








生物技术通报 ›› 2021, Vol. 37 ›› Issue (5): 197-211.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1258
收稿日期:2020-10-12
出版日期:2021-05-26
发布日期:2021-06-11
作者简介:李凯晴,女,硕士研究生,研究方向:生态学;E-mail: 基金资助:
LI Kai-qing1( ), LI Ying2, WANG Yi-lei3, ZOU Peng-fei3(
), LI Ying2, WANG Yi-lei3, ZOU Peng-fei3( )
)
Received:2020-10-12
Published:2021-05-26
Online:2021-06-11
摘要:
受体相互作用蛋白(RIP)是介导细胞死亡信号和炎症反应的关键调节因子,在调节免疫反应和维持机体稳态方面起着至关重要的作用。RIP在哺乳动物中参与了细胞死亡、炎症反应、先天免疫等不同的生物学过程,而鱼类作为重要的脊椎动物类群,关于RIP在鱼类中的功能我们仍然所知有限。因此,主要综述了RIP家族成员的分子结构、介导的信号通路和作用机制,以及其在硬骨鱼类中的研究进展,旨为深入解析鱼类乃至哺乳动物的免疫反应和抗病机制奠定基础。
李凯晴, 李莹, 王艺磊, 邹鹏飞. 受体相互作用蛋白的功能及在硬骨鱼类中的研究进展[J]. 生物技术通报, 2021, 37(5): 197-211.
LI Kai-qing, LI Ying, WANG Yi-lei, ZOU Peng-fei. The Function of Receptor-interacting Protein(RIP)Kinases and the Research Progress in Teleost Fish[J]. Biotechnology Bulletin, 2021, 37(5): 197-211.
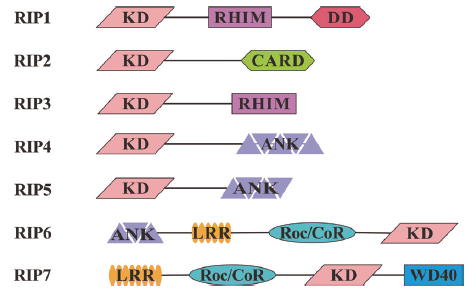
图1 哺乳动物 RIP 家族成员及蛋白结构域示意图 KD(kinase domain,激酶结构域);RHIM(receptor-interacting-protein homotypic interaction motif,RIP同源相互作用基序);DD(death domain,死亡结构域);CARD(caspase activation and recruitment domain,天冬氨酸半胱氨酸蛋白酶激活募集域);ANK(ankyrin repeat,锚蛋白重复序列);Roc/CoR结构域(ras complex protein/C-terminal of Roc);LRR(leucine-rich repeat,富亮氨酸重复序列)
Fig. 1 Protein domain of mammalian RIP family members KD stands for kinase domain, RHIM stands for receptor-interacting-protein homotypic interaction motif, DD stands for death domain, CARD stands for caspase activation and recruitment domain, ANK stands for ankyrin repeat, Roc/CoR stands for ras complex protein/C-terminal of Roc, LRR leucine-rich repeat
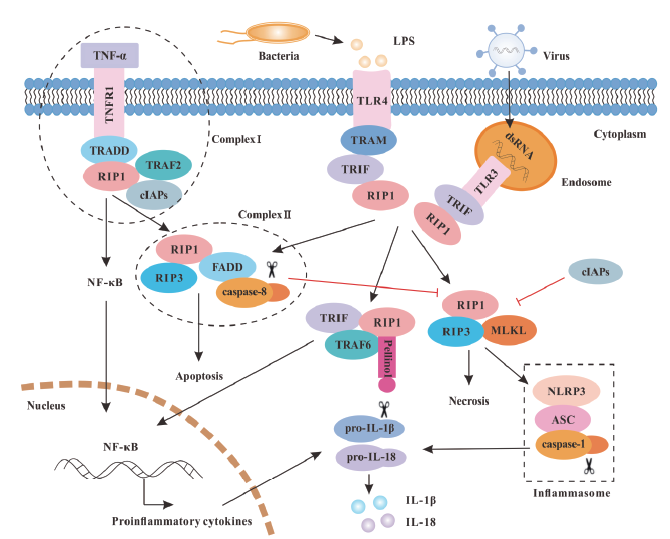
图2 哺乳动物 RIP1 和 RIP3 介导的信号通路 TNF-α与其受体TNFR1结合后,通过接头蛋白TRADD募集RIP1,激活NF-κB信号通路介导炎症反应,也可启动caspase依赖性细胞凋亡。TLR3和TLR4分别识别dsRNA和LPS,通过接头分子TRIF招募RIP1,RIP1可以与TRIF、TRAF6等结合介导下游NF-κB信号的激活从而诱导促炎细胞因子的转录;RIP1与FADD和caspase-8结合形成死亡复合物介导细胞凋亡;在caspase-8活性受到抑制时,可通过RIP1-RIP3-MLKL坏死小体的组装介导细胞程序性坏死,RIP3可以激活炎症小体介导细胞焦亡。脂多糖(lipopolysaccharide,LPS);肿瘤坏死因子-α(tumor necrosis factor alpha,TNF-α);肿瘤坏死因子受体1(TNF-receptor 1,TNFR1);肿瘤坏死因子受体相关死亡域蛋白(TNF-receptor-associated death domain,TRADD);肿瘤坏死因子受体相关因子(TNF receptor associated factor,TRAF);凋亡抑制蛋白(cellular inhibitor of apoptosis proteins,cIAPs);死亡结构域蛋白(Fas-associated death domain,FADD);核转录因子-κB(nuclear transcription factor-κB,NF-κB);Toll样受体(toll-like receptor,TLR);β干扰素TIR结构域接头蛋白相关接头分子(TIR domain-containing adaptor-inducing IFNβ-related adaptor molecule,TRAM);β干扰素TIR结构域接头蛋白(TIR domain-containing adaptor-inducing IFNβ,TRIF); NOD样受体家族含吡啶结构域蛋白3(NOD-like receptor family pyrin domain containing 3,NLRP3);凋亡相关斑点样蛋白(apoptosis-associated speck-like protein containing CARD,ASC);白介素-1(Interleukin-1,IL-1)
Fig. 2 RIP1 and RIP3-mediated signaling pathways in mammals RIP1 is recruited via the adaptor protein TRADD to the TNFR1 upon the stimulation of TNF-α, and then activates NF-κB signaling pathway to mediate inflammation and initiates caspase-dependent cell apoptosis. The dsRNA and LPS could be recognized by TLR3 and TLR4, respectively, which then recruit the adaptor molecule TRIF to interact with RIP1, and then associate with TRAF6 to participate in the activation of downstream NF-κB signals, thereby inducing the transcription of pro-inflammatory cytokines. RIP1 mediates cell apoptosis by binding to FADD and caspase-8 to form a death complex. When the activity of caspase-8 is inhibited, the assembly of RIP1-RIP3-MLKL necrosomes mediates cell necrosis, and then RIP3 activates inflammasomes to mediate cell pyrotosis. LPS stands for Lipopolysaccharide. TNF-α stands for Tumor necrosis factor alpha. TNFR1 stands for TNF-receptor 1. TRADD stands for TNF-receptor-associated death domain. TRAF stands for TNF receptor associated factor. cIAPs stands for Cellular inhibitor of apoptosis proteins. FADD stands for Fas-associated death domain. NF-κB stands for Nuclear transcription factor-κB. TLR stands for Toll-like receptor. TRAM stands for TIR domain-containing adaptor-inducing IFNβ-related adaptor molecule. TRIF stands for TIR domain-containing adaptor-inducing IFNβ. NLRP3 stands for NOD-like receptor family pyrin domain containing 3. ASC stands for Apoptosis-associated speck-like protein containing CARD. IL-1 stands for Interleukin-1

图3 哺乳动物 RIP2 介导的信号通路 NNOD1和NOD2分别识别病原菌iE-DAP和MDP,招募并激活下游RIP2,从而活化TAB-TAK和IKK复合体并诱导NF-κB和MAPK信号通路激活;TRIP6和RIP7正调控NOD1/2-RIP2介导的信号通路,caspase-12、A20、OTULIN和CYLD负调控NOD1/2-RIP2介导的信号通路;RIP2也可与caspase-1相互作用介导NF-κB的激活,而ASC可与RIP2竞争caspase-1抑制其对NF-κB的激活,促进促炎细胞因子的加工。iE-DAP(γ-d-glutamyl-meso-diaminopimelic acid, γ-d-谷氨酰-间二氨基丙酸);MDP(muramyl dipeptide,胞壁酰二肽);NOD(nucleotide-binding oligomerization domains,核苷酸结合寡聚化结构域蛋白);XIAP(X-linked inhibitor of apoptosis protein,X连锁凋亡抑制蛋白);TRIP6(thyroid hormone receptor interactor 6,甲状腺激素受体相互作用蛋白6);CYLD(cylindromatosis,头帕肿瘤综合征蛋白);TAK(TGFβ-activated kinase,转化生长因子激酶);TAB(TGFβ-activated kinase binding protein,转化生长因子激酶靶蛋白);IKK(inhibitor of nuclear factor kappa-B kinase,IκB激酶);MAPK(mitogen-activated protein kinase,促分裂原活化蛋白激酶)
Fig. 3 RIP2-mediated signaling pathways in mammals RIP2 is recruited and activated by NOD1 and NOD2, which recognize iE-DAP and MDP, respectively, thereby activating TAB-TAK and IKK complexes and inducing activation of NF-κB and MAPK signaling pathways. TRIP6 and RIP7 positively regulate NOD1/2-RIP2 mediated signaling pathway, whereas caspase-12, A20, OTULIN and CYLD negatively regulate the NOD1/2-RIP2 mediated signaling pathway. RIP2 can interact with caspase-1 to mediate the activation of NF-κB, whereas ASC competes with RIP2 to inhibit the activation of NF-κB by caspase-1 and promotes the processing of proinflammatory cytokines. iE-DAP stands for γ-d-glutamyl-meso-diaminopimelic acid. MDP stands for muramyl dipeptide. NOD stands for nucleotide-binding oligomerization domains. XIAP stands for X-linked inhibitor of apoptosis protein. TRIP6 stands for thyroid hormone receptor interactor 6. CYLD stands for cylindromatosis. TAK stands for TGFβ-activated kinase. TAB stands for TGFβ-activated kinase binding protein. IKK stands for inhibitor of nuclear factor kappa-B kinase. and MAPK stands for mitogen-activated protein kinase

图4 硬骨鱼类 RIP1、RIP2 和 RIP3 介导的信号通路 硬骨鱼类RIP1通过与TRIF相互作用参与TLR3、TLR19和TLR22介导的NF-κB信号通路的激活,与下游TRAF3结合活化TBK1和IKKε介导IFN的激活;RIG-I和MDA5识别病毒dsRNA,通过接头分子MAVS介导下游IRF3和IRF7信号转导,而RIP1可负调控MAVS介导的RLRs抗病毒信号通路;在TNF-α的刺激下,RIP1、TRADD和TRAF2结合激活NF-κB信号通路,也可介导细胞凋亡;RIP1可与RIP3和MLKL形成坏死小体诱导细胞程序性坏死。RIP2与NOD1/2相互作用活化TAB-TAK1和IKK复合体并诱导NF-κB信号通路激活,促进促炎细胞因子产生;RIP2可参与MHC抗原呈递和自噬过程。RIG-Ⅰ(retinoic acid-inducible gene Ⅰ,维甲酸诱导基因蛋白Ⅰ);MDA5(melanoma differentiation associated gene 5,黑色素瘤分化相关基因5);MAVS(mitochondrial antiviral-signaling protein,线粒体抗病毒信号蛋白);MHC(major histocompatibility complex,主要组织相容性复合体);TBK1(TANK binding kinase 1,TANK结合激酶1);IRF3(interferon regulatory factor 3,干扰素调节因子3);IRF7(interferon regulatory factor 7,干扰素调节因子7);ISGs(interferon stimulated genes,干扰素诱导基因)
Fig. 4 RIP1, RIP2, and RIP3-mediated signaling pathways in teleosts RIP1 is involved in activation of TLR3, TLR19, and TLR22 mediated NF-κB signaling pathway through interaction with TRIF, and also activates TBK1 and IKKε-mediated production of IFN by binding to downstream TRAF3 in teleosts. Recognition of viral dsRNA activates RIG-I and MDA5, which then mediate downstream IRF3 and IRF7 signal transduction through the adaptor molecule MAVS, whereas RIP1 negatively regulates MAVS-mediated RLRs antiviral signal pathway. The combination of RIP1, TRAD and TRAF2 activates NF-κB signaling pathway and mediates cell apoptosis upon the stimulation of TNF-α. RIP1 form necrosomes with RIP3 and MLKL to induce cell necrosis. RIP2 interacts with NOD1/2 to activate the TAB-TAK1 and IKK complexes and induce the activation of the NF-κB signaling pathway to promote the production of proinflammatory cytokines. RIP2 is involved in MHC antigen presentation and autophagy. RIG-Ⅰstands for retinoic acid-inducible geneⅠ. MDA5 stands for melanoma differentiation associated gene 5. MAVS stands for mitochondrial antiviral-signaling protein. MHC stands for major histocompatibility complex. TBK1 stands for TANK binding kinase 1. IRF3 stands for interferon regulatory factor 3. IRF7 stands for interferon regulatory factor 7. and ISGs stands for interferon stimulated genes
| [1] | Nelson JS. Fishes of the World[M]. 4th edition. New York:John Wiley and Sons, 2006. |
| [2] |
Zhu LY, Nie L, Zhu G, et al. Advances in research of fish immune-relevant genes:a comparative overview of innate and adaptive immunity in teleosts[J]. Developmental and Comparative Immunology, 2013,39(1-2):39-62.
doi: 10.1016/j.dci.2012.04.001 URL |
| [3] |
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses[J]. Clinical Microbiology Reviews, 2009,22(2):240-273.
doi: 10.1128/CMR.00046-08 pmid: 19366914 |
| [4] |
Tsuda H, Ning Z, Yamaguchi Y, et al. Programmed cell death and its possible relationship with periodontal disease[J]. Journal of Oral Science, 2012,54(2):137-149.
doi: 10.2334/josnusd.54.137 URL |
| [5] |
Siqueira MDS, Ribeiro RM, Travassos LH. Autophagy and its interaction with intracellular bacterial pathogens[J]. Frontiers in Immunology, 2018,9:935.
doi: 10.3389/fimmu.2018.00935 pmid: 29875765 |
| [6] |
Vanden Berghe T, Vanlangenakker N, Parthoens E, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features[J]. Cell Death and Differentiation, 2010,17(6):922-930.
doi: 10.1038/cdd.2009.184 pmid: 20010783 |
| [7] |
Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines:recommendations of the Nomenclature Committee on Cell Death 2012[J]. Cell Death and Differentiation, 2012,19(1):107-120.
doi: 10.1038/cdd.2011.96 pmid: 21760595 |
| [8] |
Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways[J]. Nature Reviews Immunology, 2011,12(2):79-88.
doi: 10.1038/nri3131 URL |
| [9] |
Festjens N, Vanden Berghe T, Cornelis S, et al. RIP1, a kinase on the crossroads of a cell’s decision to live or die[J]. Cell Death and Differentiation, 2007,14(3):400-410.
doi: 10.1038/sj.cdd.4402085 URL |
| [10] |
Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival[J]. Cell, 2009,138(2):229-232.
doi: 10.1016/j.cell.2009.07.006 pmid: 19632174 |
| [11] |
Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation[J]. Nature Immunology, 2015,16(7):689-697.
doi: 10.1038/ni.3206 pmid: 26086143 |
| [12] |
Stanger BZ, Leder P, Lee TH, et al. RIP - a novel protein containing a death domain that interacts with Fas/APO-1(CD95)in yeast and causes cell-death[J]. Cell, 1995,81(4):513-523.
pmid: 7538908 |
| [13] |
Sun XQ, Yin JP, Starovasnik MA, et al. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein(RIP)by RIP3[J]. Journal of Biological Chemistry, 2002,277(11):9505-9511.
doi: 10.1074/jbc.M109488200 URL |
| [14] |
Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif[J]. Journal of Immunology, 2005,174(8):4942-4952.
doi: 10.4049/jimmunol.174.8.4942 URL |
| [15] |
Rebsamen M, Heinz LX, Meylan E, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappa B[J]. Embo Reports, 2009,10(8):916-922.
doi: 10.1038/embor.2009.109 pmid: 19590578 |
| [16] |
O’Donnell MA, Legarda-Addison D, Skountzos P, et al. Ubiquitination of RIP1 regulates an NF-kappa B independent cell-death switch in TNF signaling[J]. Current Biology, 2007,17(5):418-424.
doi: 10.1016/j.cub.2007.01.027 URL |
| [17] |
Park HH, Lo YC, Lin SC, et al. The death domain superfamily in intracellular signaling of apoptosis and inflammation[J]. Annual Review of Immunology, 2007,25:561-586.
doi: 10.1146/annurev.immunol.25.022106.141656 URL |
| [18] | Dowling JP, Cai Y, Bertin J, et al. Kinase-independent function of RIP1, critical for mature T-cell survival and proliferation[J]. Cell Death & Disease, 2016,7:e2379. |
| [19] |
Kelliher MA, Grimm S, Ishida Y, et al. The death domain kinase RIP mediates the TNF-induced NF-kappa B signal[J]. Immunity, 1998,8(3):297-303.
pmid: 9529147 |
| [20] |
Newton K. RIPK1 and RIPK3:critical regulators of inflammation and cell death[J]. Trends in Cell Biology, 2015,25(6):347-353.
doi: 10.1016/j.tcb.2015.01.001 pmid: 25662614 |
| [21] |
Ting AT, PimentelMuinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappa B but not Fas/APO-1-initiated apoptosis[J]. Embo Journal, 1996,15(22):6189-6196.
pmid: 8947041 |
| [22] | Christofferson DE, Li Y, Hitomi J, et al. A novel role for RIP1 kinase in mediating TNF alpha production[J]. Cell Death & Disease, 2012,3:e320. |
| [23] | de Almagro MC, Goncharov T, Newton K, et al. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination[J]. Cell Death & Disease, 2015,6:e1800. |
| [24] | Lin X, Chen Q, Huang C, et al. CYLD promotes TNF-alpha-Induced cell necrosis mediated by RIP-1 in human lung cancer cells[J]. Mediators of Inflammation, 2016,2016:1542786. |
| [25] | Zhang D, Lin J, Han J. Receptor-interacting protein(RIP)kinase family[J]. Cellular & Molecular Immunology, 2010,7(4):243-249. |
| [26] |
Yang S, Wang B, Tang LS, et al. Pellino3 targets RIP1 and regulates the pro-apoptotic effects of TNF-α[J]. Nature Communications, 2013,4:2583.
doi: 10.1038/ncomms3583 URL |
| [27] |
Najjar M, Saleh D, Zelic M, et al. RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by Toll-like receptor 4[J]. Immunity, 2016,45(1):46-59.
doi: 10.1016/j.immuni.2016.06.007 pmid: 27396959 |
| [28] | Buchrieser J, Jose Oliva-Martin M, Moore MD, et al. RIPK1 is a critical modulator of both tonic and TLR-responsive inflammatory and cell death pathways in human macrophage differentiation[J]. Cell Death & Disease, 2018,9(10):973. |
| [29] |
Witt A, Vucic D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling[J]. Cell Death and Differentiation, 2017,24(7):1160-1171.
doi: 10.1038/cdd.2017.33 URL |
| [30] |
Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3[J]. Cell, 2014,157(5):1189-1202.
doi: 10.1016/j.cell.2014.04.018 URL |
| [31] |
Christofferson DE, Li Y, Yuan J. Control of life-or-death decisions by RIP1 kinase[J]. Annual Review of Physiology, 2014,76:129-150.
doi: 10.1146/annurev-physiol-021113-170259 pmid: 24079414 |
| [32] |
Rajput A, Kovalenko A, Bogdanov K, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein[J]. Immunity, 2011,34(3):340-351.
doi: 10.1016/j.immuni.2010.12.018 pmid: 21419663 |
| [33] |
Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis[J]. Nature, 2014,513(7516):90-94.
doi: 10.1038/nature13608 pmid: 25132550 |
| [34] |
Lukens JR, Vogel P, Johnson GR, et al. RIP1-driven autoinflammation targets IL-1 alpha independently of inflammasomes and RIP3[J]. Nature, 2013,498(7453):224-227.
doi: 10.1038/nature12174 pmid: 23708968 |
| [35] |
Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3[J]. Molecular Cell, 2014,54(1):133-146.
doi: 10.1016/j.molcel.2014.03.003 URL |
| [36] |
Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis[J]. Nature Cell Biology, 2014,16(1):55-65.
doi: 10.1038/ncb2883 URL |
| [37] |
Cho Y, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation[J]. Cell, 2009,137(6):1112-1123.
doi: 10.1016/j.cell.2009.05.037 URL |
| [38] |
Rodriguez DA, Weinlich R, Brown S, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis[J]. Cell Death and Differentiation, 2016,23(1):76-88.
doi: 10.1038/cdd.2015.70 pmid: 26024392 |
| [39] | Nogusa S, Thapa RJ, Dillon CP, et al. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza a virus[J]. Cell Host & Microbe, 2016,20(1):13-24. |
| [40] |
Rickard JA, O’Donnell JA, Evans JM, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis[J]. Cell, 2014,157(5):1175-1188.
doi: 10.1016/j.cell.2014.04.019 pmid: 24813849 |
| [41] | Kaiser WJ, Daley-Bauer LP, Thapa RJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014,111(21):7753-7758. |
| [42] |
Petersen SL, Chen TT, Lawrence DA, et al. TRAF2 is a biologically important necroptosis suppressor[J]. Cell Death and Differentiation, 2015,22(11):1846-1857.
doi: 10.1038/cdd.2015.35 pmid: 25882049 |
| [43] |
Orning P, Weng D, Starheim K, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death[J]. Science, 2018,362(6418):1064-1069.
doi: 10.1126/science.aau2818 URL |
| [44] |
Li Y, Gong P, Kong C, et al. Bufalin engages in RIP1-dependent and ROS-dependent programmed necroptosis in breast cancer cells by targeting the RIP1/RIP3/PGAM5 pathway[J]. Anti-Cancer Drugs, 2019,30(7):706-713.
doi: 10.1097/CAD.0000000000000770 URL |
| [45] |
Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways[J]. Nature Reviews Immunology, 2012,12(2):79-88.
doi: 10.1038/nri3131 URL |
| [46] | Moujalled DM, Cook WD, Murphy JM, et al. Necroptosis induced by RIPK3 requires MLKL but not Drp1[J]. Cell Death & Disease, 2014,5:e1086. |
| [47] |
Kaiser WJ, Sridharan H, Huang C, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL[J]. Journal of Biological Chemistry, 2013,288(43):31268-31279.
doi: 10.1074/jbc.M113.462341 URL |
| [48] | Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA[J]. Cell Host & Microbe, 2012,11(3):290-297. |
| [49] |
Lawlor KE, Khan N, Mildenhall A, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL[J]. Nature Communications, 2015,6:6282.
doi: 10.1038/ncomms7282 pmid: 25693118 |
| [50] |
Shlomovitz I, Zargrian S, Gerlic M. Mechanisms of RIPK3-induced inflammation[J]. Immunology and Cell Biology, 2017,95(2):166-172.
doi: 10.1038/icb.2016.124 pmid: 27974745 |
| [51] |
Croker BA, O’Donnell JA, Gerlic M. Pyroptotic death storms and cytopenia[J]. Current Opinion in Immunology, 2014,26:128-137.
doi: 10.1016/j.coi.2013.12.002 pmid: 24556409 |
| [52] |
Duong BH, Onizawa M, Oses-Prieto JA, et al. A20 restricts ubiquitination of pro-interleukin-1 beta protein complexes and suppresses NLRP3 inflammasome activity[J]. Immunity, 2015,42(1):55-67.
doi: 10.1016/j.immuni.2014.12.031 pmid: 25607459 |
| [53] |
Weng D, Marty-Roix R, Ganesan S, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014,111(20):7391-7396.
doi: 10.1073/pnas.1403477111 pmid: 24799678 |
| [54] |
Yu PW, Huang BCB, Shen M, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NF kappa B[J]. Current Biology, 1999,9(10):539-542.
pmid: 10339433 |
| [55] |
Wei S, Zhou H, Wang Q, et al. RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-kappa B pathway in macrophages[J]. Faseb Journal, 2019,33(10):11180-11193.
doi: 10.1096/fsb2.v33.10 URL |
| [56] |
Goncharov T, Niessen K, de Almagro MC, et al. OTUB1 modulates c-IAP1 stability to regulate signalling pathways[J]. Embo Journal, 2013,32(8):1103-1114.
doi: 10.1038/emboj.2013.62 URL |
| [57] |
Wang WL, Hong JR, Lin GH, et al. Stage-specific expression of TNF alpha regulates Bad/Bid-mediated apoptosis and RIP1/ROS-mediated secondary necrosis in birnavirus-infected fish cells[J]. PLoS One, 2011,6(2):e16740.
doi: 10.1371/journal.pone.0016740 URL |
| [58] | Ge Y, Yang H, Zhao L, et al. Structural and functional conservation of half-smooth tongue sole Cynoglossus semilaevis RIP3 in cell death signalling[J]. Fish & Shellfish Immunology, 2018,82:573-578. |
| [59] |
Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species[J]. Cell, 2013,153(3):521-534.
doi: 10.1016/j.cell.2013.03.022 URL |
| [60] |
Viringipurampeer IA, Shan X, Gregory-Evans K, et al. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish[J]. Cell Death and Differentiation, 2014,21(5):665-675.
doi: 10.1038/cdd.2013.191 pmid: 24413151 |
| [61] | Zhang Q, Wang S, Zheng S, et al. Chlorpyrifos suppresses neutrophil extracellular traps in carp by promoting necroptosis and inhibiting respiratory burst caused by the PKC/MAPK pathway[J]. Oxidative Medicine and Cellular Longevity, 2019: 1763589. |
| [62] | Cui Y, Yin K, Gong Y, et al. Atrazine induces necroptosis by miR-181-5p targeting inflammation and glycometabolism in carp lymphocytes[J]. Fish & Shellfish Immunology, 2019,94:730-738. |
| [63] |
Zhang X, Liu Z, Wu S, et al. Fish RIP1 mediates innate antiviral immune responses induced by SGIV and RGNNV infection[J]. Frontiers in Immunology, 2020,11:1718.
doi: 10.3389/fimmu.2020.01718 pmid: 32849607 |
| [64] |
Xie XC, Cao YY, Dai YH, et al. Black carp RIPK1 negatively regulates MAVS-mediated antiviral signaling during the innate immune activation[J]. Developmental and Comparative Immunology, 2020,109:103726.
doi: 10.1016/j.dci.2020.103726 URL |
| [65] |
Inohara N, del Peso L, Koseki T, et al. RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis[J]. Journal of Biological Chemistry, 1998,273(20):12296-12300.
doi: 10.1074/jbc.273.20.12296 URL |
| [66] |
Thome M, Hofmann K, Burns K, et al. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1[J]. Current Biology, 1998,8(15):885-888.
pmid: 9705938 |
| [67] |
Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems[J]. Nature, 2002,416(6877):194-199.
pmid: 11894098 |
| [68] |
Inohara N, Koseki T, Lin JM, et al. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways[J]. Journal of Biological Chemistry, 2000,275(36):27823-27831.
doi: 10.1074/jbc.M003415200 URL |
| [69] |
Martinon F, Tschopp J. Inflammatory caspases:Linking an intracellular innate immune system to autoinflammatory diseases[J]. Cell, 2004,117(5):561-574.
pmid: 15163405 |
| [70] |
Anand PK, Tait SWG, Lamkanfi M, et al. TLR2 and RIP2 pathways mediate autophagy of listeria monocytogenes via extracellular signal-regulated kinase(ERK)activation[J]. Journal of Biological Chemistry, 2011,286(50):42981-42991.
doi: 10.1074/jbc.M111.310599 URL |
| [71] |
Bertrand MJM, Doiron K, Labbe K, et al. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2[J]. Immunity, 2009,30(6):789-801.
doi: 10.1016/j.immuni.2009.04.011 pmid: 19464198 |
| [72] |
Cai X, Du J, Liu Y, et al. Identification and characterization of receptor-interacting protein 2 as a TNFR-associated factor 3 binding partner[J]. Gene, 2013,517(2):205-211.
doi: 10.1016/j.gene.2012.12.026 URL |
| [73] |
Du X, Jiang S, Liu H, et al. MS80, a novel sulfated polysaccharide, inhibits CD40-NF-kappa B pathway via targeting RIP2[J]. Molecular and Cellular Biochemistry, 2010,337(1-2):277-285.
doi: 10.1007/s11010-009-0309-9 URL |
| [74] |
Chin AI, Dempsey PW, Bruhn K, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses[J]. Nature, 2002,416(6877):190-194.
doi: 10.1038/416190a URL |
| [75] | LeBlanc PM, Yeretssian G, Rutherford N, et al. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity[J]. Cell Host & Microbe, 2008,3(3):146-157. |
| [76] | Yan R, Liu Z. LRRK2 enhances Nod1/2-mediated inflammatory cytokine production by promoting Rip2 phosphorylation[J]. Protein & Cell, 2017,8(1):55-66. |
| [77] |
Li LY, Bin LH, Li F, et al. TRIP6 is a RIP2-associated common signaling component of multiple NF-kappa B activation pathways[J]. Journal of Cell Science, 2005,118(3):555-563.
doi: 10.1242/jcs.01641 URL |
| [78] |
Yang Y, Yin C, Pandey A, et al. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2[J]. Journal of Biological Chemistry, 2007,282(50):36223-36229.
doi: 10.1074/jbc.M703079200 URL |
| [79] |
Hasegawa M, Fujimoto Y, Lucas PC, et al. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappa B activation[J]. Embo Journal, 2008,27(2):373-383.
doi: 10.1038/sj.emboj.7601962 URL |
| [80] |
Damgaard RB, Nachbur U, Yabal M, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity[J]. Molecular Cell, 2012,46(6):746-758.
doi: 10.1016/j.molcel.2012.04.014 pmid: 22607974 |
| [81] | Krieg A, Correa RG, Garrison JB, et al. XIAP mediates NOD signaling via interaction with RIP2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009,106(34):14524-14529. |
| [82] |
Yang S, Wang B, Humphries F, et al. Pellino3 ubiquitinates RIP2 and mediates Nod2-induced signaling and protective effects in colitis[J]. Nature Immunology, 2013,14(9):927-936.
doi: 10.1038/ni.2669 URL |
| [83] |
Hitotsumatsu O, Ahmad R-C, Tavares R, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals[J]. Immunity, 2008,28(3):381-390.
doi: 10.1016/j.immuni.2008.02.002 pmid: 18342009 |
| [84] |
Fiil BK, Damgaard RB, Wagner SA, et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling[J]. Molecular Cell, 2013,50(6):818-830.
doi: 10.1016/j.molcel.2013.06.004 URL |
| [85] |
Hrdinka M, Fiil BK, Zucca M, et al. CYLD limits Lys63-and Met1-linked ubiquitin at receptor complexes to regulate Innate immune signaling[J]. Cell Reports, 2016,14(12):2846-2858.
doi: 10.1016/j.celrep.2016.02.062 pmid: 26997266 |
| [86] |
Sarkar A, Duncan M, Hart J, et al. ASC directs NF-kappa B activation by regulating receptor interacting protein-2(RIP2)caspase-1 interactions[J]. Journal of Immunology, 2006,176(8):4979-4986.
doi: 10.4049/jimmunol.176.8.4979 URL |
| [87] |
McCarthy JV, Ni J, Dixit VM. RIP2 is a novel NF-kappa B-activating and cell death-inducing kinase[J]. Journal of Biological Chemistry, 1998,273(27):16968-16975.
doi: 10.1074/jbc.273.27.16968 URL |
| [88] | Swain B, Basu M, Samanta M. Molecular cloning and characterization of nucleotide binding and oligomerization domain-1(NOD1)receptor in the Indian Major Carp, rohu(Labeo rohita), and analysis of its inductive expression and down-stream signalling molecules following ligands exposure and gram-negative bacterial infections[J]. Fish & Shellfish Immunology, 2012,32(5):899-908. |
| [89] |
Maharana J, Swain B, Sahoo BR, et al. Identification of MDP(muramyl dipeptide)-binding key domains in NOD2(nucleotide-binding and oligomerization domain-2)receptor of Labeo rohita[J]. Fish Physiology and Biochemistry, 2013,39(4):1007-1023.
doi: 10.1007/s10695-012-9758-2 URL |
| [90] |
Swain B, Basu M, Samanta M. NOD1 and NOD2 receptors in mrigal(Cirrhinus mrigala):Inductive expression and downstream signalling in ligand stimulation and bacterial infections[J]. Journal of Biosciences, 2013,38(3):533-548.
doi: 10.1007/s12038-013-9330-y URL |
| [91] | Hou QH, Yi SB, Ding X, et al. Differential expression analysis of nuclear oligomerization domain proteins NOD1 and NOD2 in orange-spotted grouper(Epinephelus coioides)[J]. Fish & Shellfish Immunology, 2012,33(5):1102-1111. |
| [92] |
Basu M, Paichha M, Swain B, et al. Modulation of TLR2, TLR4, TLR5, NOD1 and NOD2 receptor gene expressions and their downstream signaling molecules following thermal stress in the Indian major carp catla(Catla catla)[J]. 3 Biotech, 2015,5(6):1021-1030.
doi: 10.1007/s13205-015-0306-5 URL |
| [93] |
Xie J, Belosevic M. Functional characterization of receptor-interacting serine/threonine kinase 2(RIP2)of the goldfish(Carassius auratus L.)[J]. Developmental and Comparative Immunology, 2015,48(1):76-85.
doi: 10.1016/j.dci.2014.09.006 URL |
| [94] |
Bi D, Gao Y, Chu Q, et al. NOD1 is the innate immune receptor for iE-DAP and can activate NF-kappa B pathway in teleost fish[J]. Developmental and Comparative Immunology, 2017,76:238-246.
doi: 10.1016/j.dci.2017.06.012 URL |
| [95] | Zou PF, Chang MX, Li Y, et al. NOD2 in Zebrafish functions in antibacterial and also antiviral responses via NF-kappa B, and also MDA5, RIG-I and MAVS[J]. Fish & Shellfish Immunology, 2016,55:173-185. |
| [96] |
Wu XM, Cao L, Nie P, et al. Histone H2A cooperates with RIP2 to induce the expression of antibacterial genes and MHC related genes[J]. Developmental and Comparative Immunology, 2019,101:103455.
doi: 10.1016/j.dci.2019.103455 URL |
| [97] |
Wu XM, Chen WQ, Hu YW, et al. RIP2 is a critical regulator for NLRs signaling and MHC antigen presentation but not for MAPK and PI3K/Akt pathways[J]. Frontiers in Immunology, 2018,9:726.
doi: 10.3389/fimmu.2018.00726 URL |
| [98] | Jang JH, Kim H, Kim YJ, et al. Molecular cloning and functional analysis of nucleotide-binding oligomerization domain-containing protein 1 in rainbow trout, Oncorhynchus mykiss[J]. Fish & Shellfish Immunology, 2016,51:53-63. |
| [99] | Liu J, Cao D, Liu Y, et al. Expression and functional analysis of receptor-interacting serine/threonine kinase 2(RIP2)in Japanese flounder(Paralichthys olivaceus)[J]. Fish & Shellfish Immunology, 2018,75:327-335. |
| [100] |
Xie J, Belosevic M. Functional characterization of apoptosis-associated speck-like protein(ASC)of the goldfish(Carassius auratus L.)[J]. Developmental and Comparative Immunology, 2016,65:201-210.
doi: 10.1016/j.dci.2016.07.013 URL |
| [101] |
Bahr C, Rohwer A, Stempka L, et al. DIK, a novel protein kinase that interacts with protein kinase Cdelta. Cloning, characterization, and gene analysis[J]. Journal of Biological Chemistry, 2000,275(46):36350-36357.
doi: 10.1074/jbc.M004771200 URL |
| [102] |
Chen LJ, Haider K, Ponda M, et al. Protein kinase C-associated kinase(PKK), a novel membrane-associated, ankyrin repeat-containing protein kinase[J]. Journal of Biological Chemistry, 2001,276(24):21737-21744.
doi: 10.1074/jbc.M008069200 URL |
| [103] |
Holland PM, Willis CR, Kanaly S, et al. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation[J]. Current Biology, 2002,12(16):1424-1428.
pmid: 12194825 |
| [104] |
Cariappa A, Chen LJ, Haider K, et al. A catalytically inactive form of protein kinase C-associated kinase/receptor interacting protein 4, a protein kinase C beta-associated kinase that mediates NF-kappa B activation, interferes with early B cell development[J]. Journal of Immunology, 2003,171(4):1875-1880.
pmid: 12902489 |
| [105] |
Chen L, Oleksyn D, Pulvino M, et al. A critical role for the protein kinase PKK in the maintenance of recirculating mature B cells and the development of B1 cells[J]. Immunology Letters, 2016,172:67-78.
doi: 10.1016/j.imlet.2016.02.015 URL |
| [106] |
Meylan E, Martinon F, Thome M, et al. RIP4(DIK/PKK), a novel member of the RIP kinase family, activates NF-kappa B and is processed during apoptosis[J]. Embo Reports, 2002,3(12):1201-1208.
doi: 10.1093/embo-reports/kvf236 URL |
| [107] |
Muto A, Ruland J, McAllister-Lucas LM, et al. Protein kinase C-associated kinase(PKK)mediates Bcl10-independent NF-kappa B activation induced by phorbol ester[J]. Journal of Biological Chemistry, 2002,277(35):31871-31876.
doi: 10.1074/jbc.M202222200 URL |
| [108] |
Kim SW, Schifano M, Oleksyn D, et al. Protein kinase C-associated kinase regulates NF-kappa B activation through inducing IKK activation[J]. International Journal of Oncology, 2014,45(4):1707-1714.
doi: 10.3892/ijo.2014.2578 URL |
| [109] |
Moran ST, Haider K, Ow Y, et al. Protein kinase C-associated kinase can activate NF kappa B in both a kinase-dependent and a kinase-independent manner[J]. Journal of Biological Chemistry, 2003,278(24):21526-21533.
doi: 10.1074/jbc.M301575200 URL |
| [110] |
Kim SW, Oleksyn DW, Rossi RM, et al. Protein kinase C-associated kinase is required for NF-kappa B signaling and survival in diffuse large B-cell lymphoma cells[J]. Blood, 2008,111(3):1644-1653.
doi: 10.1182/blood-2007-05-088591 URL |
| [111] |
Bertrand MJM, Lippens S, Staes A, et al. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4(RIP1-4)[J]. PLoS One, 2011,6(9):e22356.
doi: 10.1371/journal.pone.0022356 URL |
| [112] |
Bae HC, Jeong SH, Kim JH, et al. RIP4 upregulates CCL20 expression through STAT3 signalling in cultured keratinocytes[J]. Experimental Dermatology, 2018,27(10):1126-1133.
doi: 10.1111/exd.2018.27.issue-10 URL |
| [113] |
Zha JK, Zhou QH, Xu LG, et al. RIP5 is a RIP-homologous inducer of cell death[J]. Biochemical and Biophysical Research Communications, 2004,319(2):298-303.
doi: 10.1016/j.bbrc.2004.04.194 URL |
| [114] |
Racetin A, Raguz F, Durdov MG, et al. Immunohistochemical expression pattern of RIP5, FGFR1, FGFR2 and HIP2 in the normal human kidney development[J]. Acta Histochemica, 2019,121(5):531-538.
doi: S0065-1281(19)30029-7 pmid: 31047684 |
| [115] |
Becica T, Kero D, Vukojevic K, et al. Growth factors FGF8 and FGF2 and their receptor FGFR1, transcriptional factors Msx-1 and MSX-2, and apoptotic factors p19 and RIP5 participate in the early human limb development[J]. Acta Histochemica, 2018,120(3):205-214.
doi: S0065-1281(17)30380-X pmid: 29409666 |
| [116] |
Sanna-Cherchi S, Sampogna RV, Papeta N, et al. Mutations in DSTYK and dominant urinary tract malformations[J]. New England Journal of Medicine, 2013,369(7):621-629.
doi: 10.1056/NEJMoa1214479 URL |
| [117] | Hanafusa H, Yagi T, Ikeda H, et al. LRRK1 phosphorylation of Rab7 at S72 links trafficking of EGFR-containing endosomes to its effector RILP[J]. Journal of Cell Science, 2019, 132(11):jcs228809. |
| [118] |
Guo L, Girisha KM, Iida A, et al. Identification of a novel LRRK1 mutation in a family with osteosclerotic metaphyseal dysplasia[J]. Journal of Human Genetics, 2017,62(3):437-441.
doi: 10.1038/jhg.2016.136 URL |
| [119] |
Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology[J]. Neuron, 2004,44(4):601-607.
doi: 10.1016/j.neuron.2004.11.005 URL |
| [120] | Wu B, Xiao K, Zhang Z, et al. Altered expression of EPO might underlie hepatic hemangiomas in LRRK2 knockout mice[J]. Biomed Research International, 2016: 7681259. |
| [121] |
Baptista MAS, Dave KD, Frasier MA, et al. Loss of leucine-rich repeat kinase 2(LRRK2)in Rats leads to progressive abnormal phenotypes in peripheral organs[J]. PLoS One, 2013,8(11):e80705.
doi: 10.1371/journal.pone.0080705 URL |
| [122] | Fuji RN, Flagella M, Baca M, et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung[J]. Science Translational Medicine, 2015, 7(273):273ra15. |
| [123] |
Schapansky J, Khasnavis S, DeAndrade MP, et al. Familial knockin mutation of LRRK2 causes lysosomal dysfunction and accumulation of endogenous insoluble alpha-synuclein in neurons[J]. Neurobiology of Disease, 2018,111:26-35.
doi: S0969-9961(17)30283-8 pmid: 29246723 |
| [124] |
Sheng D, See K, Hu X, et al. Disruption of LRRK2 in Zebrafish leads to hyperactivity and weakened antibacterial response[J]. Biochemical and Biophysical Research Communications, 2018,497(4):1104-1109.
doi: 10.1016/j.bbrc.2018.02.186 URL |
| [125] |
Ren G, Xin S, Li S, et al. Disruption of LRRK2 does not cause specific loss of dopaminergic neurons in Zebrafish[J]. PLoS One, 2011,6(6):e20630.
doi: 10.1371/journal.pone.0020630 URL |
| [126] |
Prabhudesai S, Bensabeur FZ, Abdullah R, et al. LRRK2 knockdown in zebrafish causes developmental defects, neuronal loss, and synuclein aggregation[J]. Journal of Neuroscience Research, 2016,94(8):717-735.
doi: 10.1002/jnr.23754 pmid: 27265751 |
| [1] | 施炜涛, 姚春鹏, 魏文康, 王蕾, 房元杰, 仝钰洁, 马晓姣, 蒋文, 张晓爱, 邵伟. 利用CRISPR/Cas9技术构建MDH2敲除细胞株及抗呕吐毒素效应研究[J]. 生物技术通报, 2023, 39(7): 307-315. |
| [2] | 陈宝强, 李莹莹, 马博雅, 肉扎古丽·马利克, 优丽图孜·乃比, 宋金迪, 刘君, 王希东. 西瓜食酸菌III型分泌效应物基因aop2功能分析[J]. 生物技术通报, 2023, 39(6): 286-297. |
| [3] | 苏雨, 李宗芸, 韩永华. 植物液泡加工酶研究进展[J]. 生物技术通报, 2021, 37(6): 181-191. |
| [4] | 李宗杰, 狄荻, 李蓓蓓. 肺部菌群与呼吸道疾病的相互关系[J]. 生物技术通报, 2020, 36(2): 188-192. |
| [5] | 刘辉, 邓治, 杨洪, 代龙军, 李德军. 橡胶树HbMC2在酵母中的表达和抗逆性分析[J]. 生物技术通报, 2018, 34(9): 202-208. |
| [6] | 罗嫚, 王宇, 胡亚, 修江帆, 王涛, 彭建, 尚小丽, 吴建伟. 家蝇肽聚糖识别蛋白(PGRP-SA)的克隆表达及细菌结合研究[J]. 生物技术通报, 2016, 32(8): 145-151. |
| [7] | 毕彩红, 张秋霞, 于珊珊, 陈学昭, 刘春影, 祝茜. 松江鲈(Trachidermus fasciatus)髓样分化因子MyD88基因克隆与组织表达分析[J]. 生物技术通报, 2015, 31(2): 135-142. |
| [8] | 李奕平,王潇. 核苷酸结合寡聚化结构域NOD样受体(NLRs)研究进展[J]. 生物技术通报, 2013, 0(7): 36-40. |
| [9] | 赵建平;姚祥坦;. 植物小孢子胚胎发生机理[J]. , 2009, 0(06): 7-11. |
| [10] | 李凤娜;陈晓安. 一氧化氮对线粒体生物合成的积极影响[J]. , 2005, 0(02): 57-57. |
| [11] | 李凤娜;陈晓安. 一氧化氮对线粒体生物合成的积极影响[J]. , 2005, 0(02): 57-57. |
| [12] | . 国外动态[J]. , 2000, 0(02): 41-46. |
| [13] | 王伟;林均民;. 细胞培养系中的编程性细胞死亡[J]. , 1997, 0(03): 4-6. |
| [14] | 孙盈盈. 抗氧化剂使土壤杆菌转化葡萄得以实现[J]. , 1997, 0(02): 31-32. |
| [15] | 李思经. Diacrin公司加速猪细胞移植体开发[J]. , 1996, 0(05): 24-25. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||