








生物技术通报 ›› 2023, Vol. 39 ›› Issue (7): 56-66.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1343
王玲1( ), 卓燊1,2, 付学森1, 刘紫璇1, 刘笑蓉1,3, 王志辉1,3, 周日宝1,3,4(
), 卓燊1,2, 付学森1, 刘紫璇1, 刘笑蓉1,3, 王志辉1,3, 周日宝1,3,4( ), 刘湘丹1,3,4(
), 刘湘丹1,3,4( )
)
收稿日期:2022-11-01
出版日期:2023-07-26
发布日期:2023-08-17
通讯作者:
周日宝,男,博士,教授,博士生导师,研究方向:中药资源与品质评价;E-mail: 1057323510@qq.com;作者简介:王玲,女,硕士研究生,研究方向:中药资源与品质评价;E-mail: 3380788828@qq.com
基金资助:
WANG Ling1( ), ZHUO Shen1,2, FU Xue-sen1, LIU Zi-xuan1, LIU Xiao-rong1,3, WANG Zhi-hui1,3, ZHOU Ri-bao1,3,4(
), ZHUO Shen1,2, FU Xue-sen1, LIU Zi-xuan1, LIU Xiao-rong1,3, WANG Zhi-hui1,3, ZHOU Ri-bao1,3,4( ), LIU Xiang-dan1,3,4(
), LIU Xiang-dan1,3,4( )
)
Received:2022-11-01
Published:2023-07-26
Online:2023-08-17
摘要:
莲的多个部位均可作药,其中苄基异喹啉类生物碱是荷叶、莲子心的主要活性成分。荷叶含有荷叶碱、莲碱等阿朴啡类生物碱,具有良好调脂减肥作用;莲子心中主要含有甲基莲心碱、莲心碱等双苄基异喹啉类生物碱,可抗心律失常。近年来,莲生物碱成分的显著药理活性及荷叶与莲子心的“同源异效”现象,激发越来越多研究工作者开展莲生物碱生源合成途径及关键酶研究。为此,本文综述了荷叶、莲子心生物碱成分类型、生物碱合成途径及关键酶基因研究进展,以期为解析莲的生物碱合成途径及荷叶、莲子心的药效分化分子机制提供参考。
王玲, 卓燊, 付学森, 刘紫璇, 刘笑蓉, 王志辉, 周日宝, 刘湘丹. 莲生物碱生物合成途径及相关基因研究进展[J]. 生物技术通报, 2023, 39(7): 56-66.
WANG Ling, ZHUO Shen, FU Xue-sen, LIU Zi-xuan, LIU Xiao-rong, WANG Zhi-hui, ZHOU Ri-bao, LIU Xiang-dan. Advances in the Biosynthetic Pathways and Related Genes of Lotus Alkaloids[J]. Biotechnology Bulletin, 2023, 39(7): 56-66.
| 编号No. | 类型Type | 化学成分Chemical composition | 部位Organ | 文献References |
|---|---|---|---|---|
| 1 | 双苄基异喹啉类 Bisbenzylisoquinolines | 莲心碱Liensinine | 莲子心、荷叶 | [ |
| 2 | 异莲心碱Isoliensinine | 莲子心、荷叶 | [ | |
| 3 | 甲基莲心碱Neferine | 莲子心、荷叶 | [ | |
| 4 | 阿朴啡类 Aporphines | 荷叶碱Nuciferine | 荷叶、莲子心 | [ |
| 5 | 莲碱Roemerine | 荷叶 | [ | |
| 6 | O-去甲基荷叶碱O-Nornuciferine | 荷叶 | [ | |
| 7 | N-去甲基荷叶碱N-Nornuciferine | 荷叶 | [ | |
| 8 | 番荔枝碱Anonaine | 荷叶 | [ | |
| 9 | 山矾碱Caaverine | 荷叶 | [ | |
| 10 | 巴婆碱Asimilobine | 荷叶、莲子心 | [ | |
| 11 | 北美鹅掌楸尼定碱Lirinidine | 荷叶 | [ | |
| 12 | 单苄基异喹啉 Monobenzylisoquinoline | 衡州乌药碱Coclaurine | 荷叶 | [ |
| 13 | O-去甲基衡州乌药碱O-Norcoclaurine | 荷叶、莲子心 | [ | |
| 14 | N-甲基衡州乌药碱N-Methylcoclaurine | 荷叶、莲子心 | [ | |
| 15 | N-甲基异衡州乌药碱N-Methylisococlaurine | 荷叶、莲子心 | [ | |
| 16 | 亚美(杏黄)罂粟碱Armepavine | 荷叶、莲子心 | [ | |
| 17 | N-去甲基亚美罂粟碱N-Norarmepavine | 荷叶 | [ | |
| 18 | 去氢阿朴啡类 Dehydroaporphine alkaloids | 去氢荷叶碱Dethydronuciferine | 荷叶 | [ |
| 19 | 去氢莲碱Dehydroroemerine | 荷叶 | [ | |
| 20 | 睡莲碱Nelumnucine | 荷叶 | [ | |
| 21 | 去氢番荔枝碱Dethydroanonaine | 荷叶 | [ | |
| 22 | 氧化阿朴啡类Oxoapor phinoid | 鹅掌楸碱Liriodenine | 荷叶 | [ |
| 23 | 观音莲明碱Lysicamine | 荷叶、莲子心 | [ | |
| 24 | 原阿朴啡类Proaporphine alkaloids | 原荷叶碱Pronuciferine | 荷叶、莲子心 | [ |
表1 荷叶、莲子心主要化学成分
Table 1 Main chemical constituents of lotus leaf and lotus plumule
| 编号No. | 类型Type | 化学成分Chemical composition | 部位Organ | 文献References |
|---|---|---|---|---|
| 1 | 双苄基异喹啉类 Bisbenzylisoquinolines | 莲心碱Liensinine | 莲子心、荷叶 | [ |
| 2 | 异莲心碱Isoliensinine | 莲子心、荷叶 | [ | |
| 3 | 甲基莲心碱Neferine | 莲子心、荷叶 | [ | |
| 4 | 阿朴啡类 Aporphines | 荷叶碱Nuciferine | 荷叶、莲子心 | [ |
| 5 | 莲碱Roemerine | 荷叶 | [ | |
| 6 | O-去甲基荷叶碱O-Nornuciferine | 荷叶 | [ | |
| 7 | N-去甲基荷叶碱N-Nornuciferine | 荷叶 | [ | |
| 8 | 番荔枝碱Anonaine | 荷叶 | [ | |
| 9 | 山矾碱Caaverine | 荷叶 | [ | |
| 10 | 巴婆碱Asimilobine | 荷叶、莲子心 | [ | |
| 11 | 北美鹅掌楸尼定碱Lirinidine | 荷叶 | [ | |
| 12 | 单苄基异喹啉 Monobenzylisoquinoline | 衡州乌药碱Coclaurine | 荷叶 | [ |
| 13 | O-去甲基衡州乌药碱O-Norcoclaurine | 荷叶、莲子心 | [ | |
| 14 | N-甲基衡州乌药碱N-Methylcoclaurine | 荷叶、莲子心 | [ | |
| 15 | N-甲基异衡州乌药碱N-Methylisococlaurine | 荷叶、莲子心 | [ | |
| 16 | 亚美(杏黄)罂粟碱Armepavine | 荷叶、莲子心 | [ | |
| 17 | N-去甲基亚美罂粟碱N-Norarmepavine | 荷叶 | [ | |
| 18 | 去氢阿朴啡类 Dehydroaporphine alkaloids | 去氢荷叶碱Dethydronuciferine | 荷叶 | [ |
| 19 | 去氢莲碱Dehydroroemerine | 荷叶 | [ | |
| 20 | 睡莲碱Nelumnucine | 荷叶 | [ | |
| 21 | 去氢番荔枝碱Dethydroanonaine | 荷叶 | [ | |
| 22 | 氧化阿朴啡类Oxoapor phinoid | 鹅掌楸碱Liriodenine | 荷叶 | [ |
| 23 | 观音莲明碱Lysicamine | 荷叶、莲子心 | [ | |
| 24 | 原阿朴啡类Proaporphine alkaloids | 原荷叶碱Pronuciferine | 荷叶、莲子心 | [ |

图2 苄基异喹啉类生物碱合成途径 红色表示关键中间体(S)-牛心果碱,实线表示合成途径已知,虚线和问号表示合成途径未知
Fig. 2 Synthesis pathways of benzylisoquinoline alkaloids The key intermediate(S)-carnitine is shown in red; solid lines indicate that the synthetic pathway is known; dashed lines and question marks indicate that the synthetic pathway is unknown

图3 荷叶阿朴啡类生物碱合成途径 红色表示可能的关键酶,实线表示合成途径已知,虚线和问号表示合成途径未知
Fig. 3 Synthesis pathways of apomorphine alkaloids in lotus leaf Red indicates possible key enzymes, solid lines indicate that the synthetic pathway is known, and dashed lines and question marks indicate that the synthetic pathway is unknown
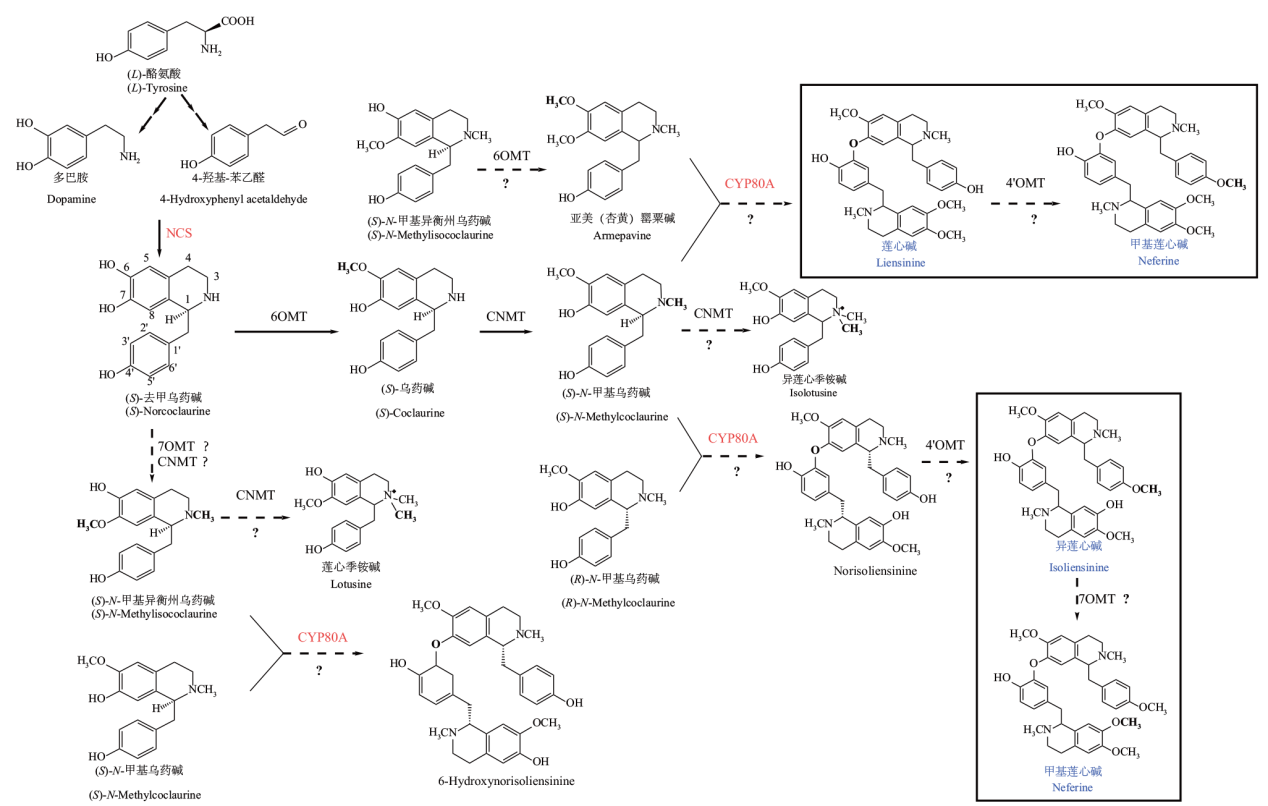
图4 莲子心双苄基异喹啉类生物碱合成途径 红色表示可能的关键酶,实线表示合成途径已知,虚线和问号表示合成途径未知
Fig. 4 Synthesis pathways of dibenzyl isoquinoline alkaloids in lotus plumule Red indicates possible key enzymes, solid lines indicate that the synthetic pathway is known, and dashed lines and question marks indicate that the synthetic pathway is unknown
| [1] |
Xue JH, Dong WP, Cheng T, et al. Nelumbonaceae: Systematic position and species diversification revealed by the complete chloroplast genome[J]. J Syst Evol, 2012, 50(6): 477-487.
doi: 10.1111/jse.2012.50.issue-6 URL |
| [2] | 刘晖晖, 陈世彬, 赵佳琛, 等. 经典名方中莲类药材的本草考证[J]. 中国实验方剂学杂志, 2022, 28(10): 42-54. |
| Liu HH, Chen SB, Zhao JC, et al. Textual research on nelumbinis in famous classical formulas[J]. Chin J Exp Tradit Med Formulae, 2022, 28(10): 42-54. | |
| [3] | 国家药典委员会. 中华人民共和国药典-一部: 2020年版[M]. 北京: 中国医药科技出版社, 2020: 285-287. |
| National Pharmacopoeia Commission.. People's republic of China(PRC)pharmacopoeia-part I: 2020 edition[M]. Beijing: China Pharmaceutical Science And Technology Press. 2020: 285-287. | |
| [4] | 单锋. 荷叶、莲子心药效分化的分子机理研究[D]. 成都: 成都中医药大学, 2015. |
| Shan F. Molecular mechanism study on efficacy difference of louts leaf and lotus plumule[D]. Chengdu: Chengdu University of TCM, 2015. | |
| [5] |
Wang MT, Shi JY, Wang L, et al. Inhibitory kinetics and mechanism of flavonoids from lotus(Nelumbo nucifera Gaertn.) leaf against pancreatic α-amylase[J]. Int J Biol Macromol, 2018, 120(Pt B): 2589-2596.
doi: 10.1016/j.ijbiomac.2018.09.035 URL |
| [6] | 高振华, 孙伶俐, 王豪, 等. 荷叶化学成分及其药理活性研究[J]. 广东化工, 2020, 47(5): 100-102. |
| Gao ZH, Sun LL, Wang H, et al. Studies on the chemical constituents and pharmacological activities of lotus leaves[J]. Guangdong Chem Ind, 2020, 47(5): 100-102. | |
| [7] | 施继尧, 王蒙, 吴弢. 莲子心的化学成分及生物活性研究进展[J]. 中医药导报, 2018, 24(21): 105-108. |
| Shi JY, Wang M, Wu T. Research progress of chemical constituents and biological activities of lanzixin(nelumbinis plumula)[J]. Guid J Tradit Chin Med Pharm, 2018, 24(21): 105-108. | |
| [8] |
赵秀玲, 党亚丽. 莲子心化学成分及其提取、药理作用的研究进展[J]. 食品科学, 2018, 39(23): 329-336.
doi: 10.7506/spkx1002-6630-201823047 |
|
Zhao XL, Dang YL. Recent advances in chemical components, extraction and pharmacological effects of nelumbinis plumula[J]. Food Sci, 2018, 39(23): 329-336.
doi: 10.1111/jfds.1974.39.issue-2 URL |
|
| [9] | 俞月, 路娟, 吕欣锴, 等. 荷叶碱药理作用及机制研究进展[J]. 中国现代中药, 2021, 23(1): 164-170. |
| Yu Y, Lu J, Lyu XK, et al. Progress in pharmacology research and mechanisms of nuciferine[J]. Mod Chin Med, 2021, 23(1): 164-170. | |
| [10] | 程禄萍, 赵玥, 周康钰, 等. 甲基莲心碱治疗心血管疾病的药理作用及其机制研究进展[J]. 现代药物与临床, 2021, 36(9): 1983-1987. |
| Cheng LP, Zhao Y, Zhou KY, et al. Rch esearprogress on pharmacological effects and mechanism of neferine in cardiovascular diseases[J]. Drugs & Clin, 2021, 36(9): 1983-1987. | |
| [11] | 商晶, 潘扬. 近年来异莲心碱化学和药理的研究进展[J]. 南京中医药大学学报, 2010, 26(3): 238-240. |
| Shang J, Pan Y. Research progress of isoliensinine in chemistry and pharmacology in recent years[J]. J Nanjing Univ Tradit Chin Med, 2010, 26(3): 238-240 | |
| [12] | 程婷婷, 原新博, 惠小涵, 等. 荷叶生物碱成分及其调脂机制研究进展[J]. 中草药, 2019, 50(8): 1998-2003. |
| Cheng TT, Yuan XB, Hui XH, et al. Research progress on chemical constituents and lipid-lowering mechanism of alkaloids in Nelumbinis Folium[J]. Chin Tradit Herb Drugs, 2019, 50(8): 1998-2003. | |
| [13] | 时伟朋, 徐怀双, 田文月, 等. 莲子心的生物碱及酸酯类成分研究[J]. 中药材, 2017, 40(10): 2347-2350. |
| Shi WP, Xu HS, Tian WY, et al. Study on alkaloids and esters of plumula nelumbinis[J]. J Chin Med Mater, 2017, 40(10): 2347-2350. | |
| [14] | 郑振佳, 王晓, 王明林, 等. 固相萃取-快速分离液相-四级杆串.联飞行时间质谱联用分析荷叶中的生物碱[J]. 中草药, 2011, 42(6): 1066-1068. |
| Zheng ZJ, Wang X, Wang ML, et al. Analysis of alkaloids in Nelumbo nucifera leaves by solid phase extraction-rapid resolution liquid chromatography-quadrupole-time of flight mass spectrometry[J]. Chin Tradit Herb Drugs, 2011, 42(6): 1066-1068. | |
| [15] | 陈曦, 戚进. 荷叶中黄酮和生物碱的研究进展[J]. 中国实验方剂学杂志, 2015, 21(18): 211-214. |
| Chen X, Qi J. Flavonoids and alkaloids in lotus leaves[J]. Chin J Exp Tradit Med Formulae, 2015, 21(18): 211-214. | |
| [16] | 姜涛, 刘子祯, 姚艺新. 莲子心化学成分测定方法研究进展[J]. 中成药, 2020, 42(2): 446-451. |
| Jiang T, Liu ZZ, Yao YX. Research progress on determination methods of chemical constituents in plumula Nelumbinis[J]. Chin Tradit Pat Med, 2020, 42(2): 446-451. | |
| [17] | 王玲玲, 刘斌, 石任兵. 荷叶的化学成分研究[J]. 天然产物研究与开发, 2009, 21(3): 416-419. |
| Wang LL, Liu B, Shi RB. Study on chemical constituents of folium nelumbinis[J]. Nat Prod Res Dev, 2009, 21(3): 416-419. | |
| [18] | 周永刚, 刘畅, 毛飞, 等. 荷叶化学成分的HPLC-TOF/MS分析[J]. 药学实践杂志, 2011, 29(5): 342-346. |
| Zhou YG, Liu C, Mao F, et al. Analysis of chemical constituents of Lotus leaf by HPLC-TOF/MS[J]. J Pharm Pract, 2011, 29(5): 342-346. | |
| [19] |
Liu CM, Kao CL, Wu HM, et al. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera gaertn. cv. Rosa-plena[J]. Molecules, 2014, 19(11): 17829-17838.
doi: 10.3390/molecules191117829 URL |
| [20] | 杨亚辉, 吴昊旻, 戚进. 荷叶化学成分研究[J]. 现代中药研究与实践, 2021, 35(5): 20-27. |
| Yang YH, Wu HM, Qi J. Study on chemical constituents of lotus leaf[J]. Res Pract Chin Med, 2021, 35(5): 20-27. | |
| [21] |
Sharma BR, Gautam LNS, Adhikari D, et al. A comprehensive review on chemical profiling of Nelumbo nucifera: potential for drug development[J]. Phytother Res, 2017, 31(1): 3-26.
doi: 10.1002/ptr.5732 pmid: 27667670 |
| [22] |
Shoji N, Umeyama A, Saito N, et al. Asimilobine and lirinidine, serotonergic receptor antagonists, from Nelumbo nucifera[J]. J Nat Prod, 1987, 50(4): 773-774.
pmid: 3430176 |
| [23] | 王婵, 杨颖博. 荷叶的化学成分与药理活性研究进展[J]. 现代中药研究与实践, 2020, 34(4): 74-81. |
| Wang C, Yang YB. Research progress on chemical constituents and pharmacological activities of nelumbinis folium[J]. Res Pract Chin Med, 2020, 34(4): 74-81. | |
| [24] | 杨勇, 李希珍, 张庆贺, 等. 莲子心化学成分及其抑制蛋白二硫键异构酶活性研究[J]. 中国中药杂志, 2017, 42(15): 3004-3010. |
| Yang Y, Li XZ, Zhang QH, et al. Studies on the chemical components of Nelumbinis Plumula and the inhibitory activity on protein disulfide isomerase[J]. China J Chin Mater Med, 2017, 42(15): 3004-3010. | |
| [25] | 肖幸华, 刘清茹, 刘曼华, 等. 莲子心水提取物正丁醇部位化学成分研究[J]. 中华中医药杂志, 2022, 37(1): 395-400. |
| Xiao XH, Liu QR, Liu MH, et al. Chemical constituents from the N-butanol faction of Nelumbinis Plumula aqueous extracts[J]. China J Tradit Chin Med Pharm, 2022, 37(1): 395-400. | |
| [26] |
Kunitomo J, Yoshikawa Y, Tanaka S, et al. Alkaloids of Nelumbo nucifera[J]. Phytochemistry, 1973, 12(3): 699-701.
doi: 10.1016/S0031-9422(00)84467-2 URL |
| [27] | 吴昊, 刘斌, 王伟, 等. 荷叶中的一个新阿朴啡型生物碱[J]. 中草药, 2010, 41(4): 514-516. |
| Wu H, Liu B, Wang W, et al. A new aporphine alkaloid in leaves of Nelumbo nucifera[J]. Chin Tradit Herb Drugs, 2010, 41(4): 514-516. | |
| [28] | 袁谱龙, 陈亮, 刘小宇, 等. 荷叶生物碱分离及相关活性研究[J]. 中成药, 2014, 36(11): 2330-2333. |
| Yuan PL, Chen L, Liu XY, et al. Alkaloids from lotus leaf and related bioactivities[J]. Chin Tradit Pat Med, 2014, 36(11): 2330-2333. | |
| [29] | 李萍, 杨光明, 张玉玲, 等. 莲子心脂溶性生物碱的分离、鉴定[J]. 食品与生物技术学报, 2016, 35(1): 19-27. |
| Li P, Yang GM, Zhang YL, et al. Isolation and identification of the lipophilic alkaloids of embryo loti[J]. J Food Sci Biotechnol, 2016, 35(1): 19-27. | |
| [30] |
Narcross L, Fossati E, Bourgeois L, et al. Microbial factories for the production of benzylisoquinoline alkaloids[J]. Trends Biotechnol, 2016, 34(3): 228-241.
doi: S0167-7799(15)00262-0 pmid: 26775900 |
| [31] | 陈应庄. 博落回属植物中苄基异喹啉类生物碱代谢组学研究[D]. 长沙: 湖南师范大学, 2008. |
| Chen YZ. Study on benzylisoquinoline alkaloids metabonomics in plant of Macleaya[D]. Changsha: Hunan Normal University, 2008. | |
| [32] |
Samanani N, Facchini PJ. The first committed enzyme in benzylisoquinoline alkaloid biosynthesis in plants Purification and characterization of norcoclaurine synthase[J]. J Biol Chem, 2002, 277(37): 33878-33883.
doi: 10.1074/jbc.M203051200 pmid: 12107162 |
| [33] |
Dastmalchi M, Park MR, Morris JS, et al. Family portraits: the enzymes behind benzylisoquinoline alkaloid diversity[J]. Phytochem Rev, 2018, 17(2): 249-277.
doi: 10.1007/s11101-017-9519-z URL |
| [34] | 张艳, 张剑, 葛海霞. 黄连中苄基异喹啉类生物碱的生源途径及其合成生物学的应用进展[J]. 药物生物技术, 2019, 26(2): 165-171. |
| Zhang Y, Zhang J, Ge HX. Progress in the biosynthetic pathway of benzylisoquinoline alkaloids in Coptis chinensis and the application of synthetic biology[J]. Pharm Biotechnol, 2019, 26(2): 165-171. | |
| [35] | 刘金凤, 黄鹏, 卿志星, 等. 苄基异喹啉类生物碱生物合成与代谢工程研究进展[J]. 基因组学与应用生物学, 2016, 35(8): 2194-2200. |
| Liu JF, Huang P, Qing ZX, et al. Advances in the biosynthesis and metabolic engineering research of benzylisoquinoline alkaloids[J]. Genom Appl Biol, 2016, 35(8): 2194-2200. | |
| [36] |
Farrow SC, Hagel JM, Beaudoin GAW, et al. Stereochemical inversion of(S)-reticuline by a cytochrome P450 fusion in opium poppy[J]. Nat Chem Biol, 2015, 11(9): 728-732.
doi: 10.1038/nchembio.1879 pmid: 26147354 |
| [37] |
Hagel JM, Facchini PJ. Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy[J]. Nat Chem Biol, 2010, 6(4): 273-275.
doi: 10.1038/nchembio.317 pmid: 20228795 |
| [38] | Ikezawa N, Iwasa K, Sato F. Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of(S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cellsJ Biol Chem, 2008, 283(14): 8810-8821. |
| [39] |
Vimolmangkang S, Deng XB, Owiti A, et al. Evolutionary origin of the NCSI gene subfamily encoding norcoclaurine synthase is associated with the biosynthesis of benzylisoquinoline alkaloids in plants[J]. Sci Rep, 2016, 6: 26323.
doi: 10.1038/srep26323 pmid: 27189519 |
| [40] |
Lee EJ, Facchini P. Norcoclaurine synthase is a member of the pathogenesis-related 10/Bet v1 protein family[J]. Plant Cell, 2010, 22(10): 3489-3503.
doi: 10.1105/tpc.110.077958 URL |
| [41] | 张翔, 马磊, 田永强, 等. 拟南芥基因组中注释为(S)-去甲乌药碱合成酶的分子克隆与异源表达(英文)[J]. 应用与环境生物学报, 2013, 19(1): 61-68. |
|
Zhang X, Ma L, et al. Molecular cloning and heterologous expression of putative(S)-norcoclaurine synthases from Arabidopsis thaliana[J]. Chin J Appl Environ Biol, 2013, 19(1): 61-68.
doi: 10.3724/SP.J.1145.2013.00061 URL |
|
| [42] | 赵力. 莲叶片生物碱合成基因的挖掘及功能鉴定[D]. 福州: 福建农林大学, 2019. |
| Zhao L. Mining and functional identification of alkaloid synthetic genes from lotus leaves[D]. Fuzhou: Fujian Agriculture and Forestry University, 2019. | |
| [43] | 朱玲平. 荷叶生物碱代谢路径关键基因的挖掘[D]. 武汉: 中国科学院研究生院(武汉植物园), 2015. |
| Zhu LP. Identification of key genes involved in metabolic pathway of lotus leaf alkaloids[D]. Wuhan: Wuhan Botanical Garden, Chinese Academy of Sciences, 2015. | |
| [44] | 李琦爽. 粉防己碱生物合成途径候选功能基因筛选及去甲乌药碱-6-O-甲基转移酶的功能研究[D]. 镇江: 江苏大学, 2020. |
| Li QS. Screening of candidate functional genes for tetrandrine biosynthesis pathway and functional study of norbrucine-6-O- methyltransferase[D]. Zhenjiang: Jiangsu University, 2020. | |
| [45] |
Liscombe DK, Facchini PJ. Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy[J]. J Biol Chem, 2007, 282(20): 14741-14751.
doi: 10.1074/jbc.M611908200 pmid: 17389594 |
| [46] |
Deng XB, Zhao L, Fang T, et al. Investigation of benzylisoquinoline alkaloid biosynthetic pathway and its transcriptional regulation in lotus[J]. Hortic Res, 2018, 5: 29.
doi: 10.1038/s41438-018-0035-0 |
| [47] |
Yang M, Zhu LP, Li L, et al. Digital gene expression analysis provides insight into the transcript profile of the genes involved in aporphine alkaloid biosynthesis in lotus(Nelumbo nucifera)[J]. Front Plant Sci, 2017, 8: 80.
doi: 10.3389/fpls.2017.00080 pmid: 28197160 |
| [48] |
Meelaph T, Kobtrakul K, et al. Coregulation of biosynthetic genes and transcription factors for aporphine-type alkaloid production in wounded lotus provides insight into the biosynthetic pathway of nuciferine[J]. ACS Omega, 2018, 3(8): 8794-8802.
doi: 10.1021/acsomega.8b00827 pmid: 31459012 |
| [49] | 周嘉裕, 廖海. 黄连小檗碱生物合成相关酶类的研究进展[J]. 时珍国医国药, 2005, 16(11): 1083-1084, 1087. |
| Zhou JY, Liao H. Progress in the study of berberine biosynthetic enzymes of Coptis[J]. Lishizhen Med Mater Med Res, 2005, 16(11): 1083-1084, 1087. | |
| [50] |
Nelson DR, Schuler MA. Cytochrome P450 genes from the sacred lotus genome[J]. Tropical Plant Biol, 2013, 6(2): 138-151.
doi: 10.1007/s12042-013-9119-z URL |
| [51] | 黄秀琼, 卿志星, 曾建国. 莲不同部位化学成分及药理作用研究进展[J]. 中草药, 2019, 50(24): 6162-6180. |
| Huang XQ, Qing ZX, Zeng JG. Research advances on chemical constituents and pharmacological effects of various parts of Nelumbo nucifera[J]. Chin Tradit Herb Drugs, 2019, 50(24): 6162-6180. | |
| [52] |
Menéndez-Perdomo IM, Facchini PJ. Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus(Nelumbo nucifera)[J]. J Biol Chem, 2020, 295(6): 1598-1612.
doi: 10.1074/jbc.RA119.011547 pmid: 31914404 |
| [53] | Yu YT, Liu Y, Dong GQ, et al. Functional characterization and key residues engineering of a regiopromiscuity O-methyltransferase involved in benzylisoquinoline alkaloid biosynthesis in Nelumbo nucifera[J]. Hortic Res, 2023, 10(2): uhac276. |
| [54] | 杨诗怡, 周小利, 陈志芸, 等. 植物基因功能验证技术概述[J]. 安徽农业科学, 2017, 45(34): 136-140. |
| Yang SY, Zhou XL, Chen ZY, et al. Technical overview of the validation of gene function in plants[J]. J Anhui Agric Sci, 2017, 45(34): 136-140. | |
| [55] |
Deng XB, Zhu LP, Fang T, et al. Analysis of isoquinoline alkaloid composition and wound-induced variation in Nelumbo using HPLC-MS/MS[J]. J Agric Food Chem, 2016, 64(5): 1130-1136.
doi: 10.1021/acs.jafc.5b06099 URL |
| [56] |
Li J, Xiong YC, Li Y, et al. Comprehensive analysis and functional studies of WRKY transcription factors in Nelumbo nucifera[J]. Int J Mol Sci, 2019, 20(20): 5006.
doi: 10.3390/ijms20205006 URL |
| [1] | 穆德添, 万凌云, 章瑶, 韦树根, 陆英, 付金娥, 田艺, 潘丽梅, 唐其. 钩藤管家基因筛选及生物碱合成相关基因的表达分析[J]. 生物技术通报, 2023, 39(2): 126-138. |
| [2] | 赵玉雪, 王芸, 余璐瑶, 刘京晶, 斯金平, 张新凤, 张磊. 植物中C-糖基转移酶的结构与应用[J]. 生物技术通报, 2022, 38(10): 18-28. |
| [3] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [4] | 汪金秀, 张琪, 丁伟, 陈拓. 核糖体肽生物合成中的典型翻译后修饰研究[J]. 生物技术通报, 2020, 36(10): 215-225. |
| [5] | 严武平, 吴友根, 于靖, 杨东梅, 张军锋. 药用植物microRNA研究现状与展望[J]. 生物技术通报, 2019, 35(8): 178-185. |
| [6] | 张礼, 孙堆, 王晓, 郑春丽. 半胱氨酸参与生物体重金属抗性的研究进展[J]. 生物技术通报, 2017, 33(5): 26-33. |
| [7] | 许蕙金兰,王翠翠,傅达奇. 甾族糖苷生物碱研究进展[J]. 生物技术通报, 2015, 31(10): 24-30. |
| [8] | 汪华;崔志峰;. 莽草酸生物合成途径的调控[J]. , 2009, 0(03): 50-53. |
| [9] | 刘蓉蓉;. 植物次生代谢途径的遗传修饰研究进展[J]. , 2008, 0(06): 10-13. |
| [10] | 马靓;丁鹏;杨广笑;何光源;. 植物类萜生物合成途径及关键酶的研究进展[J]. , 2006, 0(S1): 22-30. |
| [11] | O.J.M.Goddijn. 植物代谢工程生物反应器[J]. , 1996, 0(05): 5-10. |
| [12] | . 抗生素和干扰素[J]. , 1996, 0(05): 64-66. |
| [13] | . 食品上的应用[J]. , 1995, 0(04): 98-107. |
| [14] | . 抗生素和干扰素[J]. , 1993, 0(08): 35-35. |
| [15] | . 农业其它[J]. , 1993, 0(08): 72-74. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||