








生物技术通报 ›› 2023, Vol. 39 ›› Issue (2): 172-182.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0945
杜清洁1( ), 周璐瑶1, 杨思震1, 张嘉欣2, 陈春林1, 李娟起1, 李猛1, 赵士文1, 肖怀娟1(
), 周璐瑶1, 杨思震1, 张嘉欣2, 陈春林1, 李娟起1, 李猛1, 赵士文1, 肖怀娟1( ), 王吉庆1(
), 王吉庆1( )
)
收稿日期:2022-07-31
出版日期:2023-02-26
发布日期:2023-03-07
作者简介:杜清洁,男,博士,讲师,研究方向:蔬菜栽培逆境生理与分子生物学;E-mail: 基金资助:
DU Qing-jie1( ), ZHOU Lu-yao1, YANG Si-zhen1, ZHANG Jia-xin2, CHEN Chun-lin1, LI Juan-qi1, LI Meng1, ZHAO Shi-wen1, XIAO Huai-juan1(
), ZHOU Lu-yao1, YANG Si-zhen1, ZHANG Jia-xin2, CHEN Chun-lin1, LI Juan-qi1, LI Meng1, ZHAO Shi-wen1, XIAO Huai-juan1( ), WANG Ji-qing1(
), WANG Ji-qing1( )
)
Received:2022-07-31
Published:2023-02-26
Online:2023-03-07
摘要:
半胱氨酸蛋白酶在植物调节盐胁迫应答方面起重要作用,克隆CaCP1,研究其抗盐性功能,为辣椒抗盐品种的培育提供参考。克隆CaCP1的启动子,根据CaCP1启动子中盐胁迫相关的作用元件,构建不同长度启动子片段的重组载体,进行烟草瞬时转化。通过对CaCP1超表达烟草转基因T3代株系进行盐处理,观察其表观症状,测定生理指标,并进行胁迫相关基因的表达分析。结果表明,盐胁迫下,CaCP1启动子-410- -1 bp区段的GUS活性最强;CaCP1转基因植株叶片提前萎蔫和黄化,其中叶绿素含量和相对含水量均显著低于野生型,MDA和电解质渗漏率显著高于野生型,ROS清除酶CAT和SOD活性降低,POD活性升高;与野生型相比,转基因植株中ROS清除基因(NtSOD、NtPOD、NtCAT和NtAPX)和盐胁迫相关基因(NtLEA5、NtNHX1、NtP5CS1和NtSOS1)的表达量显著降低。CaCP1作为负调控因子介导植物对盐胁迫的防御反应。
杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182.
DU Qing-jie, ZHOU Lu-yao, YANG Si-zhen, ZHANG Jia-xin, CHEN Chun-lin, LI Juan-qi, LI Meng, ZHAO Shi-wen, XIAO Huai-juan, WANG Ji-qing. Overexpression of CaCP1 Enhances Salt Stress Sensibility in Transgenic Tobacco[J]. Biotechnology Bulletin, 2023, 39(2): 172-182.
| 引物Primer | 序列Sequence(5'-3') | 用途Purpose |
|---|---|---|
| GUS-F | TAGATCTGACTAGTTTACGTCCTGT | 构建载体检测 |
| GUS-R | TAGTCTGCCAGTTCAGTTCGT | |
| CaMV35S-F | AAGACTGGCGAACAGTTCAT | 载体构建 |
| CaMV35S-R | ATAGTGGGATTGTGCGTCAT | |
| 1381-P1F | CCGGAATTCGCGAAGGTAGTATAATTTAAAAC | 克隆启动子片段 |
| 1381-P2F | CCGGAATTCTGAATGAAATGATTTGTATTTTG | |
| 1381-P3F | CCGGAATTCTATGAAATGATCTAATATAATTG | |
| 1381-P4F | CCGGAATTCATAGAAATCATTAAGATTTTCCG | |
| 1381-P1R | CGCGTCGACTATAGAACAACTATATAGTATTATG | |
| 2307-CaCP1-F | TCTAGAATATAGTTGTTCTATAATGGCCTTT | 克隆CaCP1 |
| 2307-CaCP1-R | GGTACCTTAAGGATAAATTTTCTTTTAGGC | |
| GUS-QPCR-F | CAGTGAAGGGCCAACAGTTC | GUS基因的定量分析 |
| GUS- QPCR-R | CATGTTCATCTGCCCAGTCG | |
| NtCAT-F | AGGTACCGCTCATTCACACC | NtCAT基因的定量分析 |
| NtCAT-R | AAGCAAGCTTTTGACCCAGA | |
| NtSOD-F | AGCTACATGACGCCATTTCC | NtSOD基因的定量分析 |
| NtSOD-R | CCCTGTAAAGCAGCACCTTC | |
| NtAPX-F | CCATTTCCAGTGCTTGTGGTCTC | NtAPX基因的定量分析 |
| NtAPX-R | ATAGGTACCAGCAGAGTGCCA | |
| NtLEA5-F | CATCAGCTAGTGTGCCAGGT | NtLEA5基因的定量分析 |
| NtLEA5-R | TGGCACCCATGATGTTGTCT | |
| NtNHX1-F | CAACTGGTCTTCTTAGTGCT | NtNHX1基因的定量分析 |
| NtNHX1-R | GCCTTGTAGTGACTCTTGAA | |
| NtPOX2-F | CATCTTCACGGCTGTTCGAG | NtPOX2基因的定量分析 |
| NtPOX2-R | TGTTGGGTGGTGAGGTCTTT | |
| NtP5CS1-F | TTGCAAACTCTGTCCGTGTG | NtP5CS1基因的定量分析 |
| NtP5CS1-R | TTGGCCTCCTTTCCTCCTTT | |
| NtSOS1-F | CAAATGTTATCCCCCGAAAGC | NtSOS1基因的定量分析 |
| NtSOS1-R | CGGAGAACCTGAGGAAATGTGA | |
| NtActin-F | TGGCATCACACTTTCTACAA | RT-qPCR试验的内参基因 |
| NtActin-R | CAACGGAATCTCTCAGCTCC |
表1 本文中的引物碱基序列
Table 1 Primer base sequences used in this study
| 引物Primer | 序列Sequence(5'-3') | 用途Purpose |
|---|---|---|
| GUS-F | TAGATCTGACTAGTTTACGTCCTGT | 构建载体检测 |
| GUS-R | TAGTCTGCCAGTTCAGTTCGT | |
| CaMV35S-F | AAGACTGGCGAACAGTTCAT | 载体构建 |
| CaMV35S-R | ATAGTGGGATTGTGCGTCAT | |
| 1381-P1F | CCGGAATTCGCGAAGGTAGTATAATTTAAAAC | 克隆启动子片段 |
| 1381-P2F | CCGGAATTCTGAATGAAATGATTTGTATTTTG | |
| 1381-P3F | CCGGAATTCTATGAAATGATCTAATATAATTG | |
| 1381-P4F | CCGGAATTCATAGAAATCATTAAGATTTTCCG | |
| 1381-P1R | CGCGTCGACTATAGAACAACTATATAGTATTATG | |
| 2307-CaCP1-F | TCTAGAATATAGTTGTTCTATAATGGCCTTT | 克隆CaCP1 |
| 2307-CaCP1-R | GGTACCTTAAGGATAAATTTTCTTTTAGGC | |
| GUS-QPCR-F | CAGTGAAGGGCCAACAGTTC | GUS基因的定量分析 |
| GUS- QPCR-R | CATGTTCATCTGCCCAGTCG | |
| NtCAT-F | AGGTACCGCTCATTCACACC | NtCAT基因的定量分析 |
| NtCAT-R | AAGCAAGCTTTTGACCCAGA | |
| NtSOD-F | AGCTACATGACGCCATTTCC | NtSOD基因的定量分析 |
| NtSOD-R | CCCTGTAAAGCAGCACCTTC | |
| NtAPX-F | CCATTTCCAGTGCTTGTGGTCTC | NtAPX基因的定量分析 |
| NtAPX-R | ATAGGTACCAGCAGAGTGCCA | |
| NtLEA5-F | CATCAGCTAGTGTGCCAGGT | NtLEA5基因的定量分析 |
| NtLEA5-R | TGGCACCCATGATGTTGTCT | |
| NtNHX1-F | CAACTGGTCTTCTTAGTGCT | NtNHX1基因的定量分析 |
| NtNHX1-R | GCCTTGTAGTGACTCTTGAA | |
| NtPOX2-F | CATCTTCACGGCTGTTCGAG | NtPOX2基因的定量分析 |
| NtPOX2-R | TGTTGGGTGGTGAGGTCTTT | |
| NtP5CS1-F | TTGCAAACTCTGTCCGTGTG | NtP5CS1基因的定量分析 |
| NtP5CS1-R | TTGGCCTCCTTTCCTCCTTT | |
| NtSOS1-F | CAAATGTTATCCCCCGAAAGC | NtSOS1基因的定量分析 |
| NtSOS1-R | CGGAGAACCTGAGGAAATGTGA | |
| NtActin-F | TGGCATCACACTTTCTACAA | RT-qPCR试验的内参基因 |
| NtActin-R | CAACGGAATCTCTCAGCTCC |

图3 CaCP1启动子活性分析 A:转基因烟草叶片的GUS染色;B:转基因烟草中GUS的表达量检测。不同小写字母表示显著性(P<0.05),下同
Fig. 3 Activity analysis of promoter of CaCP1 A: GUS histochemical staining in transgenic tobacco leaves. B: Expression analysis of GUS in transgenic tobacco leaves. The lower letters indicate significant differences(P<0.05), the same below
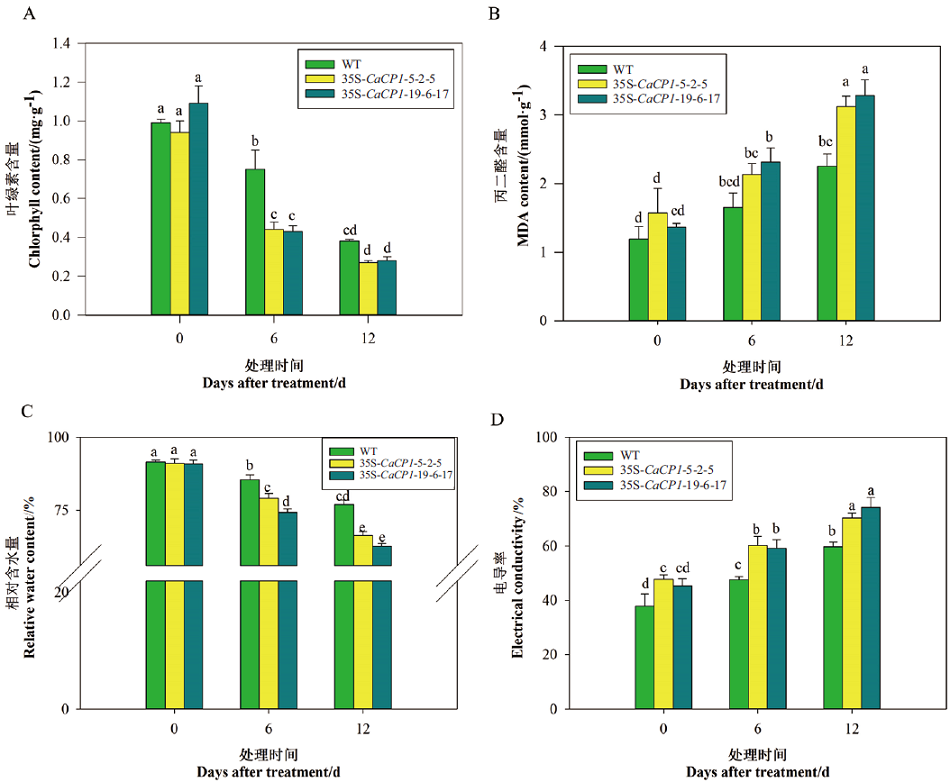
图5 盐胁迫下35S-CaCP1转基因和野生型株系的叶绿素含量、MDA含量、相对含水量和电导率
Fig. 5 Chlorophyll, MDA, relative water content and electrical conductivity of 35S-CaCP1 transgenic tobacco plants under salt stress
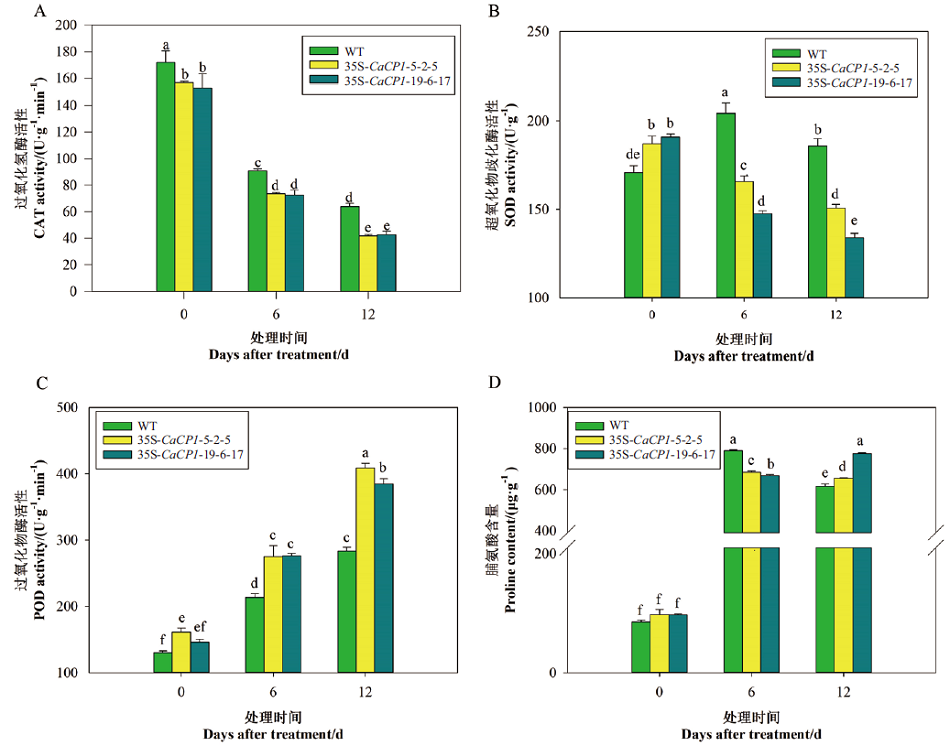
图6 盐胁迫下35S-CaCP1转基因和野生型株系的CAT、SOD、POD酶活性和脯氨酸含量
Fig. 6 CAT activity, SOD activity, POD activity, and proline content of 35S-CaCP1 transgenic tobacco plants after salt treatment
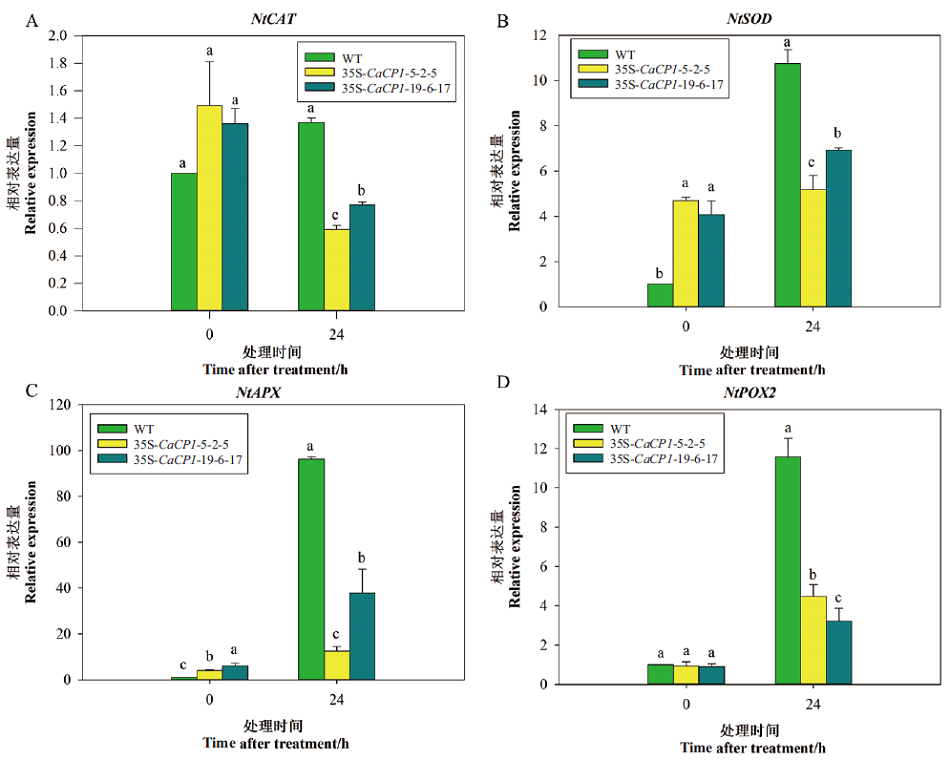
图7 盐胁迫下35S-CaCP1转基因和野生型株系抗氧化酶相关基因的表达变化
Fig. 7 Expression profiles of antioxidant-related genes in 35S-CaCP1 transgenic tobacco plants and wild plants after salt treatment
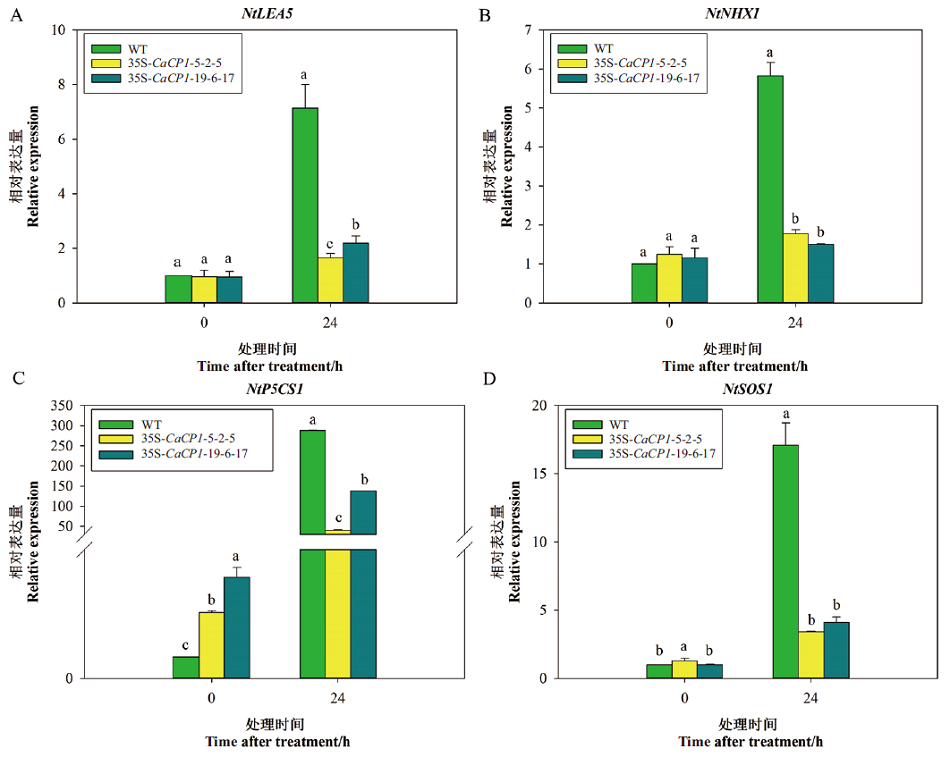
图8 盐胁迫下35S-CaCP1转基因和野生型株系胁迫相关基因的相对表达量变化
Fig. 8 Relative expression profiles of stress-related genes in 35S-CaCP1 transgenic tobacco plants and wild plants after salt treatment
| [1] | 余海英, 李廷轩, 周健民. 设施土壤次生盐渍化及其对土壤性质的影响[J]. 土壤, 2005, 37(6): 581-586. |
| Yu HY, Li TX, Zhou JM. Secondary salinization of greenhouse soil and its effects on soil properties[J]. Soils, 2005, 37(6): 581-586. | |
| [2] |
Shah ZH, Rehman HM, Akhtar T, et al. Redox and ionic homeostasis regulations against oxidative, salinity and drought stress in wheat(A systems biology approach)[J]. Front Genet, 2017, 8: 141.
doi: 10.3389/fgene.2017.00141 URL |
| [3] |
Varga B, Janda T, László E, et al. Influence of abiotic stresses on the antioxidant enzyme activity of cereals[J]. Acta Physiol Plant, 2012, 34(3): 849-858.
doi: 10.1007/s11738-011-0882-x URL |
| [4] |
Cappetta E, Andolfo G, et al. Empowering crop resilience to environmental multiple stress through the modulation of key response components[J]. J Plant Physiol, 2020, 246/247: 153134.
doi: 10.1016/j.jplph.2020.153134 URL |
| [5] |
Møller IM, Sweetlove LJ. ROS signalling—specificity is required[J]. Trends Plant Sci, 2010, 15(7): 370-374.
doi: 10.1016/j.tplants.2010.04.008 pmid: 20605736 |
| [6] |
Dai XY, Xu YY, Ma QB, et al. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis[J]. Plant Physiol, 2007, 143(4): 1739-1751.
doi: 10.1104/pp.106.094532 URL |
| [7] |
van Wyk SG, du Plessis M, Cullis CA, et al. Cysteine protease and cystatin expression and activity during soybean nodule development and senescence[J]. BMC Plant Biol, 2014, 14: 294.
doi: 10.1186/s12870-014-0294-3 pmid: 25404209 |
| [8] |
der Hoorn RAV. Plant proteases: from phenotypes to molecular mechanisms[J]. Annu Rev Plant Biol, 2008, 59: 191-223.
doi: 10.1146/annurev.arplant.59.032607.092835 pmid: 18257708 |
| [9] |
Poret M, Chandrasekar B, van der Hoorn RAL, et al. Characterization of senescence-associated protease activities involved in the efficient protein remobilization during leaf senescence of winter oilseed rape[J]. Plant Sci, 2016, 246: 139-153.
doi: S0168-9452(16)30023-1 pmid: 26993244 |
| [10] |
Deng J, Zhu FG, Liu JX, et al. Transcription factor bHLH2 represses CYSTEINE PROTEASE77 to negatively regulate nodule senescence[J]. Plant Physiol, 2019, 181(4): 1683-1703.
doi: 10.1104/pp.19.00574 pmid: 31591150 |
| [11] |
Rodríguez-Herva JJ, González-Melendi P, Cuartas-Lanza R, et al. A bacterial cysteine protease effector protein interferes with photosynthesis to suppress plant innate immune responses[J]. Cellular Microbiology, 2012, 14(5): 669-681.
doi: 10.1111/j.1462-5822.2012.01749.x pmid: 22233353 |
| [12] |
Xiao HJ, Yin YX, Chai WG, et al. Silencing of the CaCP gene delays salt- and osmotic-induced leaf senescence in Capsicum annuum L[J]. Int J Mol Sci, 2014, 15(5): 8316-8334.
doi: 10.3390/ijms15058316 URL |
| [13] |
Chen H, Nelson RS, Sherwood JL. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection[J]. BioTechniques, 1994, 16(4): 664-668, 670.
pmid: 8024787 |
| [14] |
Xu WR, Yu YH, Ding JH, et al. Characterization of a novel stilbene synthase promoter involved in pathogen- and stress-inducible expression from Chinese wild Vitis pseudoreticulata[J]. Planta, 2010, 231(2): 475-487.
doi: 10.1007/s00425-009-1062-8 URL |
| [15] |
Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves[J]. Plant J, 2000, 22(6): 543-551.
doi: 10.1046/j.1365-313x.2000.00760.x pmid: 10886774 |
| [16] | Yin YX, Guo WL, Zhang YL, et al. Cloning and characterisation of a pepper aquaporin, CaAQP, which reduces chilling stress in transgenic tobacco plants[J]. Plant Cell Tissue Organ Cult PCTOC, 2014, 118(3): 431-444. |
| [17] |
Hoekema A, Hirsch PR, Hooykaas PJJ, et al. A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid[J]. Nature, 1983, 303(5913): 179-180.
doi: 10.1038/303179a0 URL |
| [18] |
Arkus KAJ, Cahoon EB, Jez JM. Mechanistic analysis of wheat chlorophyllase[J]. Arch Biochem Biophys, 2005, 438(2): 146-155.
pmid: 15913540 |
| [19] |
Guo WL, Chen RG, Gong ZH, et al. Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper(Capsicum annuum)leaves subjected to chilling stress[J]. Genet Mol Res, 2012, 11(4): 4063-4080.
doi: 10.4238/2012.September.10.5 pmid: 23079969 |
| [20] |
Sade N, Vinocur BJ, Diber A, et al. Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion?[J]. New Phytol, 2009, 181(3): 651-661.
doi: 10.1111/j.1469-8137.2008.02689.x pmid: 19054338 |
| [21] | Zhou SY, Hu W, Deng XM, et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco[J]. PLoS One, 2012, 7(12): e52439. |
| [22] |
Irigoyen J, Einerich DW, Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa(Medicago sativd)plants[J]. Physiol Plant, 1992, 84: 55-60.
doi: 10.1111/j.1399-3054.1992.tb08764.x URL |
| [23] |
Zhang L, Xi DM, Luo L, et al. Cotton GhMPK2 is involved in multiple signaling pathways and mediates defense responses to pathogen infection and oxidative stress[J]. FEBS J, 2011, 278(8): 1367-1378.
doi: 10.1111/j.1742-4658.2011.08056.x URL |
| [24] |
Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels[J]. Anal Biochem, 1971, 44(1): 276-287.
doi: 10.1016/0003-2697(71)90370-8 pmid: 4943714 |
| [25] |
Ranieri A, Petacco F, Castagna A, et al. Redox state and peroxidase system in sunflower plants exposed to ozone[J]. Plant Sci, 2000, 159(1): 159-167.
doi: 10.1016/s0168-9452(00)00352-6 pmid: 11011103 |
| [26] |
Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, et al. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana[J]. Gene, 1993, 129(2): 175-182.
doi: 10.1016/0378-1119(93)90266-6 pmid: 8325504 |
| [27] |
Jones JT, Mullet JE. A salt- and dehydration-inducible pea gene, Cyp15a, encodes a cell-wall protein with sequence similarity to cysteine proteases[J]. Plant Mol Biol, 1995, 28(6): 1055-1065.
pmid: 7548823 |
| [28] |
Zang QW, Wang CX, Li XY, et al. Isolation and characterization of a gene encoding a polyethylene glycol-induced cysteine protease in common wheat[J]. J Biosci, 2010, 35(3): 379-388.
doi: 10.1007/s12038-010-0043-1 URL |
| [29] |
Chen HJ, Su CT, Lin CH, et al. Expression of sweet potato cysteine protease SPCP2 altered developmental characteristics and stress responses in transgenic Arabidopsis plants[J]. J Plant Physiol, 2010, 167(10): 838-847.
doi: 10.1016/j.jplph.2010.01.005 URL |
| [30] |
Niño MC, Kim MS, Kang KK, et al. Genome-wide identification and molecular characterization of cysteine protease genes in rice[J]. Plant Biotechnol Rep, 2020, 14(1): 69-87.
doi: 10.1007/s11816-019-00583-8 URL |
| [31] |
Park HC, Kim ML, Kang YH, et al. Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor[J]. Plant Physiol, 2004, 135(4): 2150-2161.
doi: 10.1104/pp.104.041442 pmid: 15310827 |
| [32] |
Gai WX, Ma X, Qiao YM, et al. Characterization of the bZIP transcription factor family in pepper(Capsicum annuum L.): CabZIP25 positively modulates the salt tolerance[J]. Front Plant Sci, 2020, 11: 139.
doi: 10.3389/fpls.2020.00139 URL |
| [33] |
Han JY, Li H, Yin B, et al. The papain-like cysteine protease CEP1 is involved in programmed cell death and secondary wall thickening during xylem development in Arabidopsis[J]. J Exp Bot, 2019, 70(1): 205-215.
doi: 10.1093/jxb/ery356 URL |
| [34] |
Zhang DD, Liu D, Lv XM, et al. The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis[J]. Plant Cell, 2014, 26(7): 2939-2961.
doi: 10.1105/tpc.114.127282 URL |
| [35] |
Alomrani S, Kunert KJ, Foyer CH. Papain-like cysteine proteases are required for the regulation of photosynthetic gene expression and acclimation to high light stress[J]. J Exp Bot, 2021, 72(9): 3441-3454.
doi: 10.1093/jxb/erab101 pmid: 33686435 |
| [36] |
Khanna-Chopra R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation[J]. Protoplasma, 2012, 249(3): 469-481.
doi: 10.1007/s00709-011-0308-z pmid: 21805384 |
| [37] |
Liu WF, Guo CL, Huang DQ, et al. The papain-like cysteine protease HpXBCP3 from Haematococcus pluvialis involved in the regulation of growth, salt stress tolerance and chlorophyll synthesis in microalgae[J]. Int J Mol Sci, 2021, 22(21): 11539.
doi: 10.3390/ijms222111539 URL |
| [38] |
Wang CL, Lu GQ, Hao YQ, et al. ABP9, a maize bZIP transcription factor, enhances tolerance to salt and drought in transgenic cotton[J]. Planta, 2017, 246(3): 453-469.
doi: 10.1007/s00425-017-2704-x pmid: 28474114 |
| [39] |
Wang DL, Lu XK, Chen XG, et al. Temporal salt stress-induced transcriptome alterations and regulatory mechanisms revealed by PacBio long-reads RNA sequencing in Gossypium hirsutum[J]. BMC Genomics, 2020, 21(1): 838.
doi: 10.1186/s12864-020-07260-z URL |
| [40] | Xu LP, Liu JB, et al. Effect of salt stress on growth and physiology in Melia Azedarach seedlings of six provenances[J]. International Journal of Agriculture and Biology, 2018, 20(2): 471-480. |
| [41] |
Choi HW, Kim YJ, Lee SC, et al. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens[J]. Plant Physiol, 2007, 145(3): 890-904.
doi: 10.1104/pp.107.103325 pmid: 17905862 |
| [42] |
Hasegawa PM, Bressan RA, Zhu JK, et al. Plant cellular and molecular responses to high salinity[J]. Annu Rev Plant Physiol Plant Mol Biol, 2000, 51: 463-499.
doi: 10.1146/annurev.arplant.51.1.463 URL |
| [43] |
Huda KMK, Banu MSA, Garg B, et al. OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes[J]. Plant J, 2013, 76(6): 997-1015.
doi: 10.1111/tpj.12352 URL |
| [44] |
Liu J, Zhu JK. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis[J]. Plant Physiol, 1997, 114(2): 591-596.
doi: 10.1104/pp.114.2.591 pmid: 9193091 |
| [45] |
Kant S, Kant P, Raveh E, et al. Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. Halophila[J]. Plant Cell Environ, 2006, 29(7): 1220-1234.
doi: 10.1111/j.1365-3040.2006.01502.x URL |
| [46] |
Ben Saad R, Ben Halima N, et al. AlSRG1, a novel gene encoding an RRM-type RNA-binding protein(RBP)from Aeluropus Litto-ralis, confers salt and drought tolerance in transgenic tobacco[J]. Environment and Experiment Botany, 2018, 150: 25-36.
doi: 10.1016/j.envexpbot.2018.03.002 URL |
| [47] |
Zhang Y, et al. Expression of TaGF14b, a 14-3-3 adaptor protein gene from wheat, enhances drought and salt tolerance in transgenic tobacco[J]. Planta, 2018, 248(1): 117-137.
doi: 10.1007/s00425-018-2887-9 pmid: 29616395 |
| [48] |
Liu QL, Zhong M, Li S, et al. Overexpression of a Chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress[J]. Plant Physiol Biochem, 2013, 69: 27-33.
doi: 10.1016/j.plaphy.2013.04.016 URL |
| [49] |
Han D, Hou YJ, Wang YF, et al. Overexpression of a Malus baccata WRKY transcription factor gene(Mbwrky5)increases drought and salt tolerance in transgenic tobacco[J]. Canadian Journal of Plant Science, 2019, 99(2): 173-183.
doi: 10.1139/cjps-2018-0053 URL |
| [1] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [2] | 王贵芳, 姚元涛, 许海峰, 相昆, 梁家慧, 张淑辉, 王文茹, 张明娟, 张美勇, 陈新. 核桃JrSnRK1α1.1调控种子油脂合成与积累[J]. 生物技术通报, 2023, 39(9): 183-191. |
| [3] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [4] | 赵志祥, 王殿东, 周亚林, 王培, 严婉荣, 严蓓, 罗路云, 张卓. 枯草芽孢杆菌Ya-1对辣椒枯萎病的防治及其对根际真菌群落的影响[J]. 生物技术通报, 2023, 39(9): 213-224. |
| [5] | 刘珍银, 段郅臻, 彭婷, 王童欣, 王健. 基于三角梅的病毒诱导基因沉默体系的建立与优化[J]. 生物技术通报, 2023, 39(7): 123-130. |
| [6] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [7] | 李文辰, 刘鑫, 康越, 李伟, 齐泽铮, 于璐, 王芳. TRV病毒诱导大豆基因沉默体系优化及应用[J]. 生物技术通报, 2023, 39(7): 143-150. |
| [8] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [9] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [10] | 张蓓, 任福森, 赵洋, 郭志伟, 孙强, 刘贺娟, 甄俊琦, 王童童, 程相杰. 辣椒响应热胁迫机制的研究进展[J]. 生物技术通报, 2023, 39(7): 37-47. |
| [11] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [12] | 张路阳, 韩文龙, 徐晓雯, 姚健, 李芳芳, 田效园, 张智强. 烟草TCP基因家族的鉴定及表达分析[J]. 生物技术通报, 2023, 39(6): 248-258. |
| [13] | 王羽, 尹铭绅, 尹晓燕, 奚家勤, 杨建伟, 牛秋红. 烟草甲体内烟碱降解菌的筛选、鉴定及降解特性[J]. 生物技术通报, 2023, 39(6): 308-315. |
| [14] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [15] | 李月, 余婉贤, 李宁, 姚明华, 李峰, 邓颖天. 辣椒苗期炭疽菌接种方法[J]. 生物技术通报, 2023, 39(4): 221-226. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||