








• 综述与专论 • 下一篇
收稿日期:2025-03-17
出版日期:2025-07-17
发布日期:2025-07-17
通讯作者:
汪光义,男,博士,教授,研究方向 :微生物代谢调控与合成生物学;E-mail: gywang@tju.edu.cn作者简介:刘修平,女,博士研究生,研究方向 :微生物代谢工程;E-mail: liuxp880@tju.edu.cn基金资助:
LIU Xiu-ping1( ), ZHU Xing-yu1(
), ZHU Xing-yu1( ), WANG Guang-yi1,2(
), WANG Guang-yi1,2( )
)
Received:2025-03-17
Published:2025-07-17
Online:2025-07-17
摘要:
发展微生物固碳技术对于遏制全球气候变暖有重要意义。梭菌属(Clostridium)产乙酸菌能够固定CO2和CO等一碳气体并将其转化为乙醇等化学产品,从而打破对化石燃料的依赖,是合成气规模化定向转化的潜力菌种。本文综述了梭菌属产乙酸菌的代谢工程改造与合成气定向转化研究现状。首先,对梭菌属产乙酸菌的能量代谢方式进行了解析,阐述了梭菌属产乙酸菌的能量节约机制;其次,对梭菌属产乙酸菌的多种遗传操作工具进行了对比和总结;最后,梳理了当前梭菌属产乙酸菌代谢工程改造两个大方向的前景和存在的问题。总之,本文总结了近年来梭菌属产乙酸菌的代谢工程研究进展,以期为更全面了解该工业微生物的合成气转化潜力提供参考,推动梭菌属产乙酸菌生产可再生能源的平台建设。
刘修平, 朱星宇, 汪光义. 梭菌属(Clostridium)产乙酸菌代谢工程改造与合成气定向转化研究现状[J]. 生物技术通报, doi: 10.13560/j.cnki.biotech.bull.1985.2025-0283.
LIU Xiu-ping, ZHU Xing-yu, WANG Guang-yi. Research Progress in Metabolic Engineering for Modification and Syngas-directed Conversion by Acetogenic Clostridium[J]. Biotechnology Bulletin, doi: 10.13560/j.cnki.biotech.bull.1985.2025-0283.

图1 Wood-Ljungdahl途径Fdh:甲酸脱氢酶;Fhs:甲酰-四氢叶酸合成酶;FTC:甲酰四氢呋喃环水解酶;MTDH:亚甲基四氢呋喃脱氢酶;MetF:亚甲基四氢呋喃还原酶;MetR:甲基转移酶;CODH/ACS:一氧化碳脱氢酶/乙酰辅酶A合酶复合体;Ald:醛脱氢酶;AdhE:醇醛脱氢酶;Pta:磷酸乙酰转移酶;Ack:乙酸激酶;Aor:醛:铁氧还蛋白氧化还原酶
Fig. 1 Wood-Ljungdahl pathwayFdh: Formate dehydrogenase; Fhs: formyl-THF synthetase; FTC: formyl-THF cyclohydrolase; MTDH: methylene-THF dehydrogenase; MetF: methylene-THF reductase; MetR: methyltransferase; CODH/ACS: carbon monoxide dehydrogenase/acetyl-CoA synthase; Ald: acetaldehyde dehydrogenase; AdhE: acetaldehyde/alcohol dehydrogenase; Pta: phosphotransacetylase; Ack: acetate kinase; Aor: aldehyde: ferredoxin oxidoreductase
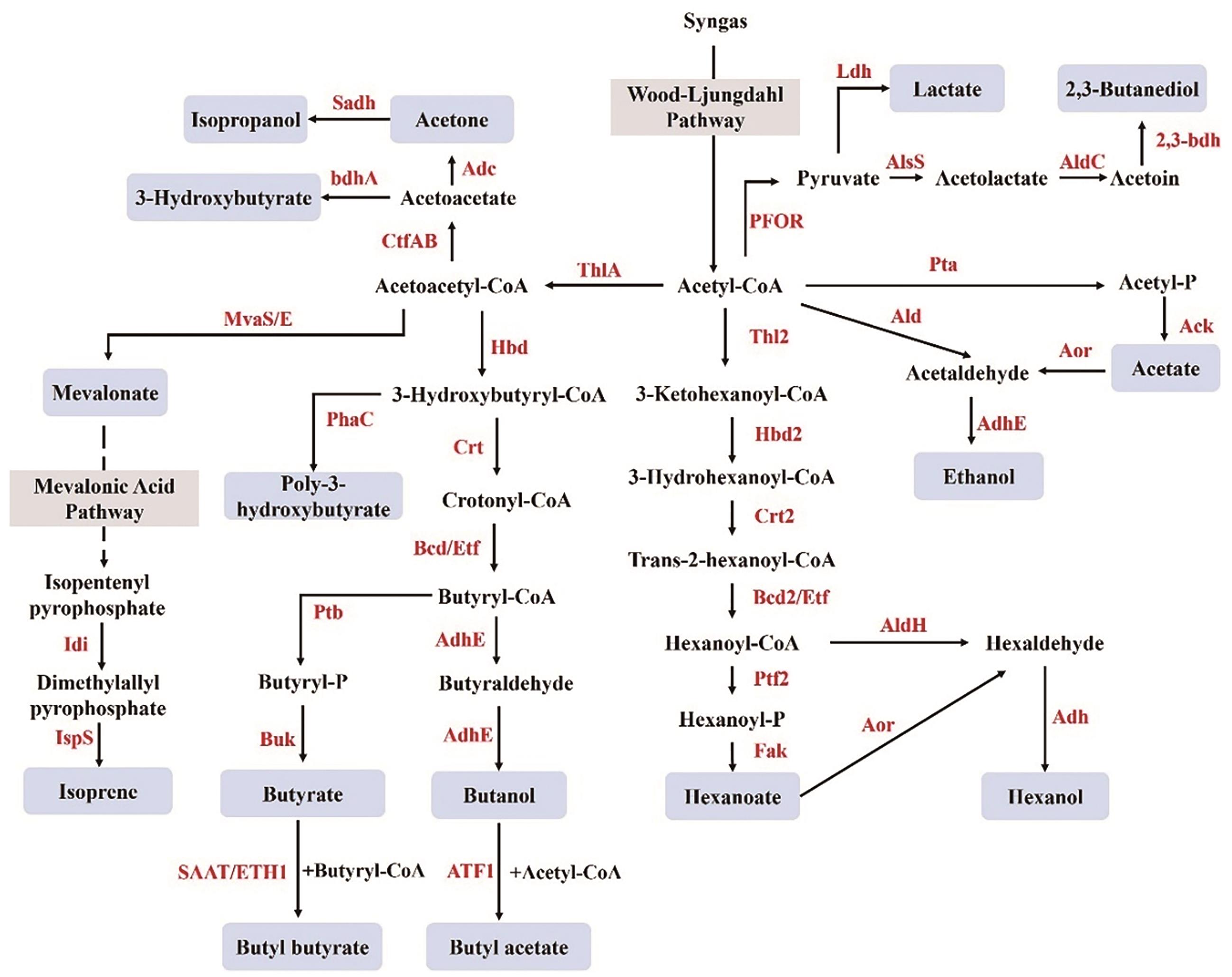
图3 梭菌属产乙酸菌合成产物的代谢途径ThlA/Thl2:硫解酶;Hbd/Hbd2:3-羟基丁酸辅酶A脱氢酶;Crt/Crt2:巴豆酸酶;Bcd/Bcd2:丁酰辅酶A脱氢酶;AdhE:醇醛脱氢酶;ATF1:乙醇酰转移酶;Ptb/Ptf2:磷酸乙酰转移酶;Buk:丁酸激酶;SAAT:草莓酒精酰转移酶;ETH1:酵母酯合成/水解酶I;MvaS/E:羟甲基戊二酰辅酶A合酶;Idi:异戊二烯焦磷酸异构酶;IspS:异戊二烯合酶;CtfAB:辅酶A亚基A/B转移酶;Adc:乙酰乙酸脱羧酶;Sadh:伯/仲醇脱氢酶;bdhA:3-羟基丁酸脱氢酶;Pta:磷酸乙酰转移酶;Ack:乙酸激酶;Ald:乙醛脱氢酶;Aor:醛氧化还原酶;Etf:电子转移黄素蛋白;Fak:脂肪酸激酶;AldH:己醛脱氢酶;Adh:醇脱氢酶;PFOR:丙酮酸铁氧还蛋白氧化还原酶;AlsS:乙酰乳酸合酶;AldC:乙偶姻脱羧酶;2,3-Bdh:2,3-丁二醇脱氢酶;Ldh:乳酸脱氢酶;PhaC:多羟基烷酸合酶
Fig. 3 Metabolic pathways of synthetic products in Acetogenic ClostridiumThlA/Thl2: Thiolase; Hbd/Hbd2: 3-hydroxybutyryl-CoA dehydrogenase; Crt/Crt2: crotonase; Bcd/Bcd2: butyryl-CoA dehydrogenase; AdhE: acetaldehyde/alcohol dehydrogenase; ATF1: alcohol Acetyltransferase; Ptb/Ptf2: Phosphotransferase; Buk: butyrate kinase; SAAT: strawberry Alcohol Acyltransferase; ETH1: ethanol hexanoyltransferase I; MvaS/E: hydroxymethylglutaryl-CoA synthase/reductase; Idi: isopentenyl pyrophosphate isomerase; IspS: isoprene synthase; CtfAB: acetoacetyl-CoA:acetate/butyrate-CoA transferase; Adc: acetoacetate decarboxylase; Sadh: secondary alcohol dehydrogenase; bdhA: 3-hydroxybutyrate dehydrogenase; Pta: Phosphotransacetylase; Ack: Acetate kinase; Ald: Acetaldehyde dehydrogenase; Aor: aldehyde: ferredoxin oxidoreductase; Etf: electron transfer flavoprotein; Fak: fatty acid kinase; AldH: aldehyde dehydrogenasel; Adh: alcohol dehydrogenase; PFOR: pyruvate: ferredoxin oxidoreductase; AlsS: acetolactate synthase; AldC: acetoin decarboxylase; 2,3-Bdh: 2,3-butanediol dehydrogenase; Ldh: lactate dehydrogenase; PhaC: Polyhydroxyalkanoate Synthase
| [1] | Kuang CY. Analysis of Green House Gases and Positive Impact of Replacing Traditional Energy with Clean Energy [C]. E3S Web of Conferences, 2021,241: 2005. |
| [2] | Salehizadeh H, Yan N, Farnood R. Recent advances in microbial CO2 fixation and conversion to value-added products [J]. Chem Eng J, 2020, 390: 124584. |
| [3] | Nguyen T, Hilliard M, Rochelle GT. Amine volatility in CO2 capture [J]. Int J Greenh Gas Control, 2010, 4(5): 707-715. |
| [4] | Sevilla M, Fuertes AB. CO2 adsorption by activated templated carbons [J]. J Colloid Interface Sci, 2012, 366(1): 147-154. |
| [5] | Mustafa J, Farhan M. CO2 separation from flue gases using different types of membranes [J]. J Membra Sci Technol, 2016, 6(2): 1-7. |
| [6] | Xu G, Li L, Yang YP, et al. A novel CO2 cryogenic liquefaction and separation system [J]. Energy, 2012, 42(1): 522-529. |
| [7] | Dashti H, Zhehao Yew L, Lou X. Recent advances in gas hydrate-based CO2 capture [J]. J Nat Gas Sci Eng, 2015, 23: 195-207. |
| [8] | 李婉麒, 杨凤娟, 贾德臣, 等. 合成气的生物利用与定向转化 [J]. 化工进展, 2023. 42(1): 73-85. |
| Li WQ, Yang FJ, Jia DC, et al. Biological utilization and conversion of syngas [J]. Chemical Industry and Engineering Progress, 2023. 42(1): 73-85. | |
| [9] | Köpke M, Held C, Hujer S, et al. Clostridium ljungdahlii represents a microbial production platform based on syngas [J]. Proc Natl Acad Sci USA, 2010, 107(29): 13087-13092. |
| [10] | Patakova P, Kolek J, Sedlar K, et al. Comparative analysis of high butanol tolerance and production in clostridia [J]. Biotechnol Adv, 2018, 36(3): 721-738. |
| [11] | 贾德臣, 姜卫红, 顾阳. 食气梭菌的研究进展 [J]. 微生物学通报, 2019, 46(2): 374-387. |
| Jia DC, Jiang WH, Gu Y. Research progresses in gas-fermenting clostridia [J]. Microbiol China, 2019, 46(2): 374-387. | |
| [12] | Liew F, Martin ME, Tappel RC, et al. Gas fermentation-a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks [J]. Front Microbiol, 2016, 7: 694. |
| [13] | Grahame DA. Acetate C-C bond formation and decomposition in the anaerobic world: the structure of a central enzyme and its key active-site metal cluster [J]. Trends Biochem Sci, 2003, 28(5): 221-224. |
| [14] | Lee S, Song Y, Choe D, et al. Reconstruction of acetogenesis pathway using short-read sequencing of Clostridium aceticum genome [J]. J Nanosci Nanotechnol, 2015, 15(5): 3852-3861. |
| [15] | Latif H, Zeidan AA, Nielsen AT, et al. Trash to treasure: production of biofuels and commodity chemicals via syngas fermenting microorganisms [J]. Curr Opin Biotechnol, 2014, 27: 79-87. |
| [16] | Zhu HF, Liu ZY, Zhou X, et al. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii [J]. Front Microbiol, 2020, 11: 416. |
| [17] | Müller V. Energy conservation in acetogenic bacteria [J]. Appl Environ Microbiol, 2003, 69(11): 6345-6353. |
| [18] | Müller V, Imkamp F, Biegel E, et al. Discovery of a ferredoxin: NAD+-oxidoreductase (rnf) in Acetobacterium woodii: a novel potential coupling site in acetogens [J]. Ann N Y Acad Sci, 2008, 1125: 137-146. |
| [19] | Tremblay PL, Zhang T, Dar SA, et al. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin: NAD+ oxidoreductase essential for autotrophic growth [J]. mBio, 2012, 4(1): e00406-12. |
| [20] | Peters JW, Miller AF, Jones AK, et al. Electron bifurcation [J]. Curr Opin Chem Biol, 2016, 31: 146-152. |
| [21] | Buckel W, Thauer RK. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation [J]. Biochim Biophys Acta BBA Bioenerg, 2013, 1827(2): 94-113. |
| [22] | Wang SN, Huang HY, Moll J, et al. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri [J]. J Bacteriol, 2010, 192(19): 5115-5123. |
| [23] | Wang SN, Huang HY, Kahnt J, et al. NADP-specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in Clostridium autoethanogenum grown on CO [J]. J Bacteriol, 2013, 195(19): 4373-4386. |
| [24] | Mock J, Zheng YN, Mueller AP, et al. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation [J]. J Bacteriol, 2015, 197(18): 2965-2980. |
| [25] | Richter H, Molitor B, Wei H, et al. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression [J]. Energy Environ Sci, 2016, 9(7): 2392-2399. |
| [26] | Bertsch J, Müller V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria [J]. Biotechnol Biofuels, 2015, 8: 210. |
| [27] | Najafpour G, Younesi H. Ethanol and acetate synthesis from waste gas using batch culture of Clostridium ljungdahlii [J]. Enzyme Microb Technol, 2006, 38(1/2): 223-228. |
| [28] | Kerby RL, Ludden PW, Roberts GP. Carbon monoxide-dependent growth of Rhodospirillum rubrum [J]. J Bacteriol, 1995, 177(8): 2241-2244. |
| [29] | Kim MS, Bae SS, Kim YJ, et al. CO-dependent H2 production by genetically engineered Thermococcus onnurineus NA1 [J]. Appl Environ Microbiol, 2013, 79(6): 2048-2053. |
| [30] | Utturkar SM, Klingeman DM, Bruno-Barcena JM, et al. Sequence data for Clostridium autoethanogenum using three generations of sequencing technologies [J]. Sci Data, 2015, 2: 150014. |
| [31] | Dong HJ, Zhang YP, Dai ZJ, et al. Engineering Clostridium strain to accept unmethylated DNA [J]. PLoS One, 2010, 5(2): e9038. |
| [32] | Cui GZ, Hong W, Zhang J, et al. Targeted gene engineering in Clostridium cellulolyticum H10 without methylation [J]. J Microbiol Meth, 2012, 89(3): 201-208. |
| [33] | Heap JT, Pennington OJ, Cartman ST, et al. A modular system for Clostridium shuttle plasmids [J]. J Microbiol Meth, 2009, 78(1): 79-85. |
| [34] | Molitor B, Kirchner K, Henrich AW, et al. Expanding the molecular toolkit for the homoacetogen Clostridium ljungdahlii [J]. Sci Rep, 2016, 6: 31518. |
| [35] | Charubin K, Hill JD, Papoutsakis ET. DNA transfer between two different species mediated by heterologous cell fusion in Clostridium coculture [J]. mBio, 2024, 15(2): e0313323. |
| [36] | Purdy D, O'Keeffe TAT, Elmore M, et al. Conjugative transfer of clostridial shuttle vectors from Escherichia coli to Clostridium difficile through circumvention of the restriction barrier [J]. Mol Microbiol, 2002, 46(2): 439-452. |
| [37] | Desai RP, Papoutsakis ET. Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum [J]. Appl Environ Microbiol, 1999, 65(3): 936-945. |
| [38] | Perret S, Maamar H, Bélaich JP, et al. Use of antisense RNA to modify the composition of cellulosomes produced by Clostridium cellulolyticum [J]. Mol Microbiol, 2004, 51(2): 599-607. |
| [39] | Raju D, Setlow P, Sarker MR. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation [J]. Appl Environ Microbiol, 2007, 73(7): 2048-2053. |
| [40] | Shao LJ, Hu SY, Yang Y, et al. Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum [J]. Cell Res, 2007, 17(11): 963-965. |
| [41] | Yoo M, Croux C, Meynial-Salles I, et al. Elucidation of the roles of adhE1 and adhE2 in the primary metabolism of Clostridium acetobutylicum by combining in-frame gene deletion and a quantitative system-scale approach [J]. Biotechnol Biofuels, 2016, 9: 92. |
| [42] | Cooksley CM, Zhang Y, Wang HZ, et al. Targeted mutagenesis of the Clostridium acetobutylicum acetone-butanol-ethanol fermentation pathway [J]. Metab Eng, 2012, 14(6): 630-641. |
| [43] | Leang C, Ueki T, Nevin KP, et al. A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen [J]. Appl Environ Microbiol, 2013, 79(4): 1102-1109. |
| [44] | Croux C, Nguyen NPT, Lee J, et al. Construction of a restriction-less, marker-less mutant useful for functional genomic and metabolic engineering of the biofuel producer Clostridium acetobutylicum [J]. Biotechnol Biofuels, 2016, 9: 23. |
| [45] | Al-Hinai MA, Fast AG, Papoutsakis ET. Novel system for efficient isolation of Clostridium double-crossover allelic exchange mutants enabling markerless chromosomal gene deletions and DNA integration [J]. Appl Environ Microbiol, 2012, 78(22): 8112-8121. |
| [46] | Cartman ST, Kelly ML, Heeg D, et al. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production [J]. Appl Environ Microbiol, 2012, 78(13): 4683-4690. |
| [47] | Ng YK, Ehsaan M, Philip S, et al. Expanding the repertoire of gene tools for precise manipulation of the Clostridium difficile genome: allelic exchange using pyrE alleles [J]. PLoS One, 2013, 8(2): e56051. |
| [48] | Nariya H, Miyata S, Suzuki M, et al. Development and application of a method for counterselectable in-frame deletion in Clostridium perfringens [J]. Appl Environ Microbiol, 2011, 77(4): 1375-1382. |
| [49] | Ueki T, Nevin KP, Woodard TL, et al. Converting carbon dioxide to butyrate with an engineered strain of Clostridium ljungdahlii [J]. mBio, 2014, 5(5): e01636-14. |
| [50] | 杨君仪, 鲍江舰, 邵瑞瑞, 等. CD630_27900基因缺失显著降低艰难拟梭菌自溶速率、毒力及对酸和抗生素的耐受性 [J]. 微生物学报, 2023. 63(6): 2440-2455. |
| Yang JY, Bao JJ, Shao RR, et al. CD630_27900 gene deletion significantly reduces autolysis rate and virulence of Clostridioides difficile and the tolerance to acids and antibiotics [J]. Acta Microbiologica Sinica, 2023. 63(6): 2440-2455. | |
| [51] | 鲍江舰, 杨君仪, 邵瑞瑞, 等. fliL基因显著影响艰难拟梭菌运动功能及产孢能力 [J]. 生物工程学报, 2023. 39(4): 1578-1595. |
| Bao JJ, Yang JY, Shao RR, et al. The fliL gene significantly affects the motility and sporulation abilities of Clostridioides difficile [J]. Chinese Journal of Biotechnology, 2023. 39(4): 1578-1595. | |
| [52] | Ehsaan M, Kuehne SA, Minton NP. Clostridium difficile genome editing using pyrE alleles [J]. Methods Mol Biol, 2016, 1476: 35-52. |
| [53] | Annan FJ, Al-Sinawi B, Humphreys CM, et al. Engineering of vitamin prototrophy in Clostridium ljungdahlii and Clostridium autoethanogenum [J]. Appl Microbiol Biotechnol, 2019, 103(11): 4633-4648. |
| [54] | Kovács K, Willson BJ, Schwarz K, et al. Secretion and assembly of functional mini-cellulosomes from synthetic chromosomal operons in Clostridium acetobutylicum ATCC 824 [J]. Biotechnol Biofuels, 2013, 6(1): 117. |
| [55] | Huang H, Chai CS, Li N, et al. CRISPR/Cas9-based efficient genome editing in Clostridium ljungdahlii, an autotrophic gas-fermenting bacterium [J]. ACS Synth Biol, 2016, 5(12): 1355-1361. |
| [56] | Xu T, Li YC, Shi Z, et al. Efficient genome editing in Clostridium cellulolyticum via CRISPR-Cas9 nickase [J]. Appl Environ Microbiol, 2015, 81(13): 4423-4431. |
| [57] | Komor AC, Kim YB, Packer MS, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage [J]. Nature, 2016, 533(7603): 420-424. |
| [58] | Kim YB, Komor AC, Levy JM, et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions [J]. Nat Biotechnol, 2017, 35(4): 371-376. |
| [59] | Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage [J]. Nature, 2017, 551(7681): 464-471. |
| [60] | Zhao R, Liu YQ, Zhang H, et al. CRISPR-Cas12a-mediated gene deletion and regulation in Clostridium ljungdahlii and its application in carbon flux redirection in synthesis gas fermentation [J]. ACS Synth Biol, 2019, 8(10): 2270-2279. |
| [61] | Banerjee A, Leang C, Ueki T, et al. Lactose-inducible system for metabolic engineering of Clostridium ljungdahlii [J]. Appl Environ Microbiol, 2014, 80(8): 2410-2416. |
| [62] | Woolston BM, Emerson DF, Currie DH, et al. Rediverting carbon flux in Clostridium ljungdahlii using CRISPR interference (CRISPRi) [J]. Metab Eng, 2018, 48: 243-253. |
| [63] | Yang GH, Jia DC, Jin L, et al. Rapid generation of universal synthetic promoters for controlled gene expression in both gas-fermenting and saccharolytic Clostridium species [J]. ACS Synth Biol, 2017, 6(9): 1672-1678. |
| [64] | Girbal L, Mortier-Barrière I, Raynaud F, et al. Development of a sensitive gene expression reporter system and an inducible promoter-repressor system for Clostridium acetobutylicum [J]. Appl Environ Microbiol, 2003, 69(8): 4985-4988. |
| [65] | Dong HJ, Tao WW, Zhang YP, et al. Development of an anhydrotetracycline-inducible gene expression system for solvent-producing Clostridium acetobutylicum: a useful tool for strain engineering [J]. Metab Eng, 2012, 14(1): 59-67. |
| [66] | Heap JT, Pennington OJ, Cartman ST, et al. The ClosTron: a universal gene knock-out system for the genus Clostridium [J]. J Microbiol Meth, 2007, 70(3): 452-464. |
| [67] | Zhang Y, Grosse-Honebrink A, Minton NP. A universal mariner transposon system for forward genetic studies in the genus Clostridium [J]. PLoS One, 2015, 10(4): e0122411. |
| [68] | Drepper T, Huber R, Heck A, et al. Flavin mononucleotide-based fluorescent reporter proteins outperform green fluorescent protein-like proteins as quantitative in vivo real-time reporters [J]. Appl Environ Microbiol, 2010, 76(17): 5990-5994. |
| [69] | Drepper T, Eggert T, Circolone F, et al. Reporter proteins for in vivo fluorescence without oxygen [J]. Nat Biotechnol, 2007, 25(4): 443-445. |
| [70] | Mukherjee A, Schroeder CM. Flavin-based fluorescent proteins: emerging paradigms in biological imaging [J]. Curr Opin Biotechnol, 2015, 31: 16-23. |
| [71] | Whitham JM, Tirado-Acevedo O, Chinn MS, et al. Metabolic response of Clostridium ljungdahlii to oxygen exposure [J]. Appl Environ Microbiol, 2015, 81(24): 8379-8391. |
| [72] | Koepke M, Liew F. Genetically engineered bacterium with altered carbon monoxide dehydrogenase (CODH) activity: US9365873 [P]. 2016-06-14. |
| [73] | Han S, Gao XY, Ying HJ, et al. NADH gene manipulation for advancing bioelectricity in Clostridium ljungdahlii microbial fuel cells [J]. Green Chem, 2016, 18(8): 2473-2478. |
| [74] | Darmawi Juminaga, Arthur Shockley, and Robert Nogle. Recombinant Microorganisms and Uses Therefor[P]. 2022, WO2022170191A1. |
| [75] | Michael Koepke, Shilpa Nagaraju, and Chen Wendy. Recombinant Microorganisms and Methods of Use Thereof[P]. 2018, US 9057071B2. |
| [76] | Liew FE, Nogle R, Abdalla T, et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale [J]. Nat Biotechnol, 2022, 40(3): 335-344. |
| [77] | Vögeli B, Schulz L, Garg S, et al. Cell-free prototyping enables implementation of optimized reverse β-oxidation pathways in heterotrophic and autotrophic bacteria [J]. Nat Commun, 2022, 13(1): 3058. |
| [78] | Jin S, Bae JY, Song Y, et al. Synthetic biology on acetogenic bacteria for highly efficient conversion of C1 gases to biochemicals [J]. Int J Mol Sci, 2020, 21(20): 7639. |
| [79] | Jia DC, He MY, Tian Y, et al. Metabolic engineering of gas-fermenting Clostridium ljungdahlii for efficient co-production of isopropanol, 3-hydroxybutyrate, and ethanol [J]. ACS Synth Biol, 2021, 10(10): 2628-2638. |
| [80] | Schiel-Bengelsdorf B, Dürre P. Pathway engineering and synthetic biology using acetogens [J]. FEBS Lett, 2012, 586(15): 2191-2198. |
| [81] | Beck Zachary Q, Cervin Marguerite A, Chotani Gopal K, et al. Recombinant Anaerobic Acetogenic Bacteria for Production of Isoprene and/or Industrial Bio-Products Using Synthesis Gas: WO2014193473(A1) [P]. 2014-12-04. |
| [82] | Feng J, Zhang J, Ma YC, et al. Renewable fatty acid ester production in Clostridium [J]. Nat Commun, 2021, 12(1): 4368. |
| [83] | 刘昊鹏, 刘超, 王雯, 等. 基于厌氧微生物的碳链延长合成高价值化学品反应机理及研究进展:不同电子供体 [J]. 北京化工大学学报(自然科学版), 2020. 47(5): 1-17. |
| Liu HP, Liu C, Wang W, et al. Advances in understanding the mechanism of chain elongation with anaerobic microbes for the synthesis of high value-added chemicals: the effect of different electron donors [J]. Journal of Beijing University of Chemical Technology (Natural Science), 2020. 47(5): 1-17. |
| [1] | 鲁天怡, 李爱朋, 费强. 生物合成聚乳酸研究进展[J]. 生物技术通报, 2025, 41(4): 47-60. |
| [2] | 饶峻, 赵晨, 李端华, 廖豪, 黄加雨, 王辂. 自诱导策略在麦角硫因生物合成中的应用[J]. 生物技术通报, 2025, 41(1): 333-346. |
| [3] | 何玙冰, 付振浩, 李仁瀚, 刘秀霞, 刘春立, 杨艳坤, 李业, 白仲虎. 利用代谢工程在酿酒酵母中高效合成2-萘乙醇[J]. 生物技术通报, 2024, 40(7): 99-107. |
| [4] | 张美玉, 赵玉斌, 王灵云, 宋元达, 赵新河, 任晓洁. 微藻破囊壶菌产功能性脂肪酸DHA研究进展[J]. 生物技术通报, 2024, 40(6): 81-94. |
| [5] | 何思成, 张紫瑗, 韩雨晴, 苗琳, 张翠英, 于爱群. 解脂耶氏酵母细胞工厂生产多不饱和脂肪酸的研究进展[J]. 生物技术通报, 2024, 40(1): 72-85. |
| [6] | 李亮, 徐姗姗, 姜艳军. 生物合成法生产麦角硫因的研究进展[J]. 生物技术通报, 2024, 40(1): 86-99. |
| [7] | 薛宁, 王瑾, 李世新, 刘叶, 程海娇, 张玥, 毛雨丰, 王猛. 多基因同步调控结合高通量筛选构建高产L-苯丙氨酸的谷氨酸棒杆菌工程菌株[J]. 生物技术通报, 2023, 39(9): 268-280. |
| [8] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [9] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [10] | 李雨真, 梅天秀, 李治文, 王淇, 李俊, 邹岳, 赵心清. 红酵母基因组和代谢工程改造研究进展[J]. 生物技术通报, 2023, 39(7): 67-79. |
| [11] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [12] | 李海宁, 张红兵, 耿革霞, 李冉, 贾振华. 非天然氨基酸的应用及生物合成策略[J]. 生物技术通报, 2023, 39(12): 43-55. |
| [13] | 邱益彬, 马艳琴, 沙媛媛, 朱逸凡, 苏二正, 雷鹏, 李莎, 徐虹. 解淀粉芽孢杆菌分子遗传操作及其应用研究进展[J]. 生物技术通报, 2022, 38(2): 205-217. |
| [14] | 马艳琴, 邱益彬, 李莎, 徐虹. 透明质酸的生物合成及其代谢工程的研究进展[J]. 生物技术通报, 2022, 38(2): 252-262. |
| [15] | 叶健文, 陈江楠, 张旭, 吴赴清, 陈国强. 动态调控:一种高效的细胞工厂工程化代谢改造策略[J]. 生物技术通报, 2020, 36(6): 1-12. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||