








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 69-76.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1062
收稿日期:2021-08-19
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:李佳乐,女,研究方向:食品质量与安全;E-mail: 基金资助:
LI Jia-le1( ), LIN Sheng-hao2, XU Wen-tao1,2(
), LIN Sheng-hao2, XU Wen-tao1,2( )
)
Received:2021-08-19
Published:2022-08-26
Online:2022-09-14
摘要:
随着转基因技术的发展,快速、稳定和简单的转基因作物检测技术的开发一直是热点。开发了以溴百里酚蓝为显色剂的基于环介导等温扩增的抗虫基因超灵敏比色生物传感器,该方法可以通用检测Cry1Ac和Cry1Ab/Ac基因,具有较好的特异性。新型pH指示剂溴百里酚蓝对于LAMP影响小,通过一步法闭管可视检测,解决了LAMP容易污染的问题,实现了自然光下裸眼可视化半定量检测。最终优化的体系为Tris终浓度3.25 mmol/L,0.1%溴百里酚蓝添加量1.5 µL,在此条件下1 h内可检测到单拷贝靶标基因。该方法兼具灵敏性、特异性和稳定性,操作简单,适合现场检测,提供了Cry1Ac和Cry1Ab/Ac基因通用检测的具体操作方法和新的LAMP比色信号输出方式,不仅完善了转基因产品的检测体系,也丰富了LAMP生物传感器信号输出方式。
李佳乐, 林晟豪, 许文涛. 基于环介导等温扩增的抗虫基因超灵敏比色生物传感器构建[J]. 生物技术通报, 2022, 38(8): 69-76.
LI Jia-le, LIN Sheng-hao, XU Wen-tao. Construction of an Ultra-sensitive Colorimetric Biosensor for Insect Resistance Genes Based on Loop-mediated Isothermal Amplification[J]. Biotechnology Bulletin, 2022, 38(8): 69-76.
| 品种 Variety | 测试样品 Testing sample | Cry1A基因信息 Cry1A gene information | 研发单位 R & D unit |
|---|---|---|---|
| 转基因水稻 | Kefeng 6 | Cry1Ac | 中国科学院遗传与发育研究所、福建省农业科学院 |
| Kefeng 8 | Cry1Ac | 中国科学院遗传与发育生物学研究所、福建省农业科学院生物技术研究所、山西省农业科学院棉花研究所 | |
| TT51 | Cry1Ab/Ac | 华中农业大学 | |
| 转基因玉米 | Bt11 | Cry1Ab | 先正达种子有限公司 |
| Bt176 | Cry1Ab | 先正达种子有限公司 | |
| MON 810 | Cry1Ab | 美国孟山都公司 | |
| 转基因棉花 | MON 15985 | Cry1Ac | 美国孟山都公司 |
| 转基因甜菜 | H7-1 | - | 美国孟山都公司 |
| 非转基因作物 | 五优稻4号 | - | 五常市龙凤山长粒香水稻研究所 |
| 粳稻糙米 | - | 五常市龙凤山长粒香水稻研究所 |
表1 测试转基因及非转基因样品Cry1A基因具体信息
Table 1 Test specific information of Cry1A gene in GMO and non-GMO samples
| 品种 Variety | 测试样品 Testing sample | Cry1A基因信息 Cry1A gene information | 研发单位 R & D unit |
|---|---|---|---|
| 转基因水稻 | Kefeng 6 | Cry1Ac | 中国科学院遗传与发育研究所、福建省农业科学院 |
| Kefeng 8 | Cry1Ac | 中国科学院遗传与发育生物学研究所、福建省农业科学院生物技术研究所、山西省农业科学院棉花研究所 | |
| TT51 | Cry1Ab/Ac | 华中农业大学 | |
| 转基因玉米 | Bt11 | Cry1Ab | 先正达种子有限公司 |
| Bt176 | Cry1Ab | 先正达种子有限公司 | |
| MON 810 | Cry1Ab | 美国孟山都公司 | |
| 转基因棉花 | MON 15985 | Cry1Ac | 美国孟山都公司 |
| 转基因甜菜 | H7-1 | - | 美国孟山都公司 |
| 非转基因作物 | 五优稻4号 | - | 五常市龙凤山长粒香水稻研究所 |
| 粳稻糙米 | - | 五常市龙凤山长粒香水稻研究所 |
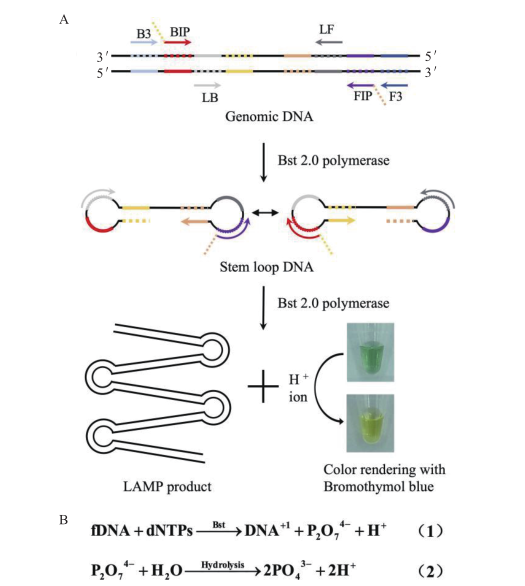
图1 检测原理图 A:实验原理图;B:LAMP反应H+产生的化学方程式示意图
Fig. 1 Detection principle diagram A:Schematic diagram of the experiment. B:Schematic diagram of the chemical equation generated by the LAMP reaction H+
| 引物名称 Primer name | 序列 Sequence(5'-3') | |
|---|---|---|
| Cry1Ac&Ab/Ac -1 | F3 | GGTGGAAGACAAGGTTCTGT |
| B3 | TCTACACCGATGCTCACAGA | |
| FIP | GGAACTATGGGAAACGCCGCT- AGACACCCTGACCTAGTTGA | |
| BIP | GAGGAAAGGTAAACTCGGGCCC- TGGTCTGGACACCAGATCA | |
| LF | CACAACAACGTATCGTTGC | |
| LB | GCTGAATCCAACTGGAGAGGC | |
| Cry1Ac&Ab/Ac -2 | F3 | TGATGCTCACGGAACTGTTG |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CCACCCAGGCAAGGATTCTCC- TGAATCCGGAACGGAACATG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | ACAGGTTGAGCCACGTGTC | |
| LB | AATCAACGGTACCGCTCTTTC | |
| Cry1Ac&Ab/Ac -3 | F3 | GGAGCTCTGATGATGCTCAC |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CAGGCAAGGATTCTCCCACAGG- GGAACTGTTGCTGAATCCGG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | CCACGTGTCCATGTTCCGTT | |
| LB | ACGGTACCGCTCTTTCTGT | |
表2 LAMP引物设计表
Table 2 LAMP primer design table
| 引物名称 Primer name | 序列 Sequence(5'-3') | |
|---|---|---|
| Cry1Ac&Ab/Ac -1 | F3 | GGTGGAAGACAAGGTTCTGT |
| B3 | TCTACACCGATGCTCACAGA | |
| FIP | GGAACTATGGGAAACGCCGCT- AGACACCCTGACCTAGTTGA | |
| BIP | GAGGAAAGGTAAACTCGGGCCC- TGGTCTGGACACCAGATCA | |
| LF | CACAACAACGTATCGTTGC | |
| LB | GCTGAATCCAACTGGAGAGGC | |
| Cry1Ac&Ab/Ac -2 | F3 | TGATGCTCACGGAACTGTTG |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CCACCCAGGCAAGGATTCTCC- TGAATCCGGAACGGAACATG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | ACAGGTTGAGCCACGTGTC | |
| LB | AATCAACGGTACCGCTCTTTC | |
| Cry1Ac&Ab/Ac -3 | F3 | GGAGCTCTGATGATGCTCAC |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CAGGCAAGGATTCTCCCACAGG- GGAACTGTTGCTGAATCCGG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | CCACGTGTCCATGTTCCGTT | |
| LB | ACGGTACCGCTCTTTCTGT | |
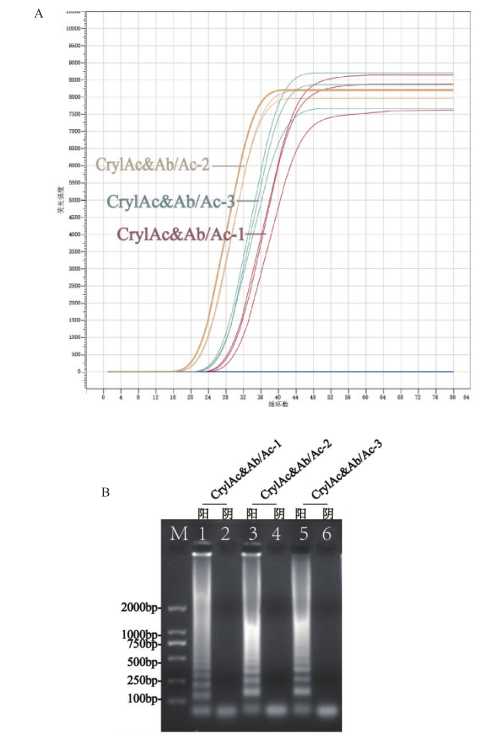
图2 引物筛选图 A:3组引物的实时扩增曲线;B:3组引物扩增终点的电泳图
Fig.2 Primer screening diagram A:Real-time amplification curve of 3 sets of primers. B:Electropherogram of the end point of 3 sets of primers amplification
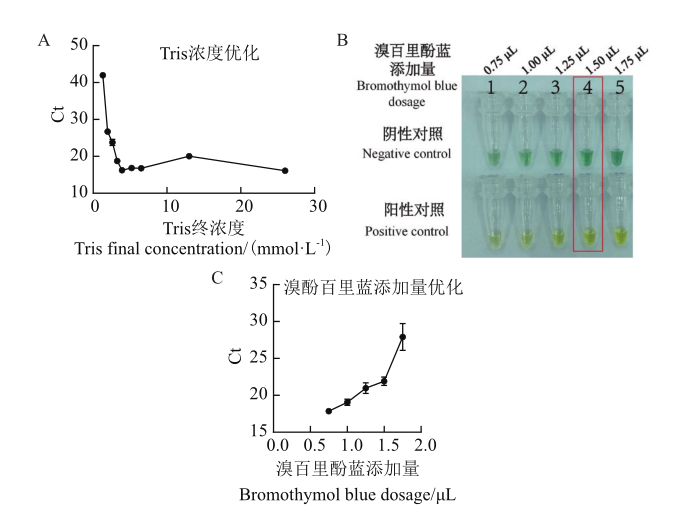
图3 比色方法可行性及优化 A:Tris浓度优化;B:不同体积BTB添加量下的指示效果比较;C:不同添加量BTB的影响图
Fig. 3 Feasibility and optimization of colorimetric method A:Tris concentration optimization. B:Comparison of indication effects under different volumes of bromothymol blue addition. C:Influence diagram of different addition amounts of bromothymol blue
| [1] | 李葱葱, 闫伟, 夏蔚, 等. 应用简并PCR方法检测转cry1A基因作物[J]. 食品科学, 2018, 39(14):317-322. |
|
Li CC, Yan W, Xia, et al. Detection of genetically modified crops with cry1A gene by PCR with degenerate primers[J]. Food Sci, 2018, 39(14):317-322.
doi: 10.1111/j.1365-2621.1974.tb02884.x URL |
|
| [2] | H·安德森, J·杜格拉斯, J·格罗亚特, 等. 对应于转基因事件MON89034的玉米植物和种子及其检测和使用方法:中国, CN101495635[P]. 2009-07-29. |
| Anderson H, Duglas J, Groat J, et al. Maize plants and seeds corresponding to the transgenic event MON89034 and methods for their detection and use:China, CN101495635[P]. 2009-07-29. | |
| [3] | 赖锦盛, 董永彬, 宋伟彬, 等. 人工合成用于转基因抗虫植物的Bt杀虫基因:中国, CN101580843[P]. 2009-11-18. |
| Lai JS, Dong YB, Song WB, et al. Artificial synthesis of Bt insecticide genes for transgenic insect-resistant plants:China, CN101580843[P]. 2009-11-18. | |
| [4] |
Brookes G, Barfoot P. GM crop technology use 1996-2018:farm income and production impacts[J]. GM Crops Food, 2020, 11(4):242-261.
doi: 10.1080/21645698.2020.1779574 pmid: 32706314 |
| [5] |
Kumar K, Gambhir G, Dass A, et al. Genetically modified crops:current status and future prospects[J]. Planta, 2020, 251(4):91.
doi: 10.1007/s00425-020-03372-8 URL |
| [6] | 张大兵, 郭金超. 转基因生物及其产品检测技术和标准化[J]. 生命科学, 2011, 23(2):195-204. |
| Zhang DB, Guo JC. The development and standardization of testing approaches for genetically modified organisms and their derived products[J]. Chin Bull Life Sci, 2011, 23(2):195-204. | |
| [7] |
Zhao ZY, Chen YS, Xu WZ, et al. Surface plasmon resonance detection of transgenic Cry1Ac cotton(Gossypium spp. )[J]. J Agric Food Chem, 2013, 61(12):2964-2969.
doi: 10.1021/jf3050439 URL |
| [8] |
Rupula K, Kosuri T, Gul MZ, et al. Immuno-analytical method development for detection of transgenic Cry1Ac protein and its validation[J]. J Sci Food Agric, 2019, 99(15):6903-6910.
doi: 10.1002/jsfa.9976 URL |
| [9] | Jambagi P, Shankergoud J, Nidagundi A, et al. Detecting cry1Ac by loop mediated isothermal amplification by SYBR green-I[J]. 2018, 7(2):2176-2180. |
| [10] | Grohmann L, Reiting R, Mäde D, et al. Collaborative trial validation of cry1Ab/Ac and Pubi-cry TaqMan-based real-time PCR assays for detection of DNA derived from genetically modified Bt plant products[J]. Accreditation Qual Assur, 2015, 20(2):85-96. |
| [11] |
Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA[J]. Nucleic Acids Res, 2000, 28(12):E63.
doi: 10.1093/nar/28.12.e63 pmid: 10871386 |
| [12] |
Qin A, Fu LT, Wong JK, et al. Precipitation of PEG/carboxyl-modified gold nanoparticles with magnesium pyrophosphate:a new platform for real-time monitoring of loop-mediated isothermal amplification[J]. ACS Appl Mater Interfaces, 2017, 9(12):10472-10480.
doi: 10.1021/acsami.7b00046 URL |
| [13] |
Liu H, Wu W, Tan J, et al. Development and evaluation of a one-step reverse transcription loop-mediated isothermal amplification for detection of Citrus leaf blotch virus[J]. J Virol Methods, 2019, 270:150-152.
doi: 10.1016/j.jviromet.2019.05.009 URL |
| [14] | 王晨光, 许文涛, 黄昆仑, 等. 转基因食品分析检测技术研究进展[J]. 食品科学, 2014, 35(21):297-305. |
| Wang CG, Xu WT, Huang KL, et al. Recent progress in techniques for the detection and analysis of genetically modified foods[J]. Food Sci, 2014, 35(21):297-305. | |
| [15] |
Chen K, Han H, Luo Z, et al. A practicable detection system for genetically modified rice by SERS-barcoded nanosensors[J]. Biosens Bioelectron, 2012, 34(1):118-124.
doi: 10.1016/j.bios.2012.01.029 pmid: 22342698 |
| [16] |
Sarkes A, Fu H, Feindel D, et al. Development and evaluation of a loop-mediated isothermal amplification(LAMP)assay for the detection of Tomato brown rugose fruit virus(ToBRFV)[J]. PLoS One, 2020, 15(6):e0230403.
doi: 10.1371/journal.pone.0230403 URL |
| [17] |
Lam P, Keri RA, Steinmetz NF. A bioengineered positive control for rapid detection of the Ebola virus by reverse transcription loop-mediated isothermal amplification(RT-LAMP)[J]. ACS Biomater Sci Eng, 2017, 3(3):452-459.
doi: 10.1021/acsbiomaterials.6b00769 URL |
| [18] |
Tao Y, Yun J, Wang J, et al. High-performance detection of Mycobacterium bovis in milk using digital LAMP[J]. Food Chem, 2020, 327:126945.
doi: 10.1016/j.foodchem.2020.126945 URL |
| [19] |
Tanner NA, Zhang Y, Evans TC. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes[J]. Biotechniques, 2015, 58(2):59-68.
doi: 10.2144/000114253 URL |
| [20] | Charoenpanich P, Mungkung A, Seeviset N, et al. A pH sensitive, loop-mediated isothermal amplification assay for detection of Salmonella in food[J]. Science, Engineering and Health Studies, 2020, 14(3):160-168. |
| [21] |
Ferrara M, Logrieco AF, Moretti A, et al. A loop-mediated isothermal amplification(LAMP)assay for rapid detection of fumonisin producing Aspergillus species[J]. Food Microbiol, 2020, 90:103469.
doi: 10.1016/j.fm.2020.103469 URL |
| [22] |
Xiong J, Huang B, Xu JS, et al. A closed-tube loop-mediated isothermal amplification assay for the visual detection of Staphylococcus aureus[J]. Appl Biochem Biotechnol, 2020, 191(1):201-211.
doi: 10.1007/s12010-020-03278-x pmid: 32103471 |
| [23] |
Tatulli G, Cecere P, Maggioni D, et al. A rapid colorimetric assay for on-site authentication of cephalopod species[J]. Biosensors, 2020, 10(12):190.
doi: 10.3390/bios10120190 URL |
| [24] |
Li Y, Wang Y, Song K, et al. A rapid and sensitive colorimetric assay for the determination of adenosine kinase activity[J]. Biochem Biophys Res Commun, 2018, 502(2):250-254.
doi: 10.1016/j.bbrc.2018.05.152 URL |
| [25] |
Magnaghi LR, Alberti G, Capone F, et al. Development of a dye-based device to assess the poultry meat spoilage. part II:array on act[J]. J Agric Food Chem, 2020, 68(45):12710-12718.
doi: 10.1021/acs.jafc.0c03771 URL |
| [26] |
Stocker MK, Sanson ML, Bernardes AA, et al. Acid-base sensor based on sol-gel encapsulation of bromothymol blue in silica:application for milk spoilage detection[J]. J Sol Gel Sci Technol, 2021, 98(3):568-579.
doi: 10.1007/s10971-021-05529-7 URL |
| [27] |
Ong SA, Wu JC. A simple method for rapid screening of biosurfactant-producing strains using bromothymol blue alone[J]. Biocatal Agric Biotechnol, 2018, 16:121-125.
doi: 10.1016/j.bcab.2018.07.027 URL |
| [28] |
Ramadan AA, Zeino S. Development and validation of spectrophotometric determination of glimepiride in pure and tablet dosage forms through ion-pair complex formation using bromothymol blue[J]. Rese Jour Pharm And Technol, 2018, 11(7):3049.
doi: 10.5958/0974-360X.2018.00561.9 URL |
| [29] |
Gul I, Bogale TF, Deng J, et al. Enzyme-based detection of epoxides using colorimetric assay integrated with smartphone imaging[J]. Biotechnol Appl Biochem, 2020, 67(4):685-692.
doi: 10.1002/bab.1898 URL |
| [1] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [2] | 王贵芳, 姚元涛, 许海峰, 相昆, 梁家慧, 张淑辉, 王文茹, 张明娟, 张美勇, 陈新. 核桃JrSnRK1α1.1调控种子油脂合成与积累[J]. 生物技术通报, 2023, 39(9): 183-191. |
| [3] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [4] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [5] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [6] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [7] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [8] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| [9] | 陶娜, 李茂兴, 郭华春. 发根农杆菌介导的甘薯遗传转化体系优化[J]. 生物技术通报, 2023, 39(10): 175-183. |
| [10] | 于惠林, 吴孔明. 中国转基因大豆的产业化策略[J]. 生物技术通报, 2023, 39(1): 1-15. |
| [11] | 解伟, 刘春明. 生物育种产业化面临的机遇与政策保障[J]. 生物技术通报, 2023, 39(1): 16-20. |
| [12] | 李圣彦, 李香银, 李鹏程, 张明俊, 张杰, 郎志宏. 转基因玉米2HVB5的性状鉴定及遗传稳定性分析[J]. 生物技术通报, 2023, 39(1): 21-30. |
| [13] | 李东阳, 肖冰, 王晨尧, 杨现明, 梁晋刚, 吴孔明. 转基因抗虫耐除草剂玉米瑞丰125 Cry1Ab/Cry2Aj杀虫蛋白的时空表达分析[J]. 生物技术通报, 2023, 39(1): 31-39. |
| [14] | 李鹏程, 张明俊, 王银晓, 李香银, 李圣彦, 郎志宏. 转基因玉米HGK60在不同遗传背景下抗虫性鉴定及农艺性状分析[J]. 生物技术通报, 2023, 39(1): 40-47. |
| [15] | 林鹰, 杨文莉, 周玲艳, 姜大刚. 农业转基因核酸标准物质研究进展[J]. 生物技术通报, 2022, 38(8): 52-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||