








生物技术通报 ›› 2021, Vol. 37 ›› Issue (10): 1-8.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0072
• 研究报告 • 下一篇
收稿日期:2021-01-18
出版日期:2021-10-26
发布日期:2021-11-12
作者简介:方丹丹,女,硕士研究生,研究方向:基因工程;E-mail: 基金资助:
FANG Dan-dan1( ), ZHANG Ting2, WEN Xiao-peng1(
), ZHANG Ting2, WEN Xiao-peng1( )
)
Received:2021-01-18
Published:2021-10-26
Online:2021-11-12
摘要:
旨在探讨马尾松PmPT3基因在低磷胁迫条件下对拟南芥磷吸收利用的影响,实现林木优良基因的利用。通过构建马尾松PmPT3基因超表达载体pBWA(V)HS-PmPT3,采用花序浸染法遗传转化拟南芥。经筛选和检测,共获得4株T3代纯合株系;磷处理结果表明:超表达马尾松PmPT3基因显著提高了转基因拟南芥超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)的活性,分别是野生型植株的2.17倍、1.59倍、1.81倍;丙二醛(MDA)含量较野生型植株降低了47.58%。转基因拟南芥的地上部分和根部总磷含量及无机磷含量较野生型相比,分别提高了1.26倍和1.74倍及1.38倍和1.89倍。转基因植株较野生型地上部干重提高了45.46%,根干重提高了55.56%,总干重提高了46.15%。与正常供磷条件相比,处于低磷胁迫下的拟南芥转基因植株,其根部和地上部分中的PmPT3基因表达量显著上调,且在根部达到极显著水平。结果表明,超表达马尾松PmPT3基因可以提高低磷胁迫下拟南芥中保护酶活性、降低丙二醛产生,促进拟南芥对磷元素的吸收,从而提高拟南芥耐低磷胁迫的能力,为通过基因工程创制耐低磷新种质提供了科学依据。
方丹丹, 张婷, 文晓鹏. 超表达马尾松PmPT3基因提高拟南芥耐低磷能力[J]. 生物技术通报, 2021, 37(10): 1-8.
FANG Dan-dan, ZHANG Ting, WEN Xiao-peng. Overexpression of Pinus massoniana PmPT3 Gene in Arabidopsis thaliana Increasing Low Phosphorus Tolerance[J]. Biotechnology Bulletin, 2021, 37(10): 1-8.
| 引物Primer | 序列Sequence(5'-3') | 退火温度Annealing temperature/℃ |
|---|---|---|
| PmPT3-F | CAGTGGTCTCACAACATGGGC- GACAATGAGGGG | 55 |
| PmPT3-R | CAGTGGTCTCATACACTACAC- GGGCATAGTTCTGT | |
| Actin2-qRTF | ACGGTAACATTGTGCTCAGT- GGTG | 59 |
| Actin2-qRTR | CTTGGAGATCCACATCTGCTGGA | |
| PmPT3-qRTF | CTATCCGCTTTCAGCCACCA | 59 |
| PmPT3-qRTR | TTCTCCACACAAAATCCGCC |
表1 实验所用引物及序列
Table 1 Primers and sequences used in the study
| 引物Primer | 序列Sequence(5'-3') | 退火温度Annealing temperature/℃ |
|---|---|---|
| PmPT3-F | CAGTGGTCTCACAACATGGGC- GACAATGAGGGG | 55 |
| PmPT3-R | CAGTGGTCTCATACACTACAC- GGGCATAGTTCTGT | |
| Actin2-qRTF | ACGGTAACATTGTGCTCAGT- GGTG | 59 |
| Actin2-qRTR | CTTGGAGATCCACATCTGCTGGA | |
| PmPT3-qRTF | CTATCCGCTTTCAGCCACCA | 59 |
| PmPT3-qRTR | TTCTCCACACAAAATCCGCC |

图1 马尾松PmPT3基因植物表达载体的构建 M:DL2000 DNA marker;A:添加酶切位点的PmPT3基因片段;B:BLUNT-PmPT3双酶切产物;C:pBWA(V)HS双酶切产物;D:重组质粒pBWA(V)HS-PmPT3双酶切产物;E:1-11菌落PCR产物
Fig.1 Construction of plant expression vector of P. masso-niana PmPT3 Gene M: DL2000 DNA marker; A: PmPT3 fragment with adding restriction sites; B: BLUNT-PmPT3 enzyme digestion product; C: pBWA(V)HS denzyme digestion product; D: recombinant plasmid pBWA(V) HS-PmPT3 enzyme digestion product; E: 1-11 colony PCR product

图2 马尾松PmPT3遗传转化拟南芥过程 LP:低磷(0.125 mmol/L);TG:转基因植株;A,B:T1 代转基因拟南芥筛选;C,D:T2 代转基因拟南芥筛选;E,F:T3 代拟南芥纯合株系筛选;G,H:磷处理20 d后拟南芥表型
Fig.2 Process of genetic transformation of P. massoniana PmPT3 into Arabidopsis LP: Low phosphorus (0.125 mmol/L) ; TG; transgenic plants; A, B: T1 generation transgenic Arabidopsis selection; C, D: T2 generation transgenic Arabidopsis selection; E, F: screening of T3 generation pure commonzanara; G, H: phenotype of Arabidopsis thaliana after 20 days of phosphorus treatment

图3 T1代转基因拟南芥qRT-PCR分析(A)及纯合株系PCR检测(B) 图中不同小写字母表示在P<0.05水平上差异显著
Fig.3 Analysis of positive gene expression in T1 generation transgenic Arabidopsis thaliana (A) and PCR detection of homozygous strains (B) Different lowercase letters in the figure indicate significant differences at the P<0.05 level
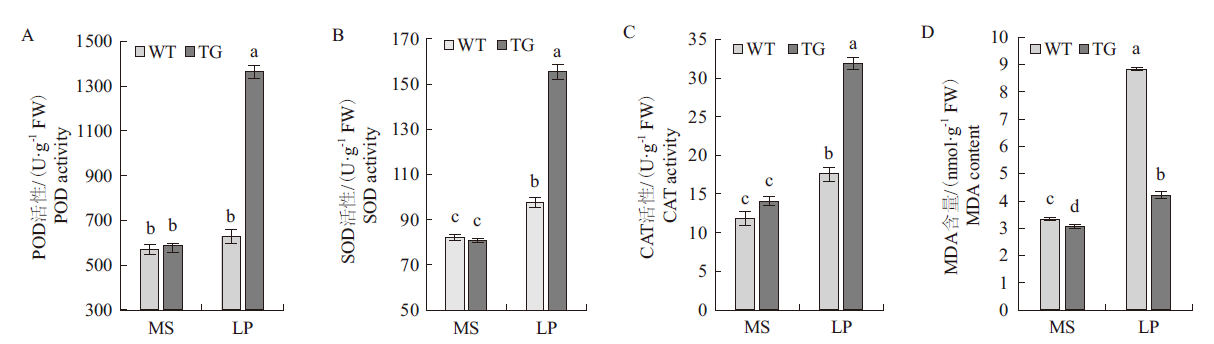
图4 低磷胁迫对转基因拟南芥POD(A)、SOD(B)、CAT(C)活性及MDA(D)含量的影响 LP:低磷(0.125 mmol/L);TG:转基因植株;图中不同小写字母表示在P<0.05水平上差异显著
Fig.4 Effect of low phosphorus stress on the POD(A), SOD(B), CAT(C) activity and MDA(D) content of transgenic Arabidopsis LP: Low phosphorus (0.125 mmol/L); TG: transgenic plants; different lowercase letters in the figure indicate significant differences at the P<0.05 level. The same below

图5 低磷胁迫对转基因拟南芥总磷含量(A)和无机磷含量(B)的影响
Fig.5 Effect of low phosphorus stress on the total phosphorus content (A) and inorganic phosphorus content (B) of transgenic Arabidopsis
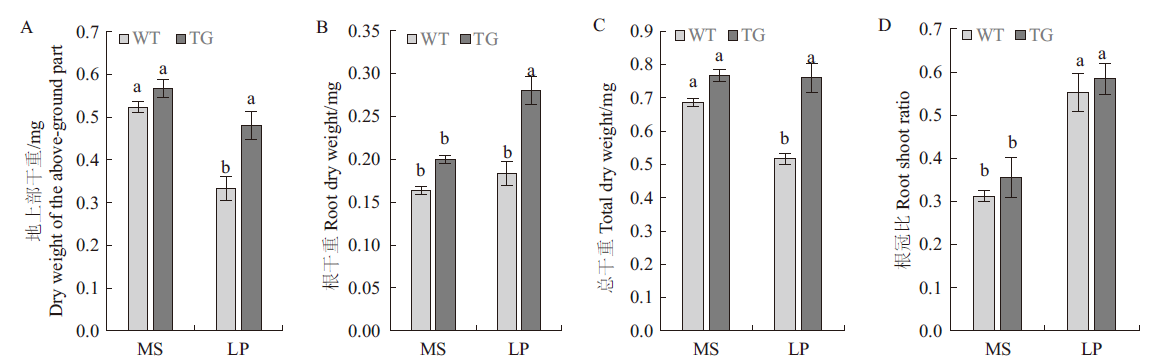
图6 低磷胁迫对转基因拟南芥地上部干重(A)、根干重(B)、总干重(C)和根冠比(D)的影响
Fig.6 Effect of low phosphorus stress on the above-ground dry weight (A), root dry weight (B), total dry weight (C) and root to shoot ratio (D) of transgenic Arabidopsis
| [1] |
Patel M, Rangani J, Kumari A, et al. Mineral nutrient homeostasis, photosynthetic performance, and modulations of antioxidative defense components in two contrasting genotypes of Arachis hypogaea L. (peanut)for mitigation of nitrogen and/or phosphorus starvation[J]. Journal of Biotechnology, 2020, 323:136-158.
doi: 10.1016/j.jbiotec.2020.08.008 URL |
| [2] | 许仙菊, 张永春. 植物耐低磷胁迫的根系适应性机制研究进展[J]. 江苏农业学报, 2018, 34(6):1425-1429. |
| Xu XJ, Zhang YC. Research progress on the root adaptation mechanism of plants under low phosphorus stress[J]. Jiangsu Journal of Agricultural Sciences, 2018, 34(6):1425-1429. | |
| [3] | 王保明, 陈永忠, 王湘南, 等. 植物低磷胁迫响应及其调控机制[J]. 福建农林大学学报:自然科学版, 2015, 44(6):567-575. |
| Wang BM, Chen YZ, Wang XN, et al. The response to low phosphorus stress and its regulation mechanism in plants[J]. Journal of Fujian Agriculture and Forestry University:Natural Science Edition, 2015, 44(6):567-575. | |
| [4] |
Liu Y, Mi GH, Chen FJ, et al. Rhizosphere effect and root growth of two maize(Zea mays L.)genotypes with contrasting P efficiency at low P availability[J]. Plant Science, 2004, 167(2):217-223.
doi: 10.1016/j.plantsci.2004.02.026 URL |
| [5] |
Zhang T, Wen XP, Ding GJ. Ectomycorrhizal symbiosis enhances tolerance to low phosphorous through expression of phosphate transporter genes in Masson pine(Pinus massoniana)[J]. Acta Physiologiae Plantarum, 2017, 39(4):1-12.
doi: 10.1007/s11738-016-2300-x URL |
| [6] | Smith FW, Rae AL, Hawkesford MJ. Molecular mechanisms of phosphate and sulphate transport in plants[J]. Biochimica et Biophysica Acta:BBA - Biomembranes, 2000, 1465(1/2):236-245. |
| [7] |
Mudge SR, Rae AL, Diatloff E, et al. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis[J]. The Plant Journal, 2002, 31(3):341-353.
doi: 10.1046/j.1365-313X.2002.01356.x URL |
| [8] | 陈丽玉, 秦璐, 赵静, 等. 豆科植物Pht1磷转运蛋白家族基因的研究进展[J]. 大豆科学, 2015, 34(6):1057-1065. |
| Chen LY, Qin L, Zhao J, et al. Advances in Pht1 phosphate transporter family genes in legumes[J]. Soybean Science, 2015, 34(6):1057-1065. 1058-1065. | |
| [9] | 李慧平, 王庆竹, 汤纬玮, 等. 超表达马尾松PmMYB169基因提高转基因拟南芥耐低磷能力[J]. 分子植物育种, 2018, 16(24):8048-8055. |
| Li HP, Wang QZ, Tang WW, et al. Low phosphorus tolerance of transgenic Arabidopsis thaliana enhanced by overexpression of Pinus massoniana PmMYB169 gene[J]. Molecular Plant Breeding, 2018, 16(24):8048-8055. | |
| [10] | 曹玉曼. 蒺藜苜蓿磷转运体基因MtPT5和MtPT6功能研究[D]. 杨凌:西北农林科技大学, 2019. |
| Cao YM. Functional analysis of phosphate transporter genes MtPT5 and MtPT6 in Medicago truncatula[D]. Yangling:Northwest A & F University, 2019. | |
| [11] | Xu YJ, Bao H, Fei HT, et al. Overexpression of a phosphate transporter gene ZmPt9 from maize influences growth of transgenic Arabidopsis thaliana[J]. Biochemical and Biophysical Research Communications, 2020 |
| [12] |
Naureen Z, Sham A, Al Ashram H, et al. Effect of phosphate nutrition on growth, physiology and phosphate transporter expression of cucumber seedlings[J]. Plant Physiology and Biochemistry, 2018, 127:211-222.
doi: S0981-9428(18)30151-7 pmid: 29614440 |
| [13] | 陈婉婷, 陈冉红, 李娇阳, 等. 杉木磷转运蛋白ClPht1;2基因克隆与表达特性分析[J]. 西北林学院学报, 2020, 35(5):1-8. |
| Chen WT, Chen RH, Li JY, et al. Cloning and expression analysis of phosphorus transporter gene ClPht1;2 in Cunninghamia lanceolata[J]. Journal of Northwest Forestry University, 2020, 35(5):1-8. | |
| [14] |
Fan XN, Che XR, Lai WZ, et al. The auxin-inducible phosphate transporter AsPT5 mediates phosphate transport and is indispensable for arbuscule formation in Chinese milk vetch at moderately high phosphate supply[J]. Environmental Microbiology, 2020, 22(6):2053-2079.
doi: 10.1111/emi.v22.6 URL |
| [15] | 孙晓波, 陈佩珍, 吴晓刚, 等. 马尾松PmAOX基因克隆与不同逆境胁迫表达分析[J]. 南京林业大学学报:自然科学版, 2020, 44(4):70-78. |
| Sun XB, Chen PZ, Wu XG, et al. The cloning and expression analysis of PmAOX gene from Pinus massoniana under different stress[J]. Journal of Nanjing Forestry University:Natural Sciences Edition, 2020, 44(4):70-78. | |
| [16] |
Yang SH, Feng Y, Zhao Y, et al. Overexpression of a Eutrema salsugineum phosphate transporter gene EsPHT1;4 enhances tolerance to low phosphorus stress in soybean[J]. Biotechnology Letters, 2020, 42(11):2425-2439.
doi: 10.1007/s10529-020-02968-0 URL |
| [17] | Zhang CX, Meng S, Li MJ, et al. Genomic identification and expression analysis of the phosphate transporter gene family in poplar[J]. Frontiers in Plant Science, 2016, 7:1398. |
| [18] | 张传玲. VIGS介导的番茄高亲和磷转运蛋白基因SlPT1功能分析[D]. 沈阳:沈阳农业大学, 2019. |
| Zhang CL. Functional analysis of SlPT1, a tomato high-affinity phosphate transporter gene by VIGS[D]. Shenyang:Shenyang Agricultural University, 2019. | |
| [19] | Sun Y, Gao L, Wang D, et al. Identification and expression analysis of the Hevea brasiliensis phosphate transporter 1 gene family[J]. Trees, 2020:1-13. |
| [20] |
Liu F, Chang XJ, Ye Y, et al. Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice[J]. Molecular Plant, 2011, 4(6):1105-1122.
doi: 10.1093/mp/ssr058 URL |
| [21] |
Cao MX, Liu HZ, Zhang C, et al. Functional analysis of StPHT1;7, a Solanum tuberosum L. phosphate transporter gene, in growth and drought tolerance[J]. Plants, 2020, 9(10):1384.
doi: 10.3390/plants9101384 URL |
| [22] |
Gechev T, Petrov V. Reactive oxygen species and abiotic stress in plants[J]. International Journal of Molecular Sciences, 2020, 21(20):7433.
doi: 10.3390/ijms21207433 URL |
| [23] | Takagi D, Miyagi A, Tazoe Y, et al. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves[J]. Plant, Cell & Environment, 2020, 43(9):2033-2053. |
| [24] |
Roch GV, Maharajan T, Krishna TPA, et al. Expression of PHT1 family transporter genes contributes for low phosphate stress tolerance in foxtail millet(Setaria italica)genotypes[J]. Planta, 2020, 252(6):1-9.
doi: 10.1007/s00425-020-03403-4 URL |
| [25] |
Wu XL, Jia QY, Ji SX, et al. Gamma-aminobutyric acid(GABA)alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synjournal and reactive oxygen species metabolism[J]. BMC Plant Biology, 2020, 20(1):1-21.
doi: 10.1186/s12870-019-2170-7 URL |
| [26] |
da Ros LM, Mansfield SD. Biotechnological mechanism for improving plant remobilization of phosphorus during leaf senescence[J]. Plant Biotechnology Journal, 2020, 18(2):470-478.
doi: 10.1111/pbi.v18.2 URL |
| [1] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [2] | 徐红云, 吕俊, 于存. 根际溶磷伯克霍尔德菌Paraburkholderia spp.对马尾松苗的促生作用[J]. 生物技术通报, 2023, 39(6): 274-285. |
| [3] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| [4] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [5] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| [6] | 陈浩婷, 张玉静, 刘洁, 代泽敏, 刘伟, 石玉, 张毅, 李天来. 低磷胁迫下番茄转录因子WRKY6功能分析[J]. 生物技术通报, 2023, 39(10): 136-147. |
| [7] | 阮航, 多浩源, 范文艳, 吕清晗, 姜述君, 朱生伟. AtERF49在拟南芥应答盐碱胁迫中的作用[J]. 生物技术通报, 2023, 39(1): 150-156. |
| [8] | 林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165. |
| [9] | 贺丽娜, 冯源, 石慧敏, 叶建仁. 具有杀线活性马尾松内生细菌的筛选与鉴定[J]. 生物技术通报, 2022, 38(8): 159-166. |
| [10] | 高聪, 萧楚健, 鲁帅, 王苏蓉, 袁卉华, 曹云英. 氧化石墨烯对拟南芥生长的促进作用[J]. 生物技术通报, 2022, 38(6): 120-128. |
| [11] | 徐红云, 张明意. GRAS转录因子AtSCL4负调控拟南芥应答渗透胁迫[J]. 生物技术通报, 2022, 38(6): 129-135. |
| [12] | 古盼, 齐学影, 李莉, 张曦, 单晓昳. AtRGS1胞吞动态调控G蛋白参与拟南芥生长发育和抗性反应[J]. 生物技术通报, 2022, 38(6): 34-42. |
| [13] | 镐青青, 姚圣, 刘佳禾, 陈佩珍, 张梦洋, 季孔庶. 马尾松NAC转录因子基因PmNAC8的克隆及表达分析[J]. 生物技术通报, 2022, 38(4): 202-216. |
| [14] | 周娟, 阎晋东, 李新梅, 刘雪晴, 赵强, 赵小英. 拟南芥F-box蛋白FKF1与转录因子FUL互作调控开花研究[J]. 生物技术通报, 2022, 38(3): 1-8. |
| [15] | 杨延, 于龙凤, 王绍梅, 李卫娜, 葛锋. 三七细胞中共超表达FPS、SS对皂苷合成的影响[J]. 生物技术通报, 2022, 38(3): 50-58. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||