








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 33-41.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0536
• 薯类作物生物技术专题(专题主编:徐建飞,尚轶) • 上一篇 下一篇
收稿日期:2024-06-07
出版日期:2024-09-26
发布日期:2024-10-12
通讯作者:
冯瑞云,男,博士,研究员,研究方向:农作物遗传改良;E-mail: fengruiyun1970@163.com作者简介:宋倩娜,女,博士,助理研究员,研究方向:植物基因组编辑;E-mail: songqianna1007@126.com
基金资助:
SONG Qian-na1,2( ), DUAN Yong-hong1, FENG Rui-yun1,2(
), DUAN Yong-hong1, FENG Rui-yun1,2( )
)
Received:2024-06-07
Published:2024-09-26
Online:2024-10-12
摘要:
【目的】基因编辑技术的发展使得马铃薯实现精准分子育种成为了可能,试管薯作为遗传转化的理想材料,但其诱导和转化具有基因型依赖性,建立高效且普遍适用的试管薯基因编辑技术体系可为其精准分子育种提供技术支撑。【方法】以四倍体栽培马铃薯品种青薯9号和并薯6号为材料,对试管薯诱导及其遗传转化体系进行摸索;同时,转化CRISPR/Cas9基因编辑载体进行基因组编辑。另外,利用筛选的条件对其他3个马铃薯品种进行测试。【结果】全黑暗条件下,采用2叶/段的扩繁方式,在含有10%蔗糖和5 mg/L激动素的培养基上,5个马铃薯品种均可成功诱导出试管薯,但诱导效果存在差异。青薯9号的最佳分化激素配方为0.5 mg/L 6-苄氨基腺嘌呤、0.2 mg/L 吲哚-3-乙酸、0.2 mg/L赤霉素、2 mg/L玉米素,再生效率、转化频率和基因编辑效率分别为41.5%、51.9%和82.1%。利用上述筛选的激素配方,在其他4个四倍体马铃薯品种中均可高效地实现试管薯的遗传转化再生,其中并薯6号、Desiree和晋薯16号的编辑效率分别为63.2%、33.3%和10%。【结论】建立了5个不同基因型四倍体马铃薯高效的试管薯遗传转化再生体系,其中的4份材料成功地实现了基因组编辑。
宋倩娜, 段永红, 冯瑞云. CRISPR/Cas9介导的高效四倍体马铃薯试管薯基因编辑体系的建立[J]. 生物技术通报, 2024, 40(9): 33-41.
SONG Qian-na, DUAN Yong-hong, FENG Rui-yun. Establishment of CRISPR/Cas9-mediated Highly Efficient Gene Editing System in Microtubers of Potatoes[J]. Biotechnology Bulletin, 2024, 40(9): 33-41.
| 蔗糖浓度 Sucrose concentration/% | 并薯6号 Bingshu No. 6 | 青薯9号 Qingshu No.9 | ||||
|---|---|---|---|---|---|---|
| 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | |||
| 6 | 0.17±0.40b | 0.02±0.04c | 0.20±0.45c | 0.02±0.03b | ||
| 8 | 2.83±1.17a | 0.29±0.19ab | 1.80±0.84ab | 0.19±0.06a | ||
| 10 | 3.50±1.64a | 0.48±0.20a | 2.60±1.34a | 0.28±0.13a | ||
| 12 | 0.67±0.82b | 0.09±0.10bc | 0.80±0.84bc | 0.05±0.05b | ||
| 15 | 0.67±1.03b | 0.06±0.11c | 0.40±0.55c | 0.02±0.03b | ||
表1 不同蔗糖浓度处理下2个马铃薯品种试管薯的诱导
Table 1 Micro tuberization under different sucrose concentrations for two potato varieties
| 蔗糖浓度 Sucrose concentration/% | 并薯6号 Bingshu No. 6 | 青薯9号 Qingshu No.9 | ||||
|---|---|---|---|---|---|---|
| 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | |||
| 6 | 0.17±0.40b | 0.02±0.04c | 0.20±0.45c | 0.02±0.03b | ||
| 8 | 2.83±1.17a | 0.29±0.19ab | 1.80±0.84ab | 0.19±0.06a | ||
| 10 | 3.50±1.64a | 0.48±0.20a | 2.60±1.34a | 0.28±0.13a | ||
| 12 | 0.67±0.82b | 0.09±0.10bc | 0.80±0.84bc | 0.05±0.05b | ||
| 15 | 0.67±1.03b | 0.06±0.11c | 0.40±0.55c | 0.02±0.03b | ||
| 不同激素配比 Different hormone combination | 并薯6号 Bingshu No. 6 | 青薯9号 Qingshu No.9 | ||||
|---|---|---|---|---|---|---|
| 单瓶平均薯数/粒Number of microtubers per bottle | 单瓶平均薯重Weight of microtubers per bottle/g | 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | |||
| 0 | 3.50±1.64a | 0.48±0.20a | 2.60±1.34a | 0.28±0.13a | ||
| 4 mg/L 6-BA | 0.67±0.58b | 0.04±0.03b | 0.33±0.58b | 0.02±0.03b | ||
| 4 mg/L 6-BA + 3.5 mg/L NAA | 0.67±0.58b | 0.03±0.03b | 0.00±0.00b | 0.00±0.00b | ||
| 5 mg/L KT | 4.67±0.58a | 0.62±0.07a | 3.30±0.82a | 0.37±0.15a | ||
表2 不同激素配比处理下2个马铃薯品种试管薯的诱导
Table 2 Micro tuberization under different hormone combinations for two potato varieties
| 不同激素配比 Different hormone combination | 并薯6号 Bingshu No. 6 | 青薯9号 Qingshu No.9 | ||||
|---|---|---|---|---|---|---|
| 单瓶平均薯数/粒Number of microtubers per bottle | 单瓶平均薯重Weight of microtubers per bottle/g | 单瓶平均薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | |||
| 0 | 3.50±1.64a | 0.48±0.20a | 2.60±1.34a | 0.28±0.13a | ||
| 4 mg/L 6-BA | 0.67±0.58b | 0.04±0.03b | 0.33±0.58b | 0.02±0.03b | ||
| 4 mg/L 6-BA + 3.5 mg/L NAA | 0.67±0.58b | 0.03±0.03b | 0.00±0.00b | 0.00±0.00b | ||
| 5 mg/L KT | 4.67±0.58a | 0.62±0.07a | 3.30±0.82a | 0.37±0.15a | ||
| 品种 Variety | 处理 Treatment | 单瓶平均结薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | 大薯重Weight of the large tuber/g | 大薯直径 Diameter of the large tuber/cm |
|---|---|---|---|---|---|
| 并薯6号 | 顶部1个节段 | 2.00±2.00a | 0.13±0.15a | 0.08±0.08ab | 0.43±0.38a |
| 底部1个节段 | 0.67±0.58a | 0.04±0.04a | 0.04±0.04b | 0.38±0.34a | |
| 顶部2个节段 | 3.33±0.58a | 0.23±0.05a | 0.09±0.05ab | 0.65±0.09a | |
| 底部2个节段 | 2.67±1.53a | 0.45±0.37a | 0.22±0.13a | 0.80±0.18a | |
| 青薯9号 | 顶部1个节段 | 1.33±2.16a | 0.10±0.20b | 0.02±0.04b | 0.18±0.29b |
| 底部1个节段 | 2.83±1.72a | 0.20±0.17ab | 0.10±0.07ab | 0.44±0.06ab | |
| 顶部2个节段 | 3.17±1.60a | 0.21±0.13ab | 0.10±0.04ab | 0.55±0.07a | |
| 底部2个节段 | 3.33±0.82a | 0.37±0.15a | 0.15±0.05a | 0.59±0.06a |
表3 2个马铃薯品种不同部位茎段长度处理下试管薯的诱导
Table 3 Induction of tuberization under different segment lengths for two potato varieties
| 品种 Variety | 处理 Treatment | 单瓶平均结薯数Number of microtubers per bottle/粒 | 单瓶平均薯重Weight of microtubers per bottle/g | 大薯重Weight of the large tuber/g | 大薯直径 Diameter of the large tuber/cm |
|---|---|---|---|---|---|
| 并薯6号 | 顶部1个节段 | 2.00±2.00a | 0.13±0.15a | 0.08±0.08ab | 0.43±0.38a |
| 底部1个节段 | 0.67±0.58a | 0.04±0.04a | 0.04±0.04b | 0.38±0.34a | |
| 顶部2个节段 | 3.33±0.58a | 0.23±0.05a | 0.09±0.05ab | 0.65±0.09a | |
| 底部2个节段 | 2.67±1.53a | 0.45±0.37a | 0.22±0.13a | 0.80±0.18a | |
| 青薯9号 | 顶部1个节段 | 1.33±2.16a | 0.10±0.20b | 0.02±0.04b | 0.18±0.29b |
| 底部1个节段 | 2.83±1.72a | 0.20±0.17ab | 0.10±0.07ab | 0.44±0.06ab | |
| 顶部2个节段 | 3.17±1.60a | 0.21±0.13ab | 0.10±0.04ab | 0.55±0.07a | |
| 底部2个节段 | 3.33±0.82a | 0.37±0.15a | 0.15±0.05a | 0.59±0.06a |
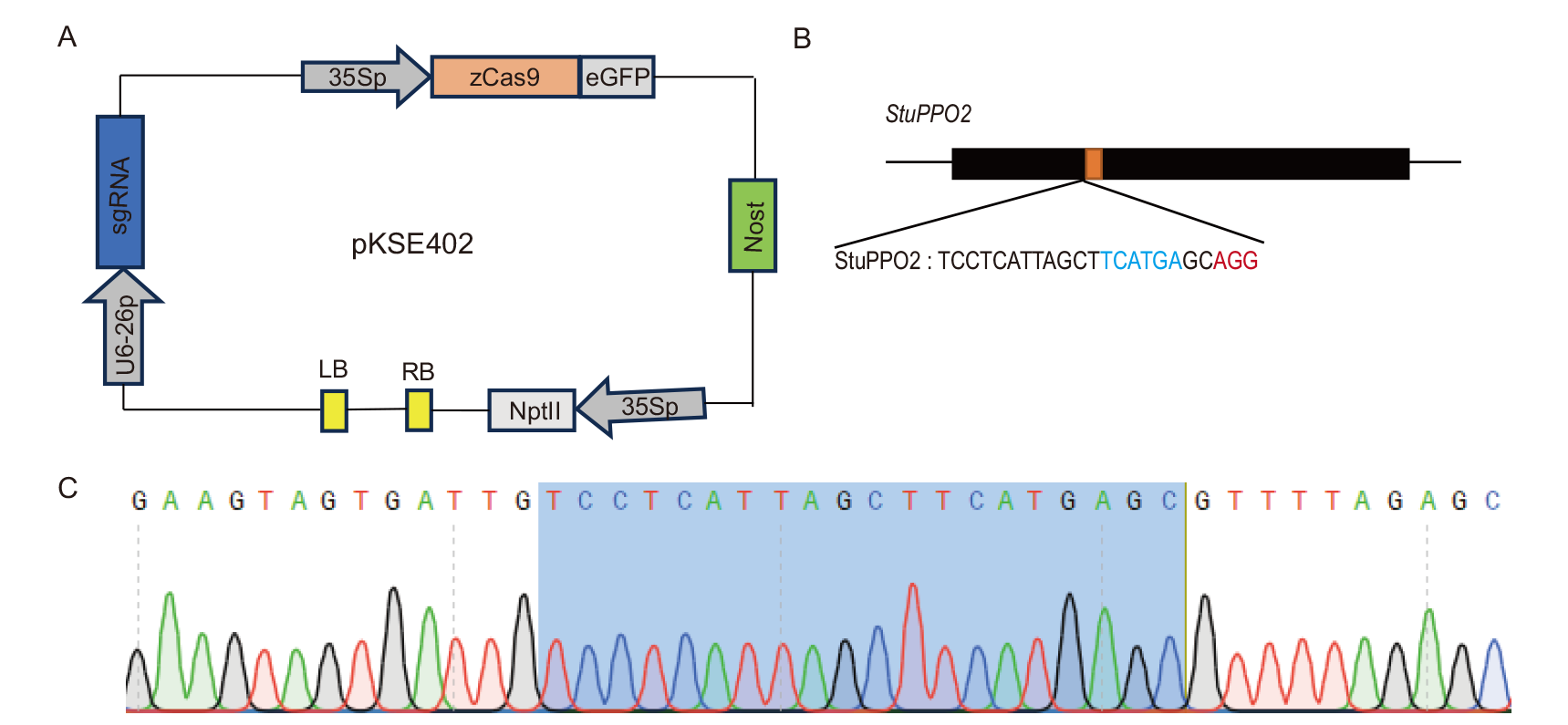
图1 CRISPR/Cas9敲除载体的构建 A:pKSE402载体示意图;B:位于StuPPO2基因上的靶位点。红色表示PAM序列;蓝色表示限制性酶切位点;C:靶位点序列测序验证
Fig. 1 Construction of CRISPR/Cas9 deletion vector A: Schematic diagram of pKSE402 vector. B: The target site in gene StuPPO2. Red is PAM sequence; blue is restriction enzyme site. C: Correct target sequence is confirmed by sequencing
| 品种Variety | 培养基类型Media types | 接种薯片数Microtubers/个 | 芽分化薯片数Differentiation microtubers/个 | 分化效率Differentiation efficiency/% |
|---|---|---|---|---|
| 青薯9号 | K1 | 106 | 8 | 7.5 |
| K2 | 94 | 39 | 41.5 | |
| 并薯6号 | K1 | 58 | 26 | 44.8 |
| K2 | 63 | 22 | 34.9 |
表4 2个马铃薯品种试管薯薄片的诱导分化再生
Table 4 Induction and differentiation of microtuber for two potato varieties
| 品种Variety | 培养基类型Media types | 接种薯片数Microtubers/个 | 芽分化薯片数Differentiation microtubers/个 | 分化效率Differentiation efficiency/% |
|---|---|---|---|---|
| 青薯9号 | K1 | 106 | 8 | 7.5 |
| K2 | 94 | 39 | 41.5 | |
| 并薯6号 | K1 | 58 | 26 | 44.8 |
| K2 | 63 | 22 | 34.9 |
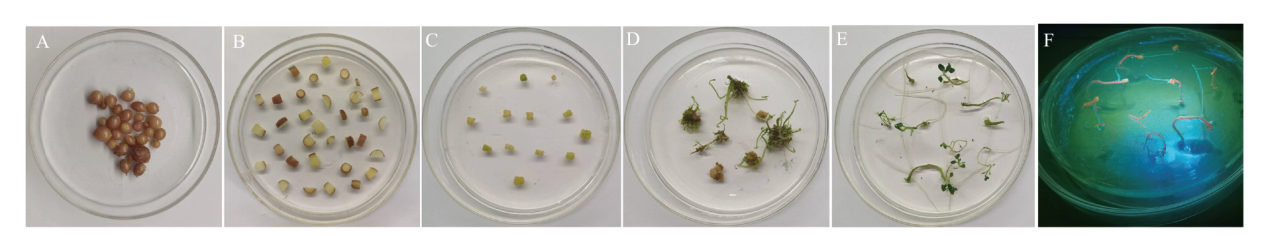
图2 农杆菌介导的马铃薯试管薯薄片遗传转化体系 A:试管薯的诱导;B:试管薯薄片的浸染;C:试管薯薄片的诱导;D:芽的分化:E:再生植物的生根;F:GFP荧光筛选阳性再生植株
Fig. 2 Agrobacterium-mediated potato microtuber genetic transformation system A: Induction of microtuber. B: Infection of microtuber thin slices. C: Induction of microtuber thin slices. D: Differentiation of buds. E: Rooting of regenerated plants. F: Positive regenerated plants are selected by GFP
| 品种 Variety | 再生植株 Regenarated plants/株 | 荧光植株 Fluorescent plants/株 | 转化效率 Transformation frequency/% | 突变植株数 Mutant plants/株 | 突变效率 Mutation frequency/% |
|---|---|---|---|---|---|
| 青薯9号 | 54 | 28 | 51.9 | 23 | 82.1 |
| 并薯6号 | 46 | 19 | 41.3 | 12 | 63.2 |
表5 荧光再生植株中StuPPO2基因编辑的鉴定与分析
Table 5 Identification and analysis of StuPPO2 gene editing for fluorescent regenerated plants
| 品种 Variety | 再生植株 Regenarated plants/株 | 荧光植株 Fluorescent plants/株 | 转化效率 Transformation frequency/% | 突变植株数 Mutant plants/株 | 突变效率 Mutation frequency/% |
|---|---|---|---|---|---|
| 青薯9号 | 54 | 28 | 51.9 | 23 | 82.1 |
| 并薯6号 | 46 | 19 | 41.3 | 12 | 63.2 |
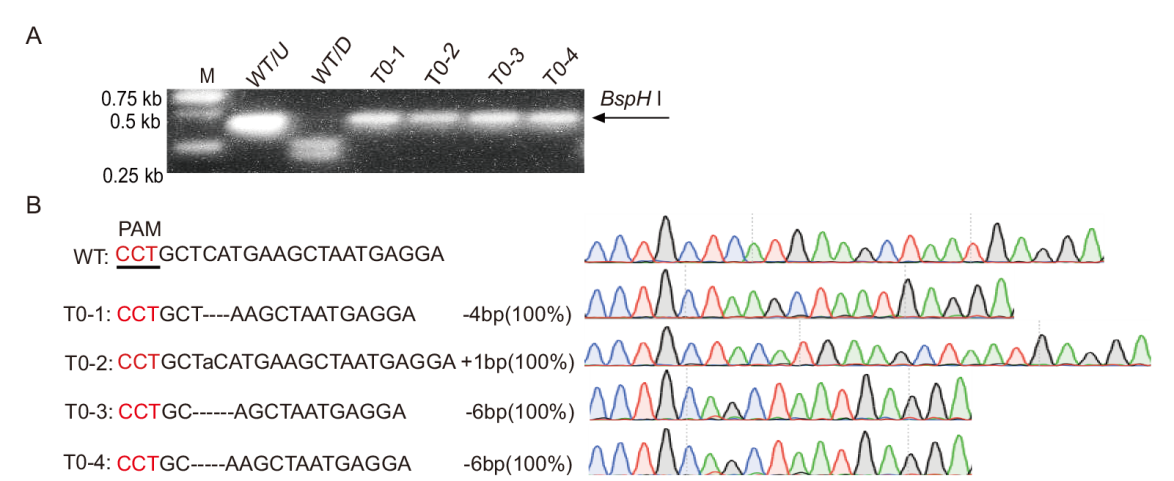
图3 StuPPO2基因敲除植株的检测 A:PCR/RE检测突变体。WT/D表示野生型被酶切;WT/U表示野生型未被酶切;T0-1-T0-4表示突变体植株;B:stuppo2突变体的突变类型分析。红色序列表示PAM序列
Fig. 3 Detection of StuPPO2 knockout plants A: PCR/RE assay for mutants. WT/D indicates that wild is cut by the enzyme; WT/U indicates that wild can not be cut by the enzyme; T0-1-T0-4 are mutant plants. B: Mutation type analysis of stuppo2 mutants. The PAM sequence is highlighted in red
| 品种 Variety | 结薯效率 Potato yield/% | 最适再生培养基 Optimal medium | 芽分化效率 Differentiation efficiency/% | 遗传转化频率 Transformation frequency/% | 基因编辑效率 Mutation frequency/% |
|---|---|---|---|---|---|
| Desiree | (48/48)100 | K1 | (27/104)25.9 | (9/28)32.1 | (3/9)33.3 |
| 晋薯16号 | (15/48)31.3 | K2 | (30/51)58.8 | (10/25)40.0 | (1/10)10 |
| 并薯26号 | (31/48)64.6 | K2 | (28/154)18.2 | (7/28)25.0 | (0/7)0 |
表6 其他3个四倍体马铃薯品种基因编辑体系的测试
Table 6 Testing of gene editing system for another three tetraploid potato varieties
| 品种 Variety | 结薯效率 Potato yield/% | 最适再生培养基 Optimal medium | 芽分化效率 Differentiation efficiency/% | 遗传转化频率 Transformation frequency/% | 基因编辑效率 Mutation frequency/% |
|---|---|---|---|---|---|
| Desiree | (48/48)100 | K1 | (27/104)25.9 | (9/28)32.1 | (3/9)33.3 |
| 晋薯16号 | (15/48)31.3 | K2 | (30/51)58.8 | (10/25)40.0 | (1/10)10 |
| 并薯26号 | (31/48)64.6 | K2 | (28/154)18.2 | (7/28)25.0 | (0/7)0 |
| [1] | Zaheer K, Akhtar MH. Potato production, usage, and nutrition—a review[J]. Crit Rev Food Sci Nutr, 2016, 56(5): 711-721. |
| [2] | Scott G, Suárez V. The rise of Asia as the centre of global potato production and some implications for industry[J]. Potato J, 2012 |
| [3] | Stemerding D, Beumer K, Edelenbosch R, et al. Responsible innovation in plant breeding: the case of hybrid potato breeding[J]. Plants, 2023, 12(9): 1751. |
| [4] | Halterman D, Guenthner J, Collinge S, et al. Biotech potatoes in the 21st century: 20 years since the first biotech potato[J]. Am J Potato Res, 2016, 93(1): 1-20. |
| [5] |
Wang HF, La Russa M, Qi LS. CRISPR/Cas9 in genome editing and beyond[J]. Annu Rev Biochem, 2016, 85: 227-264.
doi: 10.1146/annurev-biochem-060815-014607 pmid: 27145843 |
| [6] | Consortium PGS, Xu X, Pan SK, et al. Genome sequence and analysis of the tuber crop potato[J]. Nature, 2011, 475(7355): 189-195. |
| [7] | Butler NM, Atkins PA, Voytas DF, et al. Generation and inheritance of targeted mutations in potato(Solanum tuberosum L.) using the CRISPR/cas system[J]. PLoS One, 2015, 10(12): e0144591. |
| [8] |
Kieu NP, Lenman M, Wang ES, et al. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes[J]. Sci Rep, 2021, 11(1): 4487.
doi: 10.1038/s41598-021-83972-w pmid: 33627728 |
| [9] |
Ye MW, Peng Z, Tang D, et al. Generation of self-compatible diploid potato by knockout of S-RNase[J]. Nat Plants, 2018, 4(9): 651-654.
doi: 10.1038/s41477-018-0218-6 pmid: 30104651 |
| [10] |
Zhao X, Jayarathna S, Turesson H, et al. Amylose starch with no detectable branching developed through DNA-free CRISPR-Cas9 mediated mutagenesis of two starch branching enzymes in potato[J]. Sci Rep, 2021, 11(1): 4311.
doi: 10.1038/s41598-021-83462-z pmid: 33619312 |
| [11] | Zheng ZZ, Ye GJ, Zhou Y, et al. Editing sterol side chain reductase 2 gene(StSSR2)via CRISPR/Cas9 reduces the total steroidal glycoalkaloids in potato[J]. Life, 2021, 14(1): 401-413. |
| [12] |
Sheerman S, Benan MW. A rapid transformation method for Solanum tuberosum using binary Agrobacterium tumefaciens vectors[J]. Plant Cell Rep, 1988, 7: 13-16.
doi: 10.1007/BF00272967 pmid: 24241405 |
| [13] |
Gopal J, Minocha JL, Dhaliwal HS. Microtuberization in potato(Solanum tuberosum L.)[J]. Plant Cell Rep, 1998, 17(10): 794-798.
doi: 10.1007/s002990050485 pmid: 30736594 |
| [14] | Heeres P, Schippers-Rozenboom M, Jacobsen E, et al. Transformation of a large number of potato varieties: genotype-dependent variation in efficiency and somaclonal variability[J]. Euphytica, 2002, 124(1): 13-22. |
| [15] | Kamrani M, Ebadi A, Shiri M. Effect of explant, genotype and plant growth regulators on regeneration and Agrobacterium-mediated transformation of potato[J]. J Agronomy, 2015, 14(4): 227-233. |
| [16] | Banerjee AK, Prat S, Hannapel DJ. Efficient production of transgenic potato(S. tuberosum L. ssp. andigena)plants via Agrobacterium tumefaciens-mediated transformation[J]. Plant Sci, 2006, 170(4): 732-738. |
| [17] | Han EH, Goo YM, Lee MK, et al. An efficient transformation method for a potato(Solanum tuberosum L. var. Atlantic)[J]. J Plant Biotechnol, 2015, 42(2): 77-82. |
| [18] | Cui D, Huo SS, Wang X, et al. Establishment of canine macrophages stably expressing GFP-tagged canine LC3 protein for effectively detecting autophagy[J]. Mol Cell Probes, 2020, 49: 101493. |
| [19] | 陈广侠, 马伟清, 王培伦, 等. 马铃薯试管苗茎段长度对试管薯诱导的影响[J]. 山东农业科学, 2013, 45(12): 40-42. |
| Chen GX, Ma WQ, Wang PL, et al. Effect of test-tube plantlet segment length on microtuber induction[J]. Shandong Agric Sci, 2013, 45(12): 40-42. | |
| [20] | Hossain MA. Standardization of sucrose and 6-benzyl aminopurine for in vitro micro tuberization of potato[J]. Am J Agric For, 2015, 3(2): 25. |
| [21] | 司怀军, 谢从华, 柳俊. 农杆菌介导的马铃薯试管薯遗传转化体系的优化及反义class I patatin基因的导入[J]. 作物学报, 2003, 29(6): 801-805. |
| Si HJ, Xie CH, Liu J. An efficient protocol for Agrobacterium-mediated transformation with microtuber and the introduction of an antisense class I patatin gene into potato[J]. Acta Agron Sin, 2003, 29(6): 801-805. | |
| [22] | 齐恩芳, 贾小霞, 刘石, 等. 多抗转基因马铃薯植株的获得及农杆菌介导试管薯遗传转化体系优化[J]. 甘肃农业科技, 2020(11): 1-6. |
| Qi EF, Jia XX, Liu S, et al. Obtaining of multiple resistance transgenic potato plant and optimization of Agrobacterium-mediated genetic transformation system of potato in vitro[J]. Gansu Agric Sci Technol, 2020(11): 1-6. | |
| [23] | 张薇. 马铃薯高效再生及遗传转化体系的优化[J]. 安徽农业科学, 2024, 52(3): 35-39. |
| Zhang W. Efficient regeneration of potato and optimization of genetic transformation system[J]. J Anhui Agric Sci, 2024, 52(3): 35-39. | |
| [24] | Hossain MS, Mofazzal Hossain M, Hossain T, et al. Varietal performance of potato on induction and development of microtuber in response to sucrose[J]. Ann Agric Sci, 2017, 62(1): 75-81. |
| [25] | Zakaria M, Hossain MM, Mian MK, et al. Performance of different protocols on in vitro tuberization in potato(Solanum tuberosum)[J]. Bangladesh J Agric Res, 2014, 39(1): 59-66. |
| [26] | 颉瑞霞, 张小川, 张国辉, 等. 激素配比对马铃薯试管薯诱导和块茎形成的影响[J]. 分子植物育种, 2018, 16(13): 4355-4362. |
| Xie RX, Zhang XC, Zhang GH, et al. Effect of hormone combination on induction and Tuber formation of potato microtuber[J]. Mol Plant Breed, 2018, 16(13): 4355-4362. | |
| [27] | Gautam S, Solis-Gracia N, Teale MK, et al. Development of an in vitro microtuberization and temporary immersion bioreactor system to evaluate heat stress tolerance in potatoes(Solanum tuberosum L.)[J]. Front Plant Sci, 2021, 12: 700328. |
| [28] | 赵佐敏. 马铃薯组培中不同因素对诱导试管薯的影响[J]. 中国马铃薯, 2005, 19(5): 278-280. |
| Zhao ZM. Impact of various factors on the induction of microtubers[J]. Chin Potato J, 2005, 19(5): 278-280. | |
| [29] | Aslam A, Iqbal J. Combined effect of cytokinin and sucrose on in vitro tuberization parameters of two cultivars i.e., diamant and red norland of potato(Solanum tuberosum)[J]. Pak J Bot, 2010, 42: 1093-1102. |
| [30] | 王清, 李静文, 戴朝曦, 等. 纯合四倍体马铃薯遗传转化体系优化及转基因块茎的褐化鉴定[J]. 分子植物育种, 2006, 4(4): 553-558. |
| Wang Q, Li JW, Dai CX, et al. Optimization transformation system of homozygous tetroploid potato and identification browning degree of transgenic Tuber[J]. Mol Plant Breed, 2006, 4(4): 553-558. | |
| [31] | 王丽, 杨宏羽, 张俊莲, 等. 根癌农杆菌介导马铃薯试管薯转化体系的优化及AtNHX1基因的导入[J]. 西北植物学报, 2008, 28(6): 1088-1094. |
| Wang L, Yang HY, Zhang JL, et al. Optimization of transformation conditions of potato by Agrobacterium tumefaciens and introduction of AtNHX1 gene[J]. Acta Bot Boreali Occidentalia Sin, 2008, 28(6): 1088-1094. | |
| [32] | 宋倩娜, 梅超, 霍利光, 等. 马铃薯品种‘并薯6号’遗传转化体系的建立[J]. 中国马铃薯, 2021, 35(5): 385-396. |
| Song QN, Mei C, Huo LG, et al. Establishment of genetic transformation system for potato variety ‘bingshu 6’[J]. Chin Potato J, 2021, 35(5): 385-396. | |
| [33] |
杜静雅, 陈凯园, 普金, 等. 高效GFPuv荧光筛选基因编辑载体的改造及其在马铃薯遗传转化中的应用[J]. 中国农业科学, 2023, 56(11): 2223-2236.
doi: 10.3864/j.issn.0578-1752.2023.11.015 |
|
Du JY, Chen KY, Pu J, et al. The modification of gene editing vector for efficient GFPuv fluorescence screening and its application in potato genetic transformation[J]. Sci Agric Sin, 2023, 56(11): 2223-2236.
doi: 10.3864/j.issn.0578-1752.2023.11.015 |
|
| [34] | Rao YC, Yang X, Pan CY, et al. Advance of clustered regularly interspaced short palindromic repeats-Cas9 system and its application in crop improvement[J]. Front Plant Sci, 2022, 13: 839001. |
| [35] |
Chincinska IA, Miklaszewska M, Sołtys-Kalina D. Recent advances and challenges in potato improvement using CRISPR/Cas genome editing[J]. Planta, 2022, 257(1): 25.
doi: 10.1007/s00425-022-04054-3 pmid: 36562862 |
| [36] |
叶明旺, 张春芝, 黄三文. 二倍体栽培马铃薯高效遗传转化体系的建立[J]. 中国农业科学, 2018, 51(17): 3249-3257.
doi: 10.3864/j.issn.0578-1752.2018.17.002 |
|
Ye MW, Zhang CZ, Huang SW. Construction of high efficient genetic transformation system for diploid potatoes[J]. Sci Agric Sin, 2018, 51(17): 3249-3257.
doi: 10.3864/j.issn.0578-1752.2018.17.002 |
| [1] | 申鹏, 高雅彬, 丁红. 马铃薯SAT基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(9): 64-73. |
| [2] | 宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112. |
| [3] | 王超, 白如仟, 管俊梅, 罗稷林, 何雪姣, 迟绍轶, 马玲. 马铃薯块茎变绿中StHY5对龙葵素合成的促进作用[J]. 生物技术通报, 2024, 40(9): 113-122. |
| [4] | 夏士轩, 耿泽栋, 祝光涛, 张春芝, 李大伟. 基于深度学习的马铃薯花粉活力快速检测[J]. 生物技术通报, 2024, 40(9): 123-130. |
| [5] | 毛向红, 卢瑶, 范向斌, 杜培兵, 白小东. 基于SSR荧光标记毛细管电泳的马铃薯品种遗传多样性分析及分子身份证构建[J]. 生物技术通报, 2024, 40(9): 131-140. |
| [6] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [7] | 王柯然, 闫俊杰, 刘建凤, 高玉林. RNAi技术在马铃薯害虫防控中的应用和风险[J]. 生物技术通报, 2024, 40(9): 4-10. |
| [8] | 张小妹, 周南伶, 张赛行, 王超, 沈玉龙, 管俊梅, 马玲. 马铃薯StDREBs基因的克隆及其表达分析[J]. 生物技术通报, 2024, 40(9): 42-50. |
| [9] | 满全财, 孟姿诺, 李伟, 蔡心汝, 苏润东, 付长青, 高顺娟, 崔江慧. 马铃薯AQP基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 51-63. |
| [10] | 吴娟, 武小娟, 王沛捷, 谢锐, 聂虎帅, 李楠, 马艳红. 彩色马铃薯花青素合成相关ERF基因筛选及表达分析[J]. 生物技术通报, 2024, 40(9): 82-91. |
| [11] | 乔岩, 杨芳, 任盼荣, 祁伟亮, 安沛沛, 李茜, 李丹, 肖俊飞. 马铃薯野生种烯酰水合酶超家族基因ScDHNS的克隆与功能分析[J]. 生物技术通报, 2024, 40(9): 92-103. |
| [12] | 张震, 李清, 徐菁, 陈凯园, 张春芝, 祝光涛. 马铃薯线粒体靶向表达载体的构建与应用[J]. 生物技术通报, 2024, 40(5): 66-73. |
| [13] | 张玉, 石磊, 巩檑, 聂峰杰, 杨江伟, 刘璇, 杨文静, 张国辉, 颉瑞霞, 张丽. 马铃薯WOX基因家族的鉴定及在离体再生和非生物胁迫中的表达分析[J]. 生物技术通报, 2024, 40(3): 170-180. |
| [14] | 梅显军, 宋慧洋, 李京昊, 梅超, 宋倩娜, 冯瑞云, 陈喜明. 马铃薯StDof5的克隆及表达分析[J]. 生物技术通报, 2024, 40(3): 181-192. |
| [15] | 张春芝, 周倩, 吴瑶瑶, 尚轶, 黄三文. 基因组学研究助力马铃薯育种方式的变革[J]. 生物技术通报, 2024, 40(10): 11-18. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||