








生物技术通报 ›› 2025, Vol. 41 ›› Issue (10): 222-232.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0354
张雨晴( ), 董丽雪, 张宝月, 张颖, 刘雪敖, 熊双喜(
), 董丽雪, 张宝月, 张颖, 刘雪敖, 熊双喜( ), 张洪霞(
), 张洪霞( )
)
收稿日期:2025-04-02
出版日期:2025-10-26
发布日期:2025-10-28
通讯作者:
熊双喜,男,博士,讲师,研究方向 :番茄雄性生殖发育;E-mail: sxxiong@ldu.edu.cn;作者简介:张雨晴,女,硕士研究生,研究方向 :番茄雄性生殖发育;E-mail: 1924118220@qq.com
基金资助:
ZHANG Yu-qing( ), DONG Li-xue, ZHANG Bao-yue, ZHANG Ying, LIU Xue-ao, XIONG Shuang-xi(
), DONG Li-xue, ZHANG Bao-yue, ZHANG Ying, LIU Xue-ao, XIONG Shuang-xi( ), ZHANG Hong-xia(
), ZHANG Hong-xia( )
)
Received:2025-04-02
Published:2025-10-26
Online:2025-10-28
摘要:
目的 MYB80是拟南芥和水稻中的一个花粉壁形成关键调节因子,探究番茄中同源基因SlMYB80的功能并鉴定其转录激活结构域,为进一步丰富MYB转录因子对番茄雄性不育系基因资源提供理论依据。 方法 以栽培番茄‘Moneymaker’为野生型材料,以其花苞cDNA为模板克隆SlMYB80基因。利用花椰菜花叶病毒CaMV 35s启动子驱动SlMYB80编码区(CDS)与绿色荧光蛋白eGFP编码序列融合,用于检测SlMYB80蛋白亚细胞定位。同时,将该基因编码区分成4个片段,分别连接酵母表达载体pGTBKT7,与pGTADT7共转酵母进行互作试验以确定其转录激活结构域。此外,构建拟南芥MS188(Male Sterile 188)/AtMYB80启动子驱动SlMYB80 CDS的融合双元载体pAtMYB80:SlMYB80并互补拟南芥ms188突变体,探究SlMYB80的生物学功能。 结果 MYB80氨基酸序列比对和进化树结果表明,MYB80的氨基酸序列在陆生植物中十分保守,特别是R2R3 DNA结合结构域区域;烟草亚细胞定位试验表明,SlMYB80-eGFP定位于细胞核。酵母互作试验表明,SlMYB80转录激活结构域位于肽链C末端17个氨基酸残基;拟南芥ms188突变体互补结果表明,在转基因互补突变体植株的花苞中表达SlMYB80基因,能使部分花粉正常形成,从而恢复部分育性。 结论 SlMYB80作为拟南芥MS188/AtMYB80的直系同源基因编码一个R2R3 MYB转录因子定位于细胞核中,其多肽链C末端17个氨基酸为激活结构域,能部分互补ms188突变体花粉败育的表型,揭示了MYB80转录因子功能的保守性。
张雨晴, 董丽雪, 张宝月, 张颖, 刘雪敖, 熊双喜, 张洪霞. 番茄SlMYB80转录激活结构域的鉴定及其在拟南芥花粉发育中的功能验证[J]. 生物技术通报, 2025, 41(10): 222-232.
ZHANG Yu-qing, DONG Li-xue, ZHANG Bao-yue, ZHANG Ying, LIU Xue-ao, XIONG Shuang-xi, ZHANG Hong-xia. Characterization of the Activation Domain of SlMYB80 in Tomato and Its Function Validation during Pollen Development in Arabidopsis[J]. Biotechnology Bulletin, 2025, 41(10): 222-232.
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 引物用途 Primer usage |
|---|---|---|
| β-tub qRT F | GATTTCAAAGATTAGGGAAGAGTA | RT-qPCR |
| β-tub qRT R | GTTCTGAAGCAAATGTCATAGAG | |
| SlMYB80 qRT F | ATTATCTGGTATGGGAATTGATCC | |
| SlMYB80 qRT R | TTAACATGACTTGTACTTGGTGCA | |
| SlMYB80fullCDS-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | 酵母杂交 Yeast hybrid |
| SlMYB80fullCDS-BK R | aggtcgacggatccccggTCAAACCATTGGATTCATTAGAT | |
| SlMYB80fullCDS-AD F | atggccatggaggccagtATGGGAAGAATTCCATGTTGTG | |
| SlMYB80fullCDS-AD R | tcgatgcccacccgggtgTCAAACCATTGGATTCATTAGAT | |
| SlMYB80CDS(319 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(319 aa)-BK R | aggtcgacggatccccggTCAAGTATTGAACATAGTTGTTGATCC | |
| SlMYB80CDS(298 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(298 aa)-BK R | aggtcgacggatccccggTCAACAATTATCATTGCTAATTTTC | |
| SlMYB80CDS(272 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(272 aa)-BK R | aggtcgacggatccccggTCATGCTGTGCATGTACTTCCA | |
| SlMYB80CDS-35S-GFP F | gagctcggtacccggATGGGAAGAATTCCATGTTGTGA | 亚细胞定位 Subcellular localization |
| SlMYB80CDS-35S-GFP R | catgtcgactctagaAACCATTGGATTCATTAGATCATC | |
| ProAtMS188 F | ttcgagctcggtacccggAAGTTGTGTTTTTTCCCAAGTCA | 互补验证 Complementary verification |
| ProAtMS188 R | caccattctagaggatccTTCTTCTTTCTTTCTTTCTAGTTTTT | |
| SlMYB80 full CDS F | gaaagaaagaaagaagaaATGGGAAGAATTCCATGTTGTGA | |
| SlMYB80 full CDS R | cttgctcaccattctagaTCAAACCATTGGATTCATTAGATCATC | |
| pAtMS188-SlMYB80 JD F | ATCTGAATGTAAAAGGAGTTGACC | 转基因阳性植株鉴定 Transgenic positive plants identification |
| pAtMS188-SlMYB80 JD R | ATGTTGAGCAATGTAAGATGAAAG |
表1 本文中使用的引物序列
Table 1 Primers sequences used in this article
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 引物用途 Primer usage |
|---|---|---|
| β-tub qRT F | GATTTCAAAGATTAGGGAAGAGTA | RT-qPCR |
| β-tub qRT R | GTTCTGAAGCAAATGTCATAGAG | |
| SlMYB80 qRT F | ATTATCTGGTATGGGAATTGATCC | |
| SlMYB80 qRT R | TTAACATGACTTGTACTTGGTGCA | |
| SlMYB80fullCDS-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | 酵母杂交 Yeast hybrid |
| SlMYB80fullCDS-BK R | aggtcgacggatccccggTCAAACCATTGGATTCATTAGAT | |
| SlMYB80fullCDS-AD F | atggccatggaggccagtATGGGAAGAATTCCATGTTGTG | |
| SlMYB80fullCDS-AD R | tcgatgcccacccgggtgTCAAACCATTGGATTCATTAGAT | |
| SlMYB80CDS(319 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(319 aa)-BK R | aggtcgacggatccccggTCAAGTATTGAACATAGTTGTTGATCC | |
| SlMYB80CDS(298 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(298 aa)-BK R | aggtcgacggatccccggTCAACAATTATCATTGCTAATTTTC | |
| SlMYB80CDS(272 aa)-BK F | catatggccatggaggccATGGGAAGAATTCCATGTTGTG | |
| SlMYB80CDS(272 aa)-BK R | aggtcgacggatccccggTCATGCTGTGCATGTACTTCCA | |
| SlMYB80CDS-35S-GFP F | gagctcggtacccggATGGGAAGAATTCCATGTTGTGA | 亚细胞定位 Subcellular localization |
| SlMYB80CDS-35S-GFP R | catgtcgactctagaAACCATTGGATTCATTAGATCATC | |
| ProAtMS188 F | ttcgagctcggtacccggAAGTTGTGTTTTTTCCCAAGTCA | 互补验证 Complementary verification |
| ProAtMS188 R | caccattctagaggatccTTCTTCTTTCTTTCTTTCTAGTTTTT | |
| SlMYB80 full CDS F | gaaagaaagaaagaagaaATGGGAAGAATTCCATGTTGTGA | |
| SlMYB80 full CDS R | cttgctcaccattctagaTCAAACCATTGGATTCATTAGATCATC | |
| pAtMS188-SlMYB80 JD F | ATCTGAATGTAAAAGGAGTTGACC | 转基因阳性植株鉴定 Transgenic positive plants identification |
| pAtMS188-SlMYB80 JD R | ATGTTGAGCAATGTAAGATGAAAG |
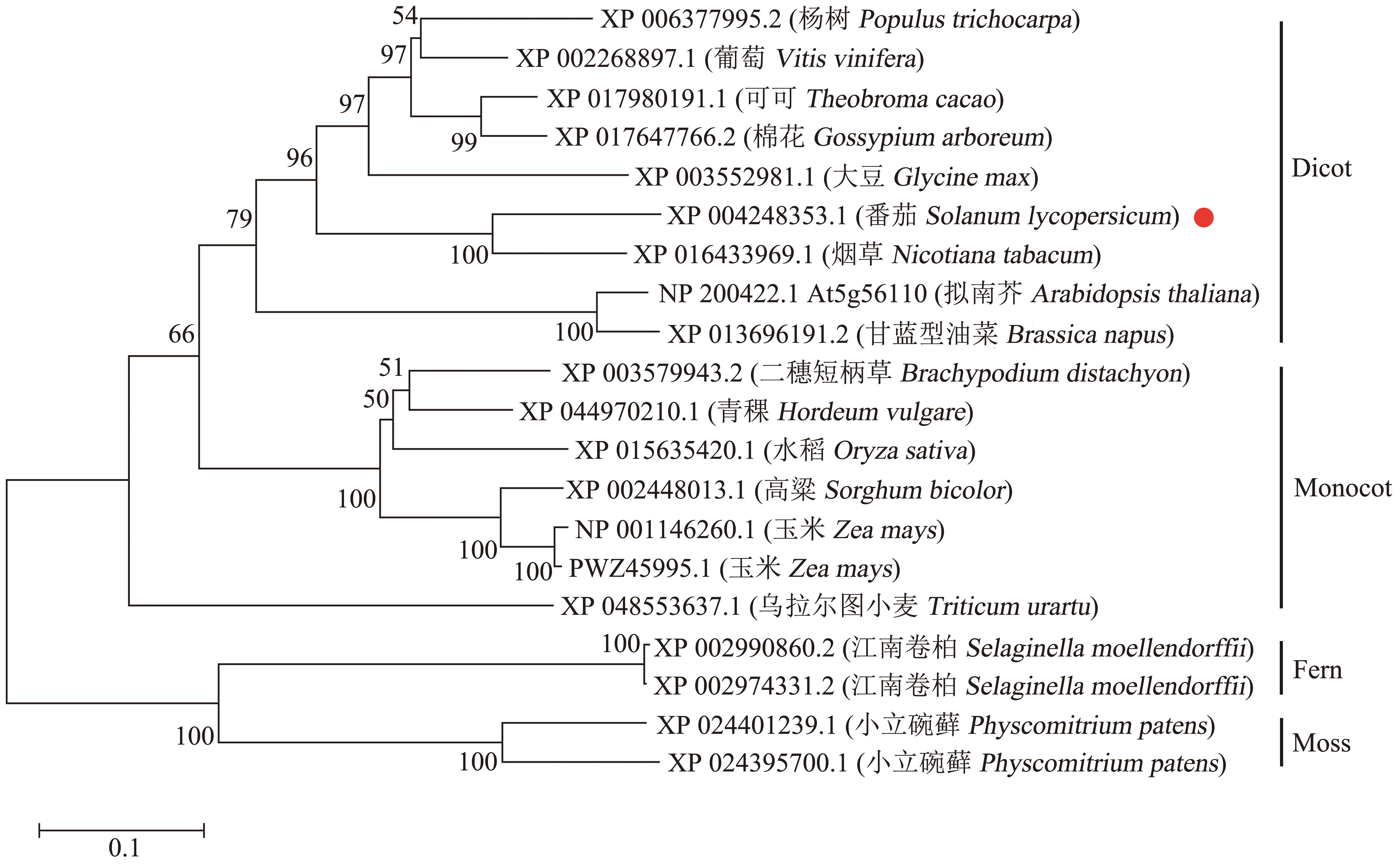
图1 SlMYB80与其他物种蛋白的系统进化分析Monocot: 单子叶植物; Dicot: 双子叶植物; 红点代表番茄SlMYB80
Fig. 1 Phylogenetic analysis of SlMYB80 with proteins from other speciesMonocot: Monocotyledon. Dicot: Dicotyledon. Red point indicates SlMYB80 from tomato

图2 PCR扩增SlMYB80全长编码区(CDS)(A)与菌落PCR检测阳性克隆(B)图A中,1:cDNA模板扩增出的SlMYB80 CDS全长;2:阴性对照(双蒸水);图B中,1、2:大肠杆菌单克隆;3:阳性对照;4:阴性对照(双蒸水);M:DL 5 000 marker
Fig. 2 PCR amplification of the full-length coding sequence (CDS) of SlMYB80 (A) and identification of positive clones by colony PCR assay (B)In Fig. A, 1: the full-length SlMYB80 CDS amplified from cDNA template; 2: negative control (ddH2O). In Fig. B, 1, 2: two single colonies of Escherichia coli; 3: positive control; 4: negative control (ddH2O). M: DL 5 000 marker

图3 SlMYB80-GFP蛋白定位于烟草叶肉细胞核中A:亚细胞定位构建示意图;B‒E:p35s:GFP pCAMBIA1300空载农杆菌注射烟草叶片激光共聚焦扫描显微镜拍摄照片;F‒I:p35s:SlMYB80CDS-eGFP pCAMBIA1300农杆菌注射烟草叶片激光共聚焦扫描显微镜拍摄照片;B和F:GFP信号通道;C和G:mCherry信号通道;D和H:叠加信号通道照片;E和I:明场通道照片;比例尺 = 20 μm
Fig. 3 SlMYB80-GFP protein specifically localized on the nucleus of tobacco mesophyll cellsA: Schematic diagram of subcellular localization construct. B‒E: Confocal microscopy images of p35s:GFP pCAMBIA1300 empty vector Agrobacterium-infiltrated tobacco leaves. F‒I: Confocal microscopy images of p35s:SlMYB80CDS-GFP pCAMBIA1300 Agrobacterium-infiltrated tobacco leaves. B and F: GFP signal channel. C and G: mCherry signal channel. D and H: Overlay signal channel images. E and I: Bright field channel images. Bar = 20 μm

图4 PCR扩增番茄SlMYB80编码区(CDS)的不同区段和菌落PCR检测阳性克隆A:1区(SlMYB80CDS第1‒272个氨基酸)DNA片段PCR扩增;B:2区(SlMYB80CDS第1‒298个氨基酸)DNA片段PCR扩增;C:3区(SlMYB80CDS第1‒319个氨基酸)DNA片段PCR扩增;D:4区(SlMYB80CDS第1‒336个氨基酸)DNA片段PCR扩增;E‒H:不同区段连接pGBKT7载体大肠杆菌菌落PCR检测阳性克隆;图A‒D中,M:DL 5 000 marker;1:扩增SlMYB80CDS不同区段DNA片段;2:阴性对照(双蒸水);图E‒H中,M:DL 5 000 marker;1‒2:大肠杆菌单克隆;3:阳性对照;4:阴性对照(双蒸水)
Fig. 4 PCR amplification of diverse fragments of SlMYB80 CDS in tomato and colony PCR screening of positive clonesA: PCR amplification of region 1 (amino acids 1‒272) DNA fragment. B: PCR amplification of region 2 (amino acids 1‒298) DNA fragment. C: PCR amplification of region 3 (amino acids 1‒319) DNA fragment. D: PCR amplification of region 4 (amino acids 1‒336) DNA fragment. E‒H: positive clones detected by colony PCR of E. coli with different segments ligated into the pGBKT7 vector. In Fig. A‒D: M: DL 5 000 marker; 1: PCR amplification of different regions of the SlMYB80CDS DNA fragment; 2: negative control (ddH2O). In Fig. E‒H: M: DL 5 000 marker; 1‒2: two single colonies of E. coli; 3: positive control; 4: negative control (ddH2O)

图5 SlMYB80激活结构域位于多肽链C末端17个氨基酸残基上A:SlMYB80不同区段DNA片段连接pGBKT7和pGADT7空载体的示意图;B:拟南芥MS188/AtMYB80氨基酸序列对比图(红线代表R2R3 DNA结合结构域,红方框代表激活结构域);C:不同缺氨基酸SD培养基上鉴定SlMYB80不同区段激活酵母报告基因表达情况;-Trp/-Leu:SD培养基缺少Trp和Leu;-Ade/-His/-Trp/-Leu:SD培养基缺少Ade、His、Trp和Leu;-Ade/-His/-Trp/-Leu/X-α-gal:SD培养基缺少Ade、His、Trp和Leu并添加X-α-gal
Fig. 5 Activation domain of SlMYB80 on the 17 amino acid residues at the C-terminus of the polypeptide chainA: Schematic diagram of the connection of diverse fragments of SlMYB80 to the vectors pGBKT7 and empty pGADT7. B: Alignment of amino acid sequences between MS188/AtMYB80 and SlMYB80 (The red line indicates the R2R3 DNA-binding domain, and the red box indicates the activation domain). C: Identification of the activation of yeast reporter gene expression by diverse fragments of SlMYB80 on SD media lacking different amino acids. -Trp/Leu: SD medium without Trp and Leu; -Ade/-His/-Trp/-Leu: SD medium without Ade, His, Trp and Leu; -Ade/-His/-Trp/-Leu/X-α-gal: SD medium added X-α-gal without Ade, His, Trp and Leu

图6 SlMYB80全长编码区(CDS)可以部分互补拟南芥ms188突变体表型A:Col-0野生型(WT)拟南芥;B:ms188突变体拟南芥;C:pAtMYB80:SlMYB80CDS p2300互补ms188突变体,比例尺=2 cm,白色箭头代表恢复的角果;D‒F:亚历山大染色(D:野生型花药;E:ms188突变体花药;F:互补植株花药,白色箭头代表恢复的花粉;比例尺=100 μm);G:PCR鉴定转基因植株(M:DL2000 marker;1‒5:转基因植株;6:阳性对照;7:阴性对照(WT);8:阴性对照(ddH2O));H:RT-qPCR检测转基因植株中SlMYB80相对表达量;t-student检验,***代表P<0.01
Fig. 6 Full-length coding sequence (CDS) of SlMYB80 can partially complement the phenotype of the Arabidopsis ms188A: Col-0 wild-type (WT). B: ms188 mutant. C: pAtMYB80:SlMYB80CDS p2300 complementation of the ms188; bar=2 cm; the white arrow indicates the recovered siliques. D‒F: Alexander staining (D: wild-type anther; E: ms188 anther; F: anther of the complemented plants (the white arrow indicates the recovered pollen grains); bar=100 μm). G: PCR identification of transgenic plants (DL2000 marker; 1‒5: independent transgenic lines; 6: positive control; 7: negative control (WT); 8: negative control (ddH2O)). H: RT-qPCR analysis of the relative expressions of SlMYB80 in transgenic plants. t-student test, *** indicates P<0.01
| [1] | 李君明, 项朝阳, 王孝宣, 等. “十三五” 我国番茄产业现状及展望 [J]. 中国蔬菜, 2021(2): 13-20. |
| Li JM, Xiang CY, Wang XX, et al. Current situation of tomato industry in China during ‘the thirteenth Five-Year Plan’ period and future prospect [J]. China Veg, 2021(2): 13-20. | |
| [2] | 胡雅丹, 伍国强, 刘晨, 等. MYB转录因子在调控植物响应逆境胁迫中的作用 [J]. 生物技术通报, 2024, 40(6): 5-22. |
| Hu YD, Wu GQ, Liu C, et al. Roles of MYB transcription factor in regulating the responses of plants to stress [J]. Biotechnol Bull, 2024, 40(6): 5-22. | |
| [3] | 李翔, 李涛, 宫超, 等. 番茄雄性不育研究现状与展望 [J]. 广东农业科学, 2023, 50(2): 49-58. |
| Li X, Li T, Gong C, et al. Current situation and prospect of research on male sterility of tomato [J]. Guangdong Agric Sci, 2023, 50(2): 49-58. | |
| [4] | Patikoglou G, Burley SK. Eukaryotic transcription factor-DNA complexes [J]. Annu Rev Biophys Biomol Struct, 1997, 26: 289-325. |
| [5] | Prouse MB, Campbell MM. The interaction between MYB proteins and their target DNA binding sites [J]. Biochim Biophys Acta Gene Regul Mech, 2012, 1819(1): 67-77. |
| [6] | Zhang ZB, Zhu J, Gao JF, et al. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis [J]. Plant J, 2007, 52(3): 528-538. |
| [7] | Xiong SX, Lu JY, Lou Y, et al. The transcription factors MS188 and AMS form a complex to activate the expression of CYP703A2 for sporopollenin biosynthesis in Arabidopsis thaliana [J]. Plant J, 2016, 88(6): 936-946. |
| [8] | Wang K, Guo ZL, Zhou WT, et al. The regulation of sporopollenin biosynthesis genes for rapid pollen wall formation [J]. Plant Physiol, 2018, 178(1): 283-294. |
| [9] | Han Y, Zhou SD, Fan JJ, et al. OsMS188 is a key regulator of tapetum development and sporopollenin synthesis in rice [J]. Rice, 2021, 14(1): 4. |
| [10] | He Y, Wang J, Hu JG, et al. Knockdown of SlEMS1 causes male sterility in tomato [J]. Hortic Plant J, 2025, 11(2): 939-942. |
| [11] | Jeong HJ, Kang JH, Zhao MA, et al. Tomato male sterile 1035 is essential for pollen development and meiosis in anthers [J]. J Exp Bot, 2014, 65(22): 6693-6709. |
| [12] | Sun ZL, Cheng BH, Zhang YH, et al. SlTDF1: a key regulator of tapetum degradation and pollen development in tomato [J]. Plant Sci, 2025, 351: 112321. |
| [13] | Bao HH, Ding YM, Yang F, et al. Gene silencing, knockout and over-expression of a transcription factor ABORTED MICROSPORES (SlAMS) strongly affects pollen viability in tomato (Solanum lycopersicum) [J]. BMC Genomics, 2022, 23(): 346. |
| [14] | Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11 [J]. Mol Biol Evol, 2021, 38(7): 3022-3027. |
| [15] | Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees [J]. Mol Biol Evol, 1987, 4(4): 406-425. |
| [16] | He SC, Ma R, Liu ZJ, et al. Overexpression of BnaAGL11, a MADS-box transcription factor, regulates leaf morphogenesis and senescence in Brassica napus [J]. J Agric Food Chem, 2022, 70(11): 3420-3434. |
| [17] | Li ZJ, Peng RH, Tian YS, et al. Genome-wide identification and analysis of the MYB transcription factor superfamily in Solanum lycopersicum [J]. Plant Cell Physiol, 2016, 57(8): 1657-1677. |
| [18] | 杨晶婷, 余艳, 高晓蓉, 等. MYB类转录因子调控植物耐逆机制的研究进展 [J]. 杭州师范大学学报: 自然科学版, 2021, 20(6): 621-627. |
| Yang JT, Yu Y, Gao XR, et al. Advances in plant MYB transcription factors regulation mechanisms to various stresses [J]. J Hangzhou Norm Univ Nat Sci Ed, 2021, 20(6): 621-627. | |
| [19] | Fang Q, Wang Q, Mao H, et al. AtDIV2, an R-R-type MYB transcription factor of Arabidopsis, negatively regulates salt stress by modulating ABA signaling [J]. Plant Cell Rep, 2018, 37(11): 1499-1511. |
| [20] | Zhang X, Chen LC, Shi QH, et al. SlMYB102, an R2R3-type MYB gene, confers salt tolerance in transgenic tomato [J]. Plant Sci, 2020, 291: 110356. |
| [21] | Wang ML, Hao J, Chen XH, et al. SlMYB102 expression enhances low-temperature stress resistance in tomato plants [J]. PeerJ, 2020, 8: e10059. |
| [22] | Liu XJ, Ban Z, Yang YY, et al. The yellowhorn MYB transcription factor MYB30 is required for wax accumulation and drought tolerance [J]. Tree Physiol, 2024, 44(10): tpae111. |
| [23] | Zhang Y, Zhang B, Yang TW, et al. The GAMYB-like gene SlMYB33 mediates flowering and pollen development in tomato [J]. Hortic Res, 2020, 7: 133. |
| [24] | Stevens VA, Murray BG. Studies on heteromorphic self-incompatibility systems: the cytochemistry and ultrastructure of the tapetum of Primula obconica [J]. J Cell Sci, 1981, 50: 419-431. |
| [25] | Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant [J]. J Exp Bot, 2006, 57(11): 2709-2717. |
| [26] | Parish RW, Li SF. Death of a tapetum: a programme of developmental altruism [J]. Plant Sci, 2010, 178(2): 73-89. |
| [27] | Fu ZZ, Yu J, Cheng XW, et al. The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development [J]. Plant Cell, 2014, 26(4): 1512-1524. |
| [28] | Wang N, Li X, Zhu J, et al. Molecular and cellular mechanisms of photoperiod- and thermo-sensitive genic male sterility in plants [J]. Mol Plant, 2025, 18(1): 26-41. |
| [29] | Gu JN, Zhu J, Yu Y, et al. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis [J]. Plant J, 2014, 80(6): 1005-1013. |
| [30] | Lou Y, Xu XF, Zhu J, et al. The tapetal AHL family protein TEK determines nexine formation in the pollen wall [J]. Nat Commun, 2014, 5: 3855. |
| [31] | Phan HA, Iacuone S, Li SF, et al. The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana [J]. Plant Cell, 2011, 23(6): 2209-2224. |
| [32] | Zhu J, Lou Y, Xu XF, et al. A genetic pathway for tapetum development and function in Arabidopsis [J]. J Integr Plant Biol, 2011, 53(11): 892-900. |
| [33] | Ghelli R, Brunetti P, Marzi D, et al. The full-length auxin response factor 8 isoform ARF8.1 controls pollen cell wall formation and directly regulates TDF1, AMS and MS188 expression [J]. Plant J, 2023, 113(4): 851-865. |
| [34] | Ko SS, Li MJ, Ho YC, et al. Rice transcription factor GAMYB modulates bHLH142 and is homeostatically regulated by TDR during anther tapetal and pollen development [J]. J Exp Bot, 2021, 72(13): 4888-4903. |
| [35] | Lu JY, Xiong SX, Yin WZ, et al. MS1 a direct target of MS188 regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis [J]. J Exp Bot, 2020, 71(16): 4877-4889. |
| [36] | Millar AA, Gubler F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development [J]. Plant Cell, 2005, 17(3): 705-721. |
| [37] | 朱骏, 杨俊, 王晨, 等. 拟南芥转录因子AtMYB103的转录激活域鉴定 [J]. 上海交通大学学报: 农业科学版, 2009, 27(6): 611-614. |
| Zhu J, Yang J, Wang C, et al. Characterization of activation domain of the Arabidopsis transcription factor AtMYB103 [J]. J Shanghai Jiao Tong Univ Agric Sci, 2009, 27(6): 611-614. |
| [1] | 陈强, 于璎霏, 张颖, 张冲. 茉莉酸甲酯对薄皮甜瓜‘绿宝石’采后冷害的调控[J]. 生物技术通报, 2025, 41(9): 105-114. |
| [2] | 苏秀敏, 韩文清, 王佼, 李鹏, 王秋兰, 李万星, 曹晋军. 哈茨木霉M408的分离鉴定、生物学特性及对番茄早疫病的生防效果[J]. 生物技术通报, 2025, 41(9): 277-288. |
| [3] | 王斌, 林冲, 袁晓, 蒋园园, 王玉昆, 肖艳辉. bHLH转录因子UNE10克隆及其在丁香罗勒挥发性化合物合成调控中的功能[J]. 生物技术通报, 2025, 41(9): 207-218. |
| [4] | 黄诗宇, 田姗姗, 杨天为, 高曼熔, 张尚文. 赤苍藤WRI1基因家族的全基因组鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(8): 242-254. |
| [5] | 曾丹, 黄园, 王健, 张艳, 刘庆霞, 谷荣辉, 孙庆文, 陈宏宇. 铁皮石斛bZIP转录因子家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 197-210. |
| [6] | 李开杰, 吴瑶, 李丹丹. 红花CtbHLH128基因克隆及调控干旱胁迫应答功能研究[J]. 生物技术通报, 2025, 41(8): 234-241. |
| [7] | 牛景萍, 赵婧, 郭茜, 王书宏, 赵晋忠, 杜维俊, 殷丛丛, 岳爱琴. 基于WGCNA鉴定大豆抗大豆花叶病毒NAC转录因子及其诱导表达分析[J]. 生物技术通报, 2025, 41(7): 95-105. |
| [8] | 李霞, 张泽伟, 刘泽军, 王楠, 郭江波, 辛翠花, 张彤, 简磊. 马铃薯转录因子StMYB96的克隆及功能研究[J]. 生物技术通报, 2025, 41(7): 181-192. |
| [9] | 魏雨佳, 李岩, 康语涵, 弓晓楠, 杜敏, 涂岚, 石鹏, 于子涵, 孙彦, 张昆. 白颖苔草CrMYB4基因的克隆和表达分析[J]. 生物技术通报, 2025, 41(7): 248-260. |
| [10] | 李小欢, 陈相宇, 陶麒宇, 朱玲, 唐铭, 姚银安, 汪丽君. PtoMYB61对毛白杨木质素合成及耐盐性的影响[J]. 生物技术通报, 2025, 41(6): 284-296. |
| [11] | 李锐, 胡婷, 陈树溦, 王尧, 王计平. 紫苏PfMYB80转录因子正向调控花青素的生物合成[J]. 生物技术通报, 2025, 41(6): 243-255. |
| [12] | 郭涛, 艾丽皎, 邹世慧, 周玲, 李学梅. 山茶CjRAV1调控开花延迟的功能研究[J]. 生物技术通报, 2025, 41(6): 208-217. |
| [13] | 程珊, 王会伟, 陈晨, 朱雅婧, 李春鑫, 别海, 王树峰, 陈献功, 张向歌. 油莎豆MYB转录因子基因CeMYB154克隆及耐盐功能分析[J]. 生物技术通报, 2025, 41(6): 218-228. |
| [14] | 程慧娟, 王昕, 石小涛, 马东旭, 龚大春, 胡骏鹏, 谢智文. 转录因子CREA敲除对黑曲霉形态和分泌β-葡萄糖苷酶的影响[J]. 生物技术通报, 2025, 41(6): 344-354. |
| [15] | 罗嗣芳, 张祖铭, 谢丽芳, 郭紫晶, 陈兆星, 杨月华, 严翔, 张洪铭. 山金柑GATA基因家族全基因组鉴定及在果实发育中的表达分析[J]. 生物技术通报, 2025, 41(5): 218-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||