2016, 15(2): 15027848. |
| [30] |
Sun YL, Liu X, Fu L, et al. Overexpression of TaBADH increases salt tolerance in Arabidopsis[J]. Can J Plant Sci, 2019, 99: 546-555.
doi: 10.1139/cjps-2018-0190 URL |
| [31] |
Wakeel A, Asif AR, Pitann B, et al. Proteome analysis of sugar beet(Beta vulgaris L.)elucidates constitutive adaptation during the first phase of salt stress[J]. J Plant Physiol, 2011, 168(6): 519-526.
doi: 10.1016/j.jplph.2010.08.016 URL |
| [32] |
Liu H, Wang QQ, Yu MM, et al. Transgenic salt-tolerant sugar beet(Beta vulgaris L.)constitutively expressing an Arabidopsis thalia-na vacuolar Na/H antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots[J]. Plant Cell Environ, 2008, 31(9): 1325-1334.
doi: 10.1111/pce.2008.31.issue-9 URL |
| [33] | Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server[M]//The Proteomics Protocols Handbook. Totowa, NJ: Humana Press, 2005: 571-607. |
| [34] |
Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms[J]. Mol Biol Evol, 2018, 35(6): 1547-1549.
doi: 10.1093/molbev/msy096 pmid: 29722887 |
| [35] |
Hu B, Jin JP, Guo AY, et al. GSDS 2.0: an upgraded gene feature visualization server[J]. Bioinformatics, 2015, 31(8): 1296-1297.
doi: 10.1093/bioinformatics/btu817 pmid: 25504850 |
| [36] |
Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers[J]. Proc Int Conf Intell Syst Mol Biol, 1994, 2: 28-36.
pmid: 7584402 |
| [37] |
Lescot M, Déhais P, Thijs G, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences[J]. Nucleic Acids Res, 2002, 30(1): 325-327.
doi: 10.1093/nar/30.1.325 pmid: 11752327 |
| [38] |
Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0[J]. Bioinformatics, 2007, 23(21): 2947-2948.
doi: 10.1093/bioinformatics/btm404 pmid: 17846036 |
| [39] |
Pan SM, Moreau RA, Yu C, et al. Betaine accumulation and betaine-aldehyde dehydrogenase in spinach leaves[J]. Plant Physiol, 1981, 67(6): 1105-1108.
doi: 10.1104/pp.67.6.1105 pmid: 16661818 |
| [40] |
Weigel P, Weretilnyk EA, Hanson AD. Betaine aldehyde oxidation by spinach chloroplasts[J]. Plant Physiol, 1986, 82(3): 753-759.
doi: 10.1104/pp.82.3.753 pmid: 16665106 |
| [41] |
Min MH, Maung TZ, Cao Y, et al. Haplotype analysis of BADH1 by next-generation sequencing reveals association with salt tolerance in rice during domestication[J]. Int J Mol Sci, 2021, 22(14): 7578.
doi: 10.3390/ijms22147578 URL |
| [42] |
Wang SB, Liang HP, Wang HL, et al. The chromosome-scale genomes of Dipterocarpus turbinatus and Hopea hainanensis(Dipterocarpaceae)provide insights into fragrant oleoresin biosynthesis and hardwood formation[J]. Plant Biotechnol J, 2022, 20(3): 538-553.
doi: 10.1111/pbi.v20.3 URL |
| [43] | 武映宏, 黄东益, 王永, 等. 椰子ALDH基因家族的鉴定及生物信息学分析[J]. 热带生物学报, 2022, 13(6): 541-549. |
| Wu YH, Huang DY, Wang Y, et al. Identification and bioinformatics analysis of ALDH gene family in coconut[J]. J Trop Biol, 2022, 13(6): 541-549. | |
| [44] |
Basanagouda G, Ramesh S, Siddu CB, et al. A non-synonymous SNP in homolog of BADH2 gene is associated with fresh pod fragrance in dolichos bean(Lablab purpureus var. lignosus(Prain)Kumari)[J]. Genet Resour Crop Evol, 2023, 70(2): 373-380.
doi: 10.1007/s10722-022-01535-y |
| [45] |
Luo ML, Sun HJ, Ge WY, et al. Effect of glycine betaine treatment on aroma production of ‘Nanguo’ pears after long-term cold storage-possible involvement of ethylene synthesis and signal transduction pathways[J]. Food Bioprocess Technol, 2022, 15(6): 1327-1342.
doi: 10.1007/s11947-022-02813-4 |
| [46] |
Saensuk C, Ruangnam S, Pitaloka MK, et al. A SNP of betaine aldehyde dehydrogenase(BADH)enhances an aroma(2-acetyl-1-pyrroline)in sponge gourd(Luffa cylindrica)and ridge gourd(Luffa acutangula)[J]. Sci Rep, 2022, 12(1): 3718.
doi: 10.1038/s41598-022-07478-9 |
| [47] |
Moghaieb REA, Saneoka H, Fujita K. Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritima[J]. Plant Sci, 2004, 166(5): 1345-1349.
doi: 10.1016/j.plantsci.2004.01.016 URL |
| [48] | Senthilkumar M, Amaresan N, Sankaranarayanan A. Estimation of betaine aldehyde dehydrogenase(BADH)activity[M]// Plant-Microbe Interactions. New York: Humana, 2021: 101-102. |
| [49] | 何晓兰, 侯喜林, 吴纪中, 等. 三角叶滨藜甜菜碱醛脱氢酶(BADH)基因的克隆及序列分析[J]. 南京农业大学学报, 2004, 27(1): 15-19. |
| He XL, Hou XL, Wu JZ, et al. Molecular cloning and sequence analysis of betaine-aldehyde dehydrogenase(BADH)in Atriplex triangularis[J]. J Nanjing Agric Univ, 2004, 27(1): 15-19. | |
| [50] | 陈翠萍. 藜麦BADH基因家族鉴定及生物信息学分析[J]. 分子植物育种, 2023, 21(19): 6276-6284. |
| Chen CP. Identification and bioinformatics analysis of BADH gene family in quinoa[J]. Mol Plant Breed, 2023, 21(19): 6276-6284. | |
| [51] | 刘振林, 曹华雯, 夏新莉, 等. 甘菊BADH基因cDNA的克隆及在盐胁迫下的表达[J]. 武汉植物学研究, 2009, 27(1): 1-7. |
| Liu ZL, Cao HW, Xia XL, et al. Cloning and expression analysis of betaine aldehyde dehydrogenase gene from Dendranthema lavan-dulifolium on salinity[J]. J Wuhan Bot Res, 2009, 27(1): 1-7. | |
| [52] | 唐露, 杨振, 白蓓蓓, 等. 香蕉BADH基因序列及表达特性分析[J]. 分子植物育种, 2019, 17(3): 719-728. |
| Tang L, Yang Z, Bai BB, et al. Sequence analysis and expression characteristics of BADH gene in banana[J]. Mol Plant Breed, 2019, 17(3): 719-728. | |
| [53] |
Ding X, Li JH, Pan Y, et al. Genome-wide identification and expression analysis of the UGlcAE gene family in tomato[J]. Int J Mol Sci, 2018, 19(6): 1583.
doi: 10.3390/ijms19061583 URL |
| [54] |
Liu Z, Ge XY, Yang ZR, et al. Genome-wide identification and characterization of SnRK2 gene family in cotton(Gossypium hirsu-tum L.)[J]. BMC Genet, 2017, 18(1): 54.
doi: 10.1186/s12863-017-0517-3 URL |
| [55] |
Zhang Y, Yin H, Li D, et al. Functional analysis of BADH gene promoter from Suaeda liaotungensis K[J]. Plant Cell Rep, 2008, 27(3): 585-592.
doi: 10.1007/s00299-007-0459-8 pmid: 17924116 |
| [56] |
Li CY, Zhang TP, Feng P, et al. Genetic engineering of glycinebetaine synthesis enhances cadmium tolerance in BADH-transgenic tobacco plants via reducing cadmium uptake and alleviating cadmium stress damage[J]. Environ Exp Bot, 2021, 191: 104602.
doi: 10.1016/j.envexpbot.2021.104602 URL |
| [57] |
Shahzad A, Qian MC, Sun BY, et al. Genome-wide association study identifies novel loci and candidate genes for drought stress tolerance in rapeseed[J]. Oil Crop Sci, 2021, 6(1): 12-22.
doi: 10.1016/j.ocsci.2021.01.002 URL |
| [58] |
Zhang L, Tian JJ, Ye LZ, et al. Transcriptomic analysis reveals the mechanism of low/high temperature resistance in an outstanding diet alga Nannochloropsis oceanica[J]. Aquaculture Reports, 2022, 27: 101365.
doi: 10.1016/j.aqrep.2022.101365 URL |
| [59] |
Ming RH, Zhang Y, Wang Y, et al. The JA-responsive MYC2-BADH-like transcriptional regulatory module in Poncirus trifoliata contributes to cold tolerance by modulation of glycine betaine biosynthesis[J]. New Phytol, 2021, 229(5): 2730-2750.
doi: 10.1111/nph.v229.5 URL |
| [60] |
Feng CG, Liu RY, Xu WJ, et al. The genome of a new anemone species(Actiniaria: Hormathiidae)provides insights into deep-sea adaptation[J]. Deep Sea Res Part I Oceanogr Res Pap, 2021, 170:103492.
doi: 10.1016/j.dsr.2021.103492 URL |
| [61] |
Jacques F, Zhao YJ, Kopečná M, et al. Roles for ALDH10 enzymes in γ-butyrobetaine synthesis, seed development, germination, and salt tolerance in Arabidopsis[J]. J Exp Bot, 2020, 71(22): 7088-7102.
doi: 10.1093/jxb/eraa394 URL |
| [1] | XU Yang, ZHANG Rui-ying, DAI Liang-xiang, ZHANG Guan-chu, DING Hong, ZHANG Zhi-meng. Regulation of Nitrogen Application on Peanut Seed Germination and Spermosphere Bacterial Community Structure Under Salt Stress [J]. Biotechnology Bulletin, 2024, 40(2): 253-265. |
| [2] | WANG Yu-qing, MA Zi-qi, HOU Jia-xin, ZONG Yu-qi, HAO Han-rui, LIU Guo-yuan, WEI Hui, LIAN Bo-lin, CHEN Yan-hong, ZHANG Jian. Research Progress in the Composition Analysis and Ecological Function of Plant Root Exudates Under Salt Stress [J]. Biotechnology Bulletin, 2024, 40(1): 12-23. |
| [3] | BI Fang-ling, ZHAO Shuang, LI Bin, LI Ai-qin, ZHANG Jian-heng, HE Pei-min. Research Progresses and Application in the Growth-promoting Effect of Symbiotic and Epiphytic Bacteria on Green Tide-causing Ulva prolifera [J]. Biotechnology Bulletin, 2024, 40(1): 32-44. |
| [4] | WANG Shuai, FENG Yu-mei, BAI Miao, DU Wei-jun, YUE Ai-qin. Functional Analysis of Soybean Gene GmHMGR Responding to Exogenous Hormones and Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(7): 131-142. |
| [5] | WEI Xi-ya, QIN Zhong-wei, LIANG La-mei, LIN Xin-qi, LI Ying-zhi. Mechanism of Melatonin Seed Priming in Improving Salt Tolerance of Capsicum annuum [J]. Biotechnology Bulletin, 2023, 39(7): 160-172. |
| [6] | WANG Hai-long, LI Yu-qian, WANG Bo, XING Guo-fang, ZHANG Jie-wei. Isolation and Expression Analysis of SiMAPK3 in Setaria italica L. [J]. Biotechnology Bulletin, 2023, 39(3): 123-132. |
| [7] | DU Qing-jie, ZHOU Lu-yao, YANG Si-zhen, ZHANG Jia-xin, CHEN Chun-lin, LI Juan-qi, LI Meng, ZHAO Shi-wen, XIAO Huai-juan, WANG Ji-qing. Overexpression of CaCP1 Enhances Salt Stress Sensibility in Transgenic Tobacco [J]. Biotechnology Bulletin, 2023, 39(2): 172-182. |
| [8] | YE Hong, WANG Yu-kun. Research Progress in Immune Receptor Functions of Pattern-Recognition Receptor in Plants [J]. Biotechnology Bulletin, 2023, 39(12): 1-15. |
| [9] | YAN Xiong-ying, WANG Zhen, WANG Xia, YANG Shi-hui. Microbial Sulfur Metabolism and Stress Resistance [J]. Biotechnology Bulletin, 2023, 39(11): 150-167. |
| [10] | WANG Ming-tao, LIU Jian-wei, ZHAO Chun-zhao. Molecular Mechanisms of Cell Wall Integrity in Plants Under Salt Stress [J]. Biotechnology Bulletin, 2023, 39(11): 18-27. |
| [11] | ZHANG Yu-juan, LI Dong-hua, GONG Hui-hui, CUI Xin-xiao, GAO Chun-hua, ZHANG Xiu-rong, YOU Jun, ZHAO Jun-sheng. Cloning and Salt-tolerance Analysis of NAC Transcription Factor SiNAC77 from Sesamum indicum L. [J]. Biotechnology Bulletin, 2023, 39(11): 308-317. |
| [12] | XU Yang, DING Hong, ZHANG Guan-chu, GUO Qing, ZHANG Zhi-meng, DAI Liang-xiang. Metabolomics Analysis of Germinating Peanut Seed Under Salt Stress [J]. Biotechnology Bulletin, 2023, 39(1): 199-213. |
| [13] | ZHANG Bin, YANG Xin-xia. Identification of Key Transcription Factors in Response to Salt Stress in Rice [J]. Biotechnology Bulletin, 2022, 38(3): 9-15. |
| [14] | ZHANG Ye-meng, ZHU Li-li, CHEN Zhi-guo. Identification and Expression Analysis of NHX Gene Family in Quinoa Under Salt Stress [J]. Biotechnology Bulletin, 2022, 38(12): 184-193. |
| [15] | ZHANG Tong-tong, ZHENG Deng-yu, WU Zhong-yi, ZHANG Zhong-bao, YU Rong. Functional Analysis of ZmNF-YB13 Responding to Drought and Salt Stress [J]. Biotechnology Bulletin, 2022, 38(10): 115-123. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||