








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 142-149.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1404
收稿日期:2021-11-10
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:覃雪晶,女,硕士研究生,研究方向:经济林栽培理论与利用;E-mail: 基金资助:
QIN Xue-jing( ), WANG Yu-han, CAO Yi-bo, ZHANG Ling-yun(
), WANG Yu-han, CAO Yi-bo, ZHANG Ling-yun( )
)
Received:2021-11-10
Published:2022-08-26
Online:2022-09-14
摘要:
制备青杄(Picea wilsonii)PwHAP5基因的特异性多克隆抗体,为研究PwHAP5基因功能、分子机制奠定基础。经PCR扩增获得青杄花粉PwHAP5基因片段与载体pET-48b相连构建原核表达载体pET-48b-PwHAP5。经诱导产生重组蛋白PwHAP5,通过His-NTA镍柱纯化蛋白产物。蛋白经SDS-PAGE检测后再免疫兔子制备兔抗血清。血清经ProteinA纯化后,通过SDS-PAGE和Western blot检测抗体纯度和特异性。结果表明,成功构建了原核表达载体pET-48b-PwHAP5,制备了分子质量约为45 kD的重组蛋白PwHAP5。获得的青杄PwHAP5兔抗血清经间接ELISA法检测效价达1∶729 000以上;制备的PwHAP5蛋白兔多克隆抗体能特异性识别抗原,效价高,满足后续研究PwHAP5蛋白在青杄生长发育中的功能等需求。
覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149.
QIN Xue-jing, WANG Yu-han, CAO Yi-bo, ZHANG Ling-yun. Prokaryotic Expression and Preparation of Polyclonal Antibody of PwHAP5 Gene in Picea wilsonii[J]. Biotechnology Bulletin, 2022, 38(8): 142-149.
| 引物名称 Primer name | 序列 Sequence(5'-3') | 酶切位点 Enzyme |
|---|---|---|
| 上游引物P1 | CGCGGATCCTATGGATCAGCAGCAGC-CCACAAT | BamH I |
| 下游引物P2 | CCCAAGCTTACTGCTGCCATGAGGA- GGTG | Hind III |
表1 引物信息
Table 1 Quote sequence information
| 引物名称 Primer name | 序列 Sequence(5'-3') | 酶切位点 Enzyme |
|---|---|---|
| 上游引物P1 | CGCGGATCCTATGGATCAGCAGCAGC-CCACAAT | BamH I |
| 下游引物P2 | CCCAAGCTTACTGCTGCCATGAGGA- GGTG | Hind III |

图1 PwHAP5基因片段的PCR扩增及重组质粒鉴定 A:PwHAP5基因的RT-PCR(M:DL2000;1:RT-PCR);B:pET-48b-Pw-HAP5载体的双酶切鉴定(M:DL2000;1:pET-48b-PwHAP5载体BamH I和Hind Ⅲ双酶切产物)
Fig. 1 PCR products of PwHAP5 and identification of the recombinant plasmid A:PCR products of PwHAP5(M:DL2000; 1:RT-PCR). B:Identification of the plasmid pET-48b-PwHAP5(M:DL2000; 1:the plasmid pET-48b-PwHAP5 digested with BamH I and Hind Ⅲ)
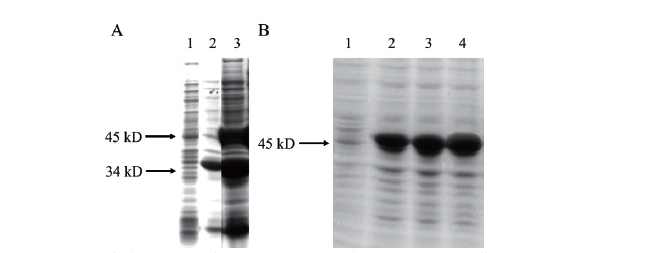
图2 目的蛋白PwHAP5表达形式和表达量鉴定 A:目的蛋白表达形式鉴定(1:未经IPTG诱导的重组大肠杆菌pET-48b-PwHAP5/BL21;2:重组大肠杆菌pET-48b-PwHAP5/BL21裂解后的上清液;3:重组大肠杆菌pET-48b-PwHAP5/BL21裂解后的沉淀物);B:目的蛋白SDS-PAGE(1:未经IPTG诱导前的蛋白;2-4:经IPTG诱导4、6、8 h后的蛋白产物)
Fig.2 Identification of expression form and expression amount of target protein PwHAP5 A:Identification on the expression form of target protein(1:Recombinant E. coli pET-48b-PwHAP5/BL2;2:the lysate supernatant of E. coli pET-48b-PwHAP5/BL2;3:the lysate precipitant of E. coli pET-48b-PwHAP5/BL2);B:SDS-PAGE of the target protein(1:the target protein without IPTG induction;2-4:the target protein induced for 4,6 and 8 h after IPTG,respectively)
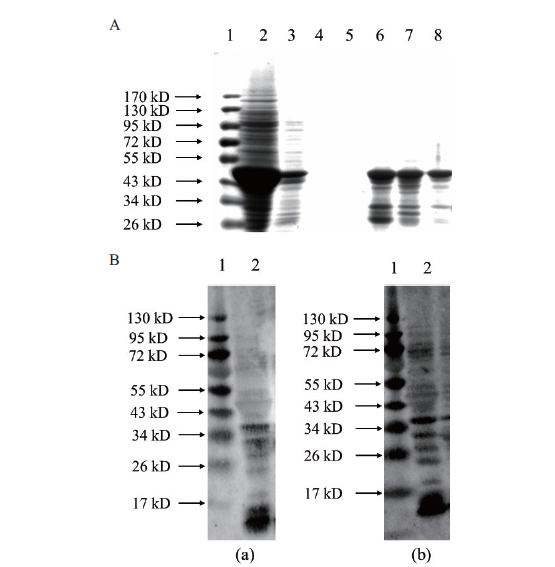
图3 纯化蛋白SDS-PAGE分析及未免疫兔血清的本底反应 A:Ni-NTA纯化重组蛋白(1:蛋白分子质量标准,2:总蛋白,3-5:分别是第1、2、3次洗脱液中的杂蛋白,6-8:分别是第1、2、3次洗脱液中的重组蛋白);B:本底反应(a,b:两只兔子血清的Western blot检测,1:蛋白分子质量标准,2:兔血清免疫印记检测条带)
Fig.3 SDS-PAGE analysis of purified recombinant protein and Western blotting assay A:Purified recombinant protein via Ni-NTA(1:protein marker;2:the total protein;3-5:the unnecessary proteins in the 1st,2nd and 3rd eluents,respectively;6-8:the necessary proteins in the 1st,2nd and 3rd eluents,respectively). B:Western blotting assay(a and b:serum from 2 different rabbits;1:protein marker;2:the rabbit antiserum)
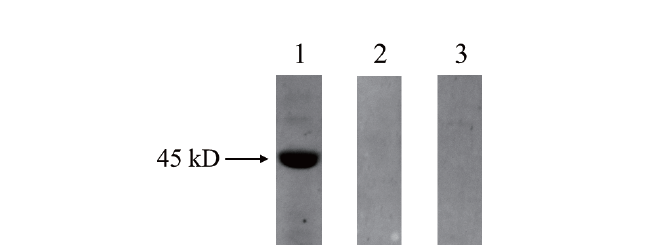
图6 抗体特异性检测结果 1:PwHAP5蛋白;2:未经诱导的PwHAP5蛋白;3:对照组
Fig. 6 Specificity of rabbit polyclonal antibody of PwHAP5 1:Protein of PwHAP5;2:uninduced protein of PwHAP5;3:the control group
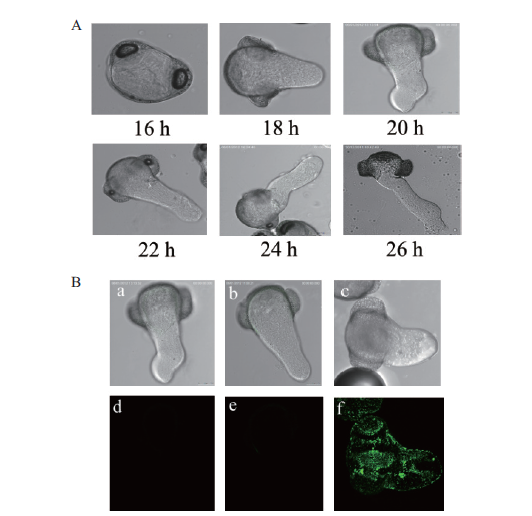
图7 花粉管萌发试验和间接免疫荧光检测 A:不同时长处理下花粉管的萌发情况;B:抗体间接免疫荧光检测(a,d:对照组;b,e:一抗处理的花粉管;e,f:一抗和二抗处理过的花粉管)
Fig.7 Pollen tube germination test and indirect immuno-fluorescence assay A:Germination situation of pollen tube under different time treatment;B:indirect immunofluorescence detection of antibody(a, d:the control group;b, e:the primary antibody treated pollen tube;e, f:pollen tubes treated with primary and secondary antibodies)
| [1] |
Mao YH, Chen CB. The hap complex in yeasts:structure, assembly mode, and gene regulation[J]. Front Microbiol, 2019, 10:1645.
doi: 10.3389/fmicb.2019.01645 URL |
| [2] |
Thirumurugan T, Ito Y, Kubo T, et al. Identification, characterization and interaction of HAP family genes in rice[J]. Mol Genet Genomics, 2008, 279(3):279-289.
doi: 10.1007/s00438-007-0312-3 URL |
| [3] |
Saha J, Gupta K, Gupta B. In silico characterization and evolutionary analyses of CCAAT binding proteins in the lycophyte plant Selaginella moellendorffii genome:a growing comparative genomics resource[J]. Comput Biol Chem, 2013, 47:81-88.
doi: 10.1016/j.compbiolchem.2013.08.001 URL |
| [4] |
Li LL, Yu YL, Wei J, et al. Homologous HAP5 subunit from Picea wilsonii improved tolerance to salt and decreased sensitivity to ABA in transformed Arabidopsis[J]. Planta, 2013, 238(2):345-356.
doi: 10.1007/s00425-013-1894-0 URL |
| [5] |
Shi HT, Ye TT, Zhong B, et al. AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21[J]. New Phytol, 2014, 203(2):554-567.
doi: 10.1111/nph.12812 URL |
| [6] |
Nardone V, Chaves-Sanjuan A, Nardini M. Structural determinants for NF-Y/DNA interaction at the CCAAT box[J]. Biochim Biophys Acta Gene Regul Mech, 2017, 1860(5):571-580.
doi: 10.1016/j.bbagrm.2016.09.006 URL |
| [7] |
Xing QK, Zheng ZM, Zhou XG, et al. Ds9 was isolated encoding as OsHAP3H and its C-Terminus was required for interaction with HAP2 and HAP5[J]. J Plant Biol, 2015, 58(1):26-37.
doi: 10.1007/s12374-014-0191-1 URL |
| [8] |
Li QP, Yan WH, Chen HX, et al. Duplication of OsHAP family genes and their association with heading date in rice[J]. J Exp Bot, 2016, 67(6):1759-1768.
doi: 10.1093/jxb/erv566 URL |
| [9] |
Zhang H, Zhu SS, Liu TZ, et al. DELAYED HEADING DATE1 interacts with OsHAP5C/D, delays flowering time and enhances yield in rice[J]. Plant Biotechnol J, 2019, 17(2):531-539.
doi: 10.1111/pbi.12996 pmid: 30107076 |
| [10] |
Nguyen VNT, Gho YS, An GH, et al. Identification of a module of HAP transcription factors for seed development in rice[J]. Plant Biotechnol Rep, 2019, 13(4):389-397.
doi: 10.1007/s11816-019-00545-0 |
| [11] |
Alam MM, Tanaka T, Nakamura H, et al. Overexpression of a rice heme activator protein gene(OsHAP2E)confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number[J]. Plant Biotechnol J, 2015, 13(1):85-96.
doi: 10.1111/pbi.12239 URL |
| [12] |
Valeeva LR, Nyamsuren C, Sharipova MR, et al. Heterologous expression of secreted bacterial BPP and HAP phytases in plants stimulates Arabidopsis thaliana growth on phytate[J]. Front Plant Sci, 2018, 9:186.
doi: 10.3389/fpls.2018.00186 pmid: 29515604 |
| [13] |
Shi HT, Chan ZL. AtHAP5A modulates freezing stress resistance in Arabidopsis independent of the CBF pathway[J]. Plant Signal Behav, 2014, 9(7):e29109.
doi: 10.4161/psb.29109 URL |
| [14] |
Zhu XF, Wu Q, Meng YT, et al. AtHAP5A regulates iron translocation in iron-deficient Arabidopsis thaliana[J]. J Integr Plant Biol, 2020, 62(12):1910-1925.
doi: 10.1111/jipb.12984 URL |
| [15] |
袁义杭, 张鹤华, 游韩莉, 等. 青杄PwNAC42基因的克隆及表达模式分析[J]. 生物技术通报, 2018, 34(3):113-120.
doi: 10.13560/j.cnki.biotech.bull.1985.2017-0924 |
| Yuan YH, Zhang HH, You HL, et al. Cloning and expression analysis of PwNAC42 in Picea wilsonii[J]. Biotechnol Bull, 2018, 34(3):113-120. | |
| [16] | 孙明洋. 玉米遗传转化体系的优化及PwHAP5基因转化的研究[D]. 长春: 吉林大学, 2011. |
| Sun MY. Study on optimalize of maize transformation system and transformation of PwHAP5[D]. Changchun: Jilin University, 2011. | |
| [17] |
Yu YL, Li YZ, Huang GX, et al. PwHAP5, a CCAAT-binding transcription factor, interacts with PwFKBP12 and plays a role in pollen tube growth orientation in Picea wilsonii[J]. J Exp Bot, 2011, 62(14):4805-4817.
doi: 10.1093/jxb/err120 URL |
| [18] | 王雨涵. 青杄HAP5和FKBP12的原核表达及多克隆抗体的制备[D]. 北京: 北京林业大学, 2012. |
| Wang YH. Prokaryotic expression and preparation of polyclonal antibodies of PwHAP5 and PwFKBP12in Picea wilsonii[D]. Beijing: Beijing Forestry University, 2012. | |
| [19] | 时智康, 王琪, 焦翠翠, 等. 克里米亚刚果出血热病毒抗体间接ELISA检测方法的建立[J]. 中国病原生物学杂志, 2021, 16(4):389-395. |
| Shi ZK, Wang Q, Jiao CC, et al. Establishment of an indirect ELISA method of detecting antibodies to the Crimean Congo hemorrhagic fever virus[J]. J Pathog Biol, 2021, 16(4):389-395. | |
| [20] | 卫静, 李长江, 刘亚静, 等. 拟南芥转录因子NF-YC的原核表达及多克隆抗体制备[J]. 生物技术通报, 2012(3):57-62. |
| Wei J, Li CJ, Liu YJ, et al. Prokaryotic expression of the Arabidopsis transcription factor NF-YC and preparation of its polyclonal antibodies[J]. Biotechnol Bull, 2012(3):57-62. | |
| [21] |
Quan SW, Niu JX, Zhou L, et al. Identification and characterization of NF-Y gene family in walnut(Juglans regia L.)[J]. BMC Plant Biol, 2018, 18(1):255.
doi: 10.1186/s12870-018-1459-2 URL |
| [22] |
Yan HL, et al. Genome-wide identification, characterization and expression analysis of NF-Y gene family in relation to fruit ripening in banana[J]. Postharvest Biol Technol, 2019, 151:98-110.
doi: 10.1016/j.postharvbio.2019.02.002 URL |
| [23] |
Ballif J, Endo S, Kotani M, et al. Over-expression of HAP3b enhances primary root elongation in Arabidopsis[J]. Plant Physiol Biochem, 2011, 49(6):579-583.
doi: 10.1016/j.plaphy.2011.01.013 URL |
| [24] | Wang RK, et al. Genome-wide analysis of poplar NF-YB gene family and identified PtNF-YB1 important in regulate flowering timing in transgenic plants[J]. BMC Plant Biol, 2019(1):251. |
| [25] | Xia WF, Zhang JJ, Shen WZ, et al. A rapid intraoperative parathyroid hormone assay based on the immune colloidal gold technique for parathyroid identification in thyroid surgery[J]. Front Endocrinol:Lausanne, 2020, 11:594745. |
| [26] |
王泓力, 焦雨铃. 染色质免疫共沉淀实验方法[J]. 植物学报, 2020, 55(4):475-480.
doi: 10.11983/CBB20076 |
| Wang HL, Jiao YL. Protocols for chromatin immunoprecipitation[J]. Chin Bull Bot, 2020, 55(4):475-480. |
| [1] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [2] | 郭文博, 路杨, 隋丽, 赵宇, 邹晓威, 张正坤, 李启云. 球孢白僵菌真菌病毒BbPmV-4外壳蛋白多克隆抗体制备及应用[J]. 生物技术通报, 2023, 39(10): 58-67. |
| [3] | 索青青, 吴楠, 杨慧, 李莉, 王锡锋. 水稻咖啡酰辅酶A-O-甲基转移酶基因的原核表达、抗体制备和应用[J]. 生物技术通报, 2022, 38(8): 135-141. |
| [4] | 王光丽, 范婵, 王辉, 卢惠芳, 夏灵尹, 黄健, 闵迅. 霍乱弧菌溶血素HlyA的原核表达、纯化及多克隆抗体制备与鉴定[J]. 生物技术通报, 2022, 38(7): 269-277. |
| [5] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [6] | 沈俊强, 张莉萍, 于瑞明, 王永录, 潘丽, 刘霞, 刘新生. 猪嵴病毒结构蛋白VP0与VP1原核表达及间接ELISA方法的建立[J]. 生物技术通报, 2022, 38(10): 243-253. |
| [7] | 山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155. |
| [8] | 曾福源, 苏泽辉, 周诗慧, 谢妙, 庞欢瑛. 溶藻弧菌PEPCK蛋白原核表达及其乙酰化、琥珀酰化修饰的鉴定[J]. 生物技术通报, 2021, 37(5): 84-91. |
| [9] | 张西西, 张怡青, 李玉林, 韩笑, 王国强, 王晓军, 王旭东, 王云龙. 新型冠状病毒(SARS-CoV-2)N蛋白C端重组蛋白的原核表达、纯化及应用[J]. 生物技术通报, 2021, 37(5): 92-97. |
| [10] | 白福美, 李至敏, 王小琴, 胡紫微, 鲍玲玲, 李志敏. 集胞藻PCC6803中N-乙酰鸟氨酸转氨酶的生化表征及结构分析[J]. 生物技术通报, 2021, 37(5): 98-107. |
| [11] | 瞿欢, 李成, 陈汭, 廖艺杰, 曹三杰, 文翼平, 颜其贵, 黄小波. 猪δ冠状病毒S1-CTD的截短表达及间接ELISA抗体方法的建立[J]. 生物技术通报, 2021, 37(5): 273-280. |
| [12] | 彭利忠, 张鹏, 周雯雯, 曾旭辉, 张小宁. 精子特异性蛋白Cabs1多克隆抗体的制备及多用途验证[J]. 生物技术通报, 2021, 37(3): 261-270. |
| [13] | 贺扬, 余巧玲, 王均, 覃川杰, 李华涛. 罗非鱼原核表达基因研究进展[J]. 生物技术通报, 2021, 37(2): 195-202. |
| [14] | 付强, 郭妍婷, 陈俊贞, 王金泉, 史慧君. 牛病毒性腹泻病毒离子孔道蛋白p7多肽多克隆抗体的制备和鉴定[J]. 生物技术通报, 2021, 37(10): 137-142. |
| [15] | 唐禄, 董丽平, 尹茉莉, 刘磊, 董媛, 王会岩. 成纤维细胞生长因子20单克隆抗体的制备及鉴定[J]. 生物技术通报, 2021, 37(10): 179-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||