








生物技术通报 ›› 2024, Vol. 40 ›› Issue (8): 164-173.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0237
收稿日期:2024-03-11
出版日期:2024-08-26
发布日期:2024-07-31
通讯作者:
杨锦昌,男,博士,研究员,研究方向:特色植物资源培育与利用;E-mail: jcyang@caf.ac.cn作者简介:余纽,女,博士,副研究员,研究方向:植物特色代谢物形成机制;E-mail: niuyu@caf.ac.cn基金资助:
YU Niu( ), LIU Fan, YANG Jin-chang(
), LIU Fan, YANG Jin-chang( )
)
Received:2024-03-11
Published:2024-08-26
Online:2024-07-31
摘要:
【目的】萜类化合物在植物生理和防御中发挥着重要作用。萜类合成酶基因(TPS)是萜类生物合成途径中的关键酶,热带泌油树种油楠(Sindora glabra)茎部含有大量萜类树脂油,探究油楠SgTPS在萜类物质合成和非生物胁迫响应中的功能,为通过合成生物学生产萜类提供重要基因资源,有助于拓展植物萜类合成途径与调控机制的认识。【方法】利用油楠基因组和转录组数据从油楠茎部组织克隆SgTPS7,并对其进行生物信息学分析;表达纯化SgTPS7蛋白利用GC-MS鉴定其催化功能;采用RT-qPCR技术分析SgTPS7的表达模式。【结果】油楠SgTPS7编码区序列全长1 650 bp。经同源比对分析发现,SgTPS7与苏木亚科古巴香胶树(Copaifera officinalis)CoTPS2的相似性最高,达92.3%,与其他科属植物的亲缘关系较远,属于苏木亚科特异的TPS基因。体外酶活试验表明,SgTPS7主要催化法尼基焦磷酸产生大根香叶烯D,同时催化香叶基焦磷酸产生芳樟醇。在非生物胁迫处理条件下,SgTPS7的表达差异显著。高温胁迫处理后,SgTPS7在叶片和茎中的表达均在24 h达到峰值,而在根部的表达则呈下降趋势;长期干旱处理显著影响SgTPS7的表达,在处理5 d后SgTPS7在叶和茎中的表达均达到最高,而根部SgTPS7的表达在10 d后达到最高。【结论】SgTPS7为多功能萜类合成酶基因,其在响应高温和干旱两种非生物胁迫中可能具有重要的作用。
余纽, 柳帆, 杨锦昌. 油楠SgTPS7的克隆及其在萜类生物合成和非生物胁迫中的功能[J]. 生物技术通报, 2024, 40(8): 164-173.
YU Niu, LIU Fan, YANG Jin-chang. Cloning of SgTPS7 in Sindora glabra and Its Function in Terpene Synthesis and Abiotic Stress[J]. Biotechnology Bulletin, 2024, 40(8): 164-173.
| 名称 Primer name | 序列 Primer sequence(5'-3') |
|---|---|
| SgTPS7-F | ATGACTAGATCCACAGCAGGC |
| SgTPS7-R | CTATTTATTGTGATCAATTGCTATGG |
| qSgTPS7-F | GCATATCTCGGTATCCGTGG |
| qSgTPS7-R | GCAGATCATTCCGAGAATCC |
| Action-2-F | CATGAAGTGTGATGTGGATA |
| Action-2-R | CCTTGCTCATTCTATCAGCA |
表1 引物序列
Table 1 Primers’ sequences
| 名称 Primer name | 序列 Primer sequence(5'-3') |
|---|---|
| SgTPS7-F | ATGACTAGATCCACAGCAGGC |
| SgTPS7-R | CTATTTATTGTGATCAATTGCTATGG |
| qSgTPS7-F | GCATATCTCGGTATCCGTGG |
| qSgTPS7-R | GCAGATCATTCCGAGAATCC |
| Action-2-F | CATGAAGTGTGATGTGGATA |
| Action-2-R | CCTTGCTCATTCTATCAGCA |
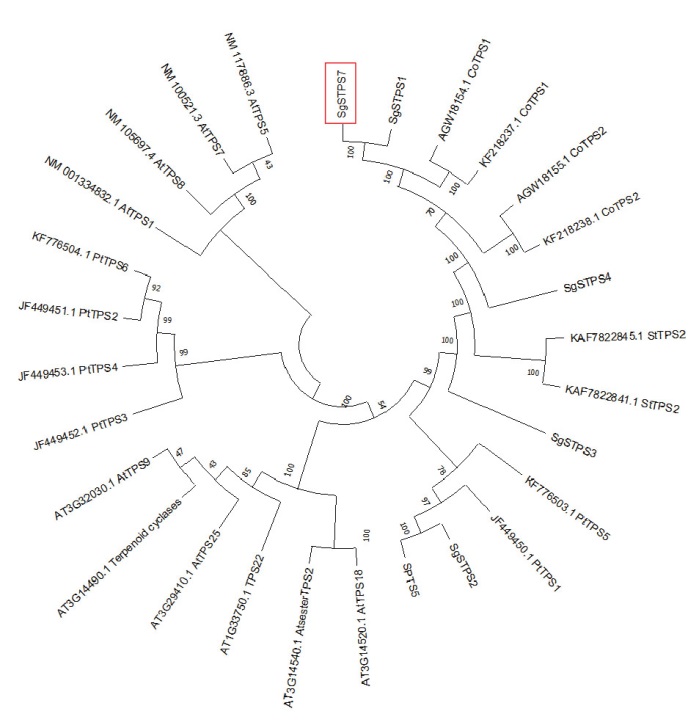
图3 不同植物TPS同源蛋白序列的系统进化树分析 PtTPS:毛果杨;CoTPS:古巴香胶树;AtTPS:拟南芥;StTPS:马铃薯
Fig. 3 Phylogenetic tree analysis of TPS homologous protein sequences in different plants PtTPS: Populus trichocarpa; CoTPS: Copaifera officinalis; AtTPS: Arabidopsis thaliana; StTPS: Solanum Tuberosum
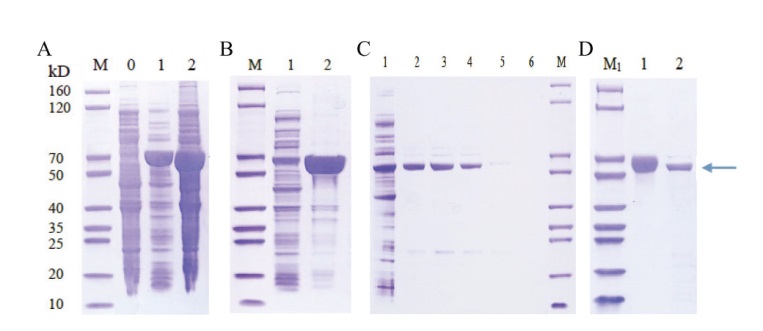
图4 SgTPS7蛋白表达纯化分析 A:SgTPS7蛋白表达[M:protein marker;0:对照(不加IPTG):1:15℃诱导16 h;2:37℃诱导16 h];B:SgTPS7蛋白可溶性(1:上清;2:沉淀);C:SgTPS7蛋白上清纯化结果(1:上清同Ni-IDA 孵育后流出液;2-4:100 mmol/L咪唑洗脱组分;5-6:300 mmol/L咪唑洗脱组分);D:SgTPS7纯化蛋白[1:BSA(2.00 μg);2:SgTPS7蛋白(2.00 μg)]
Fig. 4 Expression and purification analysis of SgTPS7 protein A: SgTPS7 protein expression[M: protein marker; 0: control(without IPTG); 1: 15℃ for 16 h; 2: 37℃ for 16 h]. B: SgTPS7 protein solubility(1: supernatant; 2: precipitation). C: SgTPS7 protein purification(1: outflow; 2-4: 100 mmol/L imidazole elution; 5-6: 300 mmol/L imidazole elution). D: Purified SgTPS7 protein[1: BSA(2.00 μg); 2: SgTPS7 protein(2.00 μg)]
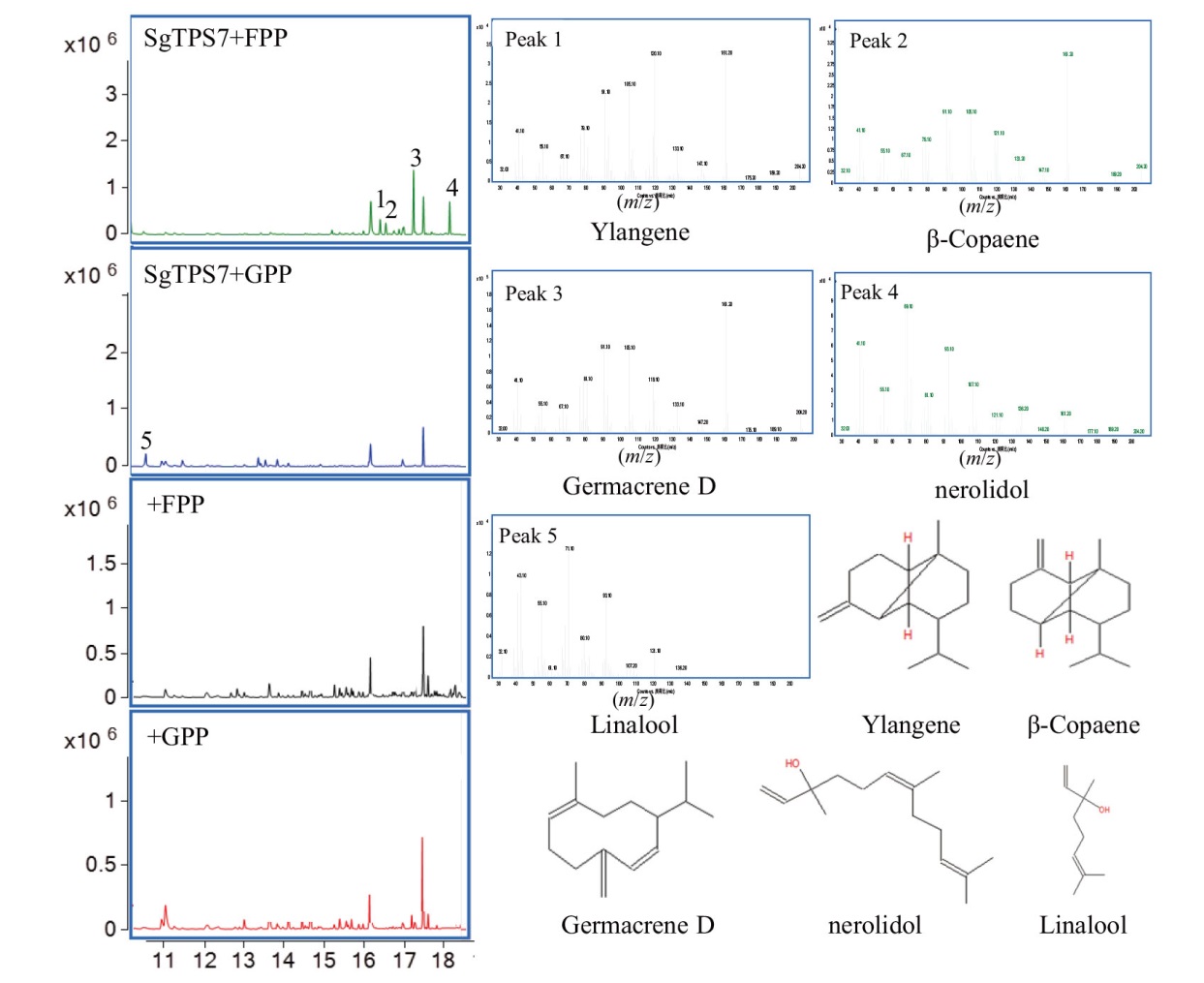
图5 GC-MS分析SgTPS7的催化产物 1、2、3、4、5分别为β-衣兰烯、β-可巴烯、大根香叶烯D、橙花叔醇和芳樟醇
Fig. 5 GC-MS analysis of enzymatic products of SgTPS7 protein 1, 2, 3, 4 and 5 are ylangene, β-copaene, germacrene D, nerolidol, and linalool, respectively
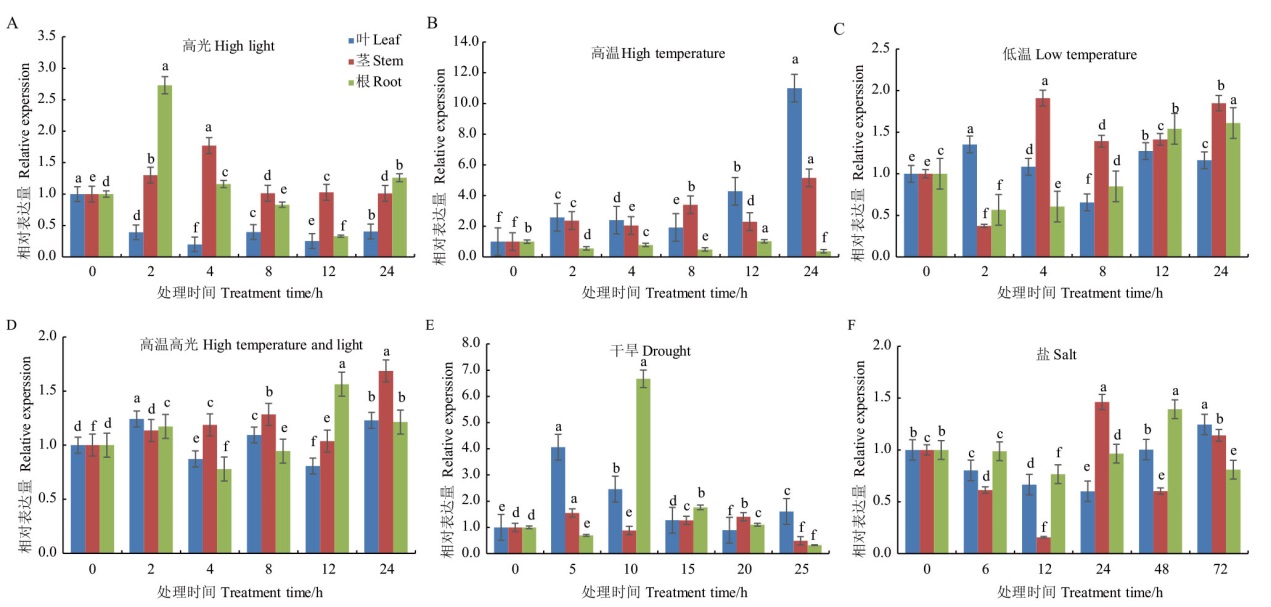
图6 不同胁迫处理下SgTPS7的表达水平 不同小写字母表示在同一组织中不同时间点SgTPS7表达量的统计学差异显著(P<0.05)
Fig. 6 Expressions of SgTPS7 under various stresses Different lowercase letters indicate significant statistical differences of SgTPS7 expressions from the same tissue at different time points(P<0.05)
| [1] |
Gershenzon J, Dudareva N. The function of terpene natural products in the natural world[J]. Nat Chem Biol, 2007, 3(7): 408-414.
doi: 10.1038/nchembio.2007.5 pmid: 17576428 |
| [2] |
Huang XR, Zhang WW, Liao YL, et al. Contemporary understanding of transcription factor regulation of terpenoid biosynthesis in plants[J]. Planta, 2023, 259(1): 2.
doi: 10.1007/s00425-023-04268-z pmid: 37971670 |
| [3] |
Tetali SD. Terpenes and isoprenoids: a wealth of compounds for global use[J]. Planta, 2019, 249(1): 1-8.
doi: 10.1007/s00425-018-3056-x pmid: 30467631 |
| [4] |
Hall DE, Zerbe P, Jancsik S, et al. Evolution of conifer diterpene synthases: diterpene resin acid biosynthesis in lodgepole pine and jack pine involves monofunctional and bifunctional diterpene synthases[J]. Plant Physiol, 2013, 161(2): 600-616.
doi: 10.1104/pp.112.208546 pmid: 23370714 |
| [5] |
Celedon JM, Bohlmann J. Oleoresin defenses in conifers: chemical diversity, terpene synthases and limitations of oleoresin defense under climate change[J]. New Phytol, 2019, 224(4): 1444-1463.
doi: 10.1111/nph.15984 pmid: 31179548 |
| [6] | 王凌健, 方欣, 杨长青, 等. 植物萜类次生代谢及其调控[J]. 中国科学: 生命科学, 2013, 43(12): 1030-1046. |
| Wang LJ, Fang X, Yang CQ, et al. Biosynthesis and regulation of secondary terpenoid metabolism in plants[J]. Sci Sin Vitae, 2013, 43(12): 1030-1046 | |
| [7] |
Wildung MR, Croteau R. A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis[J]. J Biol Chem, 1996, 271(16): 9201-9204.
doi: 10.1074/jbc.271.16.9201 pmid: 8621577 |
| [8] | Chen YC, Li Z, Zhao YX, et al. The Litsea genome and the evolution of the laurel family[J]. Nat Commun, 2020, 11(1): 1675. |
| [9] | 杨锦昌, 李琼琼, 尹光天, 等. 海南尖峰岭野生油楠不同单株树脂化学成分研究[J]. 林业科学研究, 2016, 29(2): 245-249. |
| Yang JC, Li QQ, Yin GT, et al. Study on chemical components of oleoresin from different wild Sindora glabra individuals in Jianfengling,Hainan, China[J]. For Res, 2016, 29(2): 245-249. | |
| [10] | Yu N, Chen ZL, Yang JC, et al. Integrated transcriptomic and metabolomic analyses reveal regulation of terpene biosynthesis in the stems of Sindora glabra[J]. Tree Physiol, 2021, 41(6): 1087-1102. |
| [11] | Yu N, Dong ML, Yang JC, et al. Age-dependent modulation of oleoresin production in the stem of Sindora glabra[J]. Tree Physiol, 2022, 42(10): 2050-2067. |
| [12] | Kopaczyk JM, Warguła J, Jelonek T. The variability of terpenes in conifers under developmental and environmental stimuli[J]. Environ Exp Bot, 2020, 180: 104197. |
| [13] | da Trindade R, da Silva JK, Setzer WN. Copaifera of the neotropics: A review of the phytochemistry and pharmacology[J]. Int J Mol Sci, 2018, 19(5): 1511. |
| [14] | Jia QD, Brown R, Köllner TG, et al. Origin and early evolution of the plant terpene synthase family[J]. Proc Natl Acad Sci USA, 2022, 119(15): e2100361119. |
| [15] | Yu N, Yang JC, Yin GT, et al. Transcriptome analysis of oleoresin-producing tree Sindora Glabra and characterization of sesquiterpene synthases[J]. Front Plant Sci, 2018, 9: 1619. |
| [16] | Booth JK, Yuen MMS, Jancsik S, et al. Terpene synthases and terpene variation in Cannabis sativa[J]. Plant Physiol, 2020, 184(1): 130-147. |
| [17] |
Zhou F, Pichersky E. The complete functional characterisation of the terpene synthase family in tomato[J]. New Phytol, 2020, 226(5): 1341-1360.
doi: 10.1111/nph.16431 pmid: 31943222 |
| [18] |
Sharma S, Chaurasia S, Dinday S, et al. High-level biosynthesis of enantiopure germacrene D in yeast[J]. Appl Microbiol Biotechnol, 2024, 108(1): 50.
doi: 10.1007/s00253-023-12885-7 pmid: 38183482 |
| [19] |
Lücker J, Bowen P, Bohlmann J. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding(+)-valencene synthase and(-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries[J]. Phytochemistry, 2004, 65(19): 2649-2659.
doi: 10.1016/j.phytochem.2004.08.017 pmid: 15464152 |
| [20] |
Prosser I, Altug IG, Phillips AL, et al. Enantiospecific(+)- and(-)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif[J]. Arch Biochem Biophys, 2004, 432(2): 136-144.
doi: 10.1016/j.abb.2004.06.030 pmid: 15542052 |
| [21] | Arimura GI, Huber DPW, Bohlmann J. Forest tent caterpillars(Malacosoma disstria)induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar(Populus trichocarpa ×deltoides): cDNA cloning, functional characterization, and patterns of gene expression of(-)-germacrene D synthase, PtdTPS1[J]. Plant J, 2004, 37(4): 603-616. |
| [22] | 崔萌, 刘志钦, 叶乃兴, 等. 茉莉花香气相关基因JsGDS启动子的克隆及功能分析[J]. 分子植物育种, 2021, 19(2): 441-447 |
| Cui M, Liu ZQ, Ye NX, et al. Cloning and functional analysis of the aroma-related gene JsGDS promoter from Jasminum sambac[J]. Mol Plant Breed, 2021, 19(2): 441-447 | |
| [23] | Xu YC, Wang YJ, Mattson N, et al. Genome-wide analysis of the Solanum tuberosum(potato)trehalose-6-phosphate synthase(TPS)gene family: evolution and differential expression during development and stress[J]. BMC Genomics, 2017, 18(1): 926. |
| [24] | Majroomi Senji B, Abdollahi Mandoulakani B. The impact of cold stress on genes expression pattern of mono- and sesquiterpene biosynthesis and their contents in Ocimum basilicum L.[J]. Phytochemistry, 2018, 156: 250-256. |
| [25] |
Zhao MY, Zhang N, Gao T, et al. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants[J]. New Phytol, 2020, 226(2): 362-372.
doi: 10.1111/nph.16364 pmid: 31828806 |
| [26] |
Muchlinski A, Chen XL, Lovell JT, et al. Biosynthesis and emission of stress-induced volatile terpenes in roots and leaves of switchgrass(Panicum virgatum L.)[J]. Front Plant Sci, 2019, 10: 1144.
doi: 10.3389/fpls.2019.01144 pmid: 31608090 |
| [1] | 李勇慧, 鲍星星, 段一珂, 赵运霞, 于相丽, 陈尧, 张延召. 灵宝杜鹃bZIP家族全基因组鉴定及表达特征分析[J]. 生物技术通报, 2024, 40(8): 186-198. |
| [2] | 崔原瑗, 王昭懿, 白双宇, 任毓昭, 豆飞飞, 刘彩霞, 刘凤楼, 王掌军, 李清峰. 大麦非特异性磷脂酶C基因家族全基因组鉴定及苗期胁迫表达分析[J]. 生物技术通报, 2024, 40(8): 74-82. |
| [3] | 刘丹丹, 王雷刚, 孙明慧, 焦小雨, 吴琼, 王文杰. 茶树海藻糖-6-磷酸合成酶(TPS)基因家族鉴定与表达分析[J]. 生物技术通报, 2024, 40(8): 152-163. |
| [4] | 吴丁洁, 陈盈盈, 徐静, 刘源, 张航, 李瑞丽. 植物赤霉素氧化酶及其功能研究进展[J]. 生物技术通报, 2024, 40(7): 43-54. |
| [5] | 胡雅丹, 伍国强, 刘晨, 魏明. MYB转录因子在调控植物响应逆境胁迫中的作用[J]. 生物技术通报, 2024, 40(6): 5-22. |
| [6] | 常雪瑞, 王田田, 王静. 辣椒E2基因家族的鉴定及分析[J]. 生物技术通报, 2024, 40(6): 238-250. |
| [7] | 李景艳, 周家婧, 袁媛, 苏晓艺, 乔文慧, 薛岩磊, 李国婧, 王瑞刚. 拟南芥AtiPGAM2基因参与非生物胁迫的响应[J]. 生物技术通报, 2024, 40(5): 215-224. |
| [8] | 潘萍萍, 徐志浩, 张怡雯, 李青, 王忠华. 多花黄精查尔酮合酶PcCHS的原核表达、亚细胞定位及表达分析[J]. 生物技术通报, 2024, 40(5): 280-289. |
| [9] | 杜兵帅, 邹昕蕙, 王子豪, 张馨元, 曹一博, 张凌云. 油茶SWEET基因家族的全基因组鉴定及表达分析[J]. 生物技术通报, 2024, 40(5): 179-190. |
| [10] | 郭慧妍, 董雪, 安梦楠, 夏子豪, 吴元华. 泛素化修饰关键酶在植物抗逆反应中的功能研究进展[J]. 生物技术通报, 2024, 40(4): 1-11. |
| [11] | 江林琪, 赵佳莹, 郑飞雄, 姚馨怡, 李效贤, 俞振明. 铁皮石斛14-3-3基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(3): 229-241. |
| [12] | 周宏丹, 罗晓萍, 涂米雪, 李忠光. 植物褪黑素:植物应答非生物胁迫的新兴信号分子[J]. 生物技术通报, 2024, 40(3): 41-51. |
| [13] | 李雪, 李容欧, 孔美懿, 黄磊. 解淀粉芽孢杆菌SQ-2对水稻的促生作用[J]. 生物技术通报, 2024, 40(2): 109-119. |
| [14] | 吴翠翠, 肖水平. 陆地棉HD-Zip家族全基因组鉴定及响应非生物胁迫的表达分析[J]. 生物技术通报, 2024, 40(2): 130-145. |
| [15] | 辛奇, 李压凡, 尹铮, 张晓丹, 陈霆, 刘晓华. 甘蔗CBL-CIPK基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(2): 197-211. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||