








生物技术通报 ›› 2025, Vol. 41 ›› Issue (6): 155-166.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1144
• 研究报告 • 上一篇
王斌1,2,3( ), 李健荣2, 占朝霞2, 袁晓2,3(
), 李健荣2, 占朝霞2, 袁晓2,3( )
)
收稿日期:2024-11-26
出版日期:2025-06-26
发布日期:2025-06-30
通讯作者:
袁晓,女,硕士,研究方向 :采后果蔬贮藏保鲜;E-mail: yxiao@sgu.edu.cn作者简介:王斌,男,博士,副教授,研究方向 :采后果蔬生物学;E-mail: b_wang@sgu.edu.cn
基金资助:
WANG Bin1,2,3( ), LI Jian-rong2, ZHAN Zhao-xia2, YUAN Xiao2,3(
), LI Jian-rong2, ZHAN Zhao-xia2, YUAN Xiao2,3( )
)
Received:2024-11-26
Published:2025-06-26
Online:2025-06-30
摘要:
目的 黄瓜(Cucumis sativus L.)是冷敏性蔬菜,采后贮藏易发生冷害,限制了低温贮藏技术在采后黄瓜上的应用。低温处理诱导CsGR-RBP3(glycine-rich RNA-binding protein 3)在采后黄瓜中的表达,克隆CsGR-RBP3,并研究其在采后黄瓜耐冷性中的功能,为抗冷黄瓜新品种的培育提供候选基因。 方法 以黄瓜果皮cDNA为模板克隆CsGR-RBP3编码序列,利用病毒介导的基因沉默(virus-induced gene silencing, VIGS)技术在采后黄瓜中抑制低温诱导的CsGR-RBP3表达,研究其表达降低对冷藏黄瓜冷害的影响,并利用RNA-Seq鉴定CsGR-RBP3调控采后黄瓜耐冷性的关键代谢通路。 结果 黄瓜CsGR-RBP3编码1个含有168个氨基酸残基的蛋白质,蛋白序列中含有保守的RRM(RNA-recognition motif)结构域,可能是一个线粒体相关蛋白,与拟南芥AtGR-RBPs的序列相似性较低。抑制低温诱导的CsGR-RBP3表达加重采后黄瓜冷害,并下调冷诱导相关基因的表达,降低线粒体抗氧化酶活性和相关基因的表达。此外,可能受CsGR-RBP3调控的差异表达基因(differentially expressed genes, DEGs)显著富集在苯丙氨酸代谢、苯丙烷素生物合成和植物‒病原菌互作途径中,这3个途径中的DEGs在低温诱导的CsGR-RBP3表达被抑制后整体下调。这些结果表明,CsGR-RBP3表达与冷藏黄瓜抗冷性正相关,其可能通过调控线粒体相关抗氧化酶活性,维持线粒体氧化还原平衡,并整合多个防御途径正向调控采后黄瓜抗冷性。 结论 CsGR-RBP3在黄瓜采后耐冷性中具有重要功能,其可能通过多个防御途径调控采后黄瓜耐冷性。
王斌, 李健荣, 占朝霞, 袁晓. CsGR-RBP3克隆及其在采后黄瓜耐冷性中的功能[J]. 生物技术通报, 2025, 41(6): 155-166.
WANG Bin, LI Jian-rong, ZHAN Zhao-xia, YUAN Xiao. Cloning of CsGR-RBP3 and Its Functional Roles in Cold Tolerance of Harvested Cucumber[J]. Biotechnology Bulletin, 2025, 41(6): 155-166.
基因 Gene | 正向序列 Forward sequence (5′‒3′) | 反向序列 Reverse sequence (5′‒3′) | 用途 Usage |
|---|---|---|---|
| GR-RBP3 | ATGCAATTATTCCCCACACGA | TCAGTTTTTGTCACCACCACCA | TA克隆 TA cloning |
| GR-RBP3 | atttggagaggacagggtaccATGCAATTATTCCCCACACGA | tctagaggatccccgggtaccGTTTTTGTCACCACCACCATAGC | 亚细胞定位载体构建 Construction of subcellular localization vectors |
| GR-RBP3 | agaaggcctccatggggatccTTCCAAGCTGTCTATCTTTCGAAC | cgtgagctcggtaccggatccGTTTTTGTCACCACCACCATAGC | VIGS实验载体构建 Vector construction of VIGS experiment |
| GR-RBP3 | GCAGCCTTCCAAGCTGTCTA | ACTTGCTGAACGCTACCCTC | 荧光定量PCR RT-qPCR |
| LEA5 | CTCCATTCTCTTCAGGCGGG | GTAACCGGTAACGGGGTCTG | |
| DREB1D | GCAGCTCACACGCTCTAAGT | GCGTTTGAGGAGGAGGTGTT | |
| ERD15 | CAAAGCTAAACCCGAACGCC | CCATGTCGAGGTTGTCACCA | |
| DLP4 | CTGAGGATGCGGGTTCAAGT | AGCTTTCTTGCACAGCTCCA | |
| NAA3 | GGAGAGGCTCAAACTTGGCT | ATGCCTCCAACCAGTGATGG | |
| CAT1 | GATCCTTACAGGCACCGACC | CCAACGGTCAACGAGGAGTT | |
| CAT3 | GAGAAGCTTTGCGTATGCGG | GGTGAGGACATTTGGGAGCA | |
| APX3 | TGGCCCAAAGGATGAGCTTT | GGGAGGGCGTTTGATTCGTA | |
| APX4 | TCCAGACCTGAAAACGCCAA | TTAACGCCACTTTGTGCTGC | |
| APX6 | GCCAAACTCAGCAACCTTGG | TCTGATAGCTCTCTCTTTCCGT | |
| POD41 | TTGCTTAGTGGGAGGCTGTG | GAGGTTGATTTCCGCATCGC | |
| POD42 | GTAGACCCTGTGCTGAACCC | CGTACTGTACAGCCTTGGGG | |
| POD64 | CTGTCAGGGCTGCAGCTTAT | GCCACGTTGTTCCCTACTGA | |
| Actin | AGGCCGTTCTGTCCCTCTAC | AGCAAGGTCCAAACGGAGAA |
表1 引物序列
Table 1 Primer sequence
基因 Gene | 正向序列 Forward sequence (5′‒3′) | 反向序列 Reverse sequence (5′‒3′) | 用途 Usage |
|---|---|---|---|
| GR-RBP3 | ATGCAATTATTCCCCACACGA | TCAGTTTTTGTCACCACCACCA | TA克隆 TA cloning |
| GR-RBP3 | atttggagaggacagggtaccATGCAATTATTCCCCACACGA | tctagaggatccccgggtaccGTTTTTGTCACCACCACCATAGC | 亚细胞定位载体构建 Construction of subcellular localization vectors |
| GR-RBP3 | agaaggcctccatggggatccTTCCAAGCTGTCTATCTTTCGAAC | cgtgagctcggtaccggatccGTTTTTGTCACCACCACCATAGC | VIGS实验载体构建 Vector construction of VIGS experiment |
| GR-RBP3 | GCAGCCTTCCAAGCTGTCTA | ACTTGCTGAACGCTACCCTC | 荧光定量PCR RT-qPCR |
| LEA5 | CTCCATTCTCTTCAGGCGGG | GTAACCGGTAACGGGGTCTG | |
| DREB1D | GCAGCTCACACGCTCTAAGT | GCGTTTGAGGAGGAGGTGTT | |
| ERD15 | CAAAGCTAAACCCGAACGCC | CCATGTCGAGGTTGTCACCA | |
| DLP4 | CTGAGGATGCGGGTTCAAGT | AGCTTTCTTGCACAGCTCCA | |
| NAA3 | GGAGAGGCTCAAACTTGGCT | ATGCCTCCAACCAGTGATGG | |
| CAT1 | GATCCTTACAGGCACCGACC | CCAACGGTCAACGAGGAGTT | |
| CAT3 | GAGAAGCTTTGCGTATGCGG | GGTGAGGACATTTGGGAGCA | |
| APX3 | TGGCCCAAAGGATGAGCTTT | GGGAGGGCGTTTGATTCGTA | |
| APX4 | TCCAGACCTGAAAACGCCAA | TTAACGCCACTTTGTGCTGC | |
| APX6 | GCCAAACTCAGCAACCTTGG | TCTGATAGCTCTCTCTTTCCGT | |
| POD41 | TTGCTTAGTGGGAGGCTGTG | GAGGTTGATTTCCGCATCGC | |
| POD42 | GTAGACCCTGTGCTGAACCC | CGTACTGTACAGCCTTGGGG | |
| POD64 | CTGTCAGGGCTGCAGCTTAT | GCCACGTTGTTCCCTACTGA | |
| Actin | AGGCCGTTCTGTCCCTCTAC | AGCAAGGTCCAAACGGAGAA |
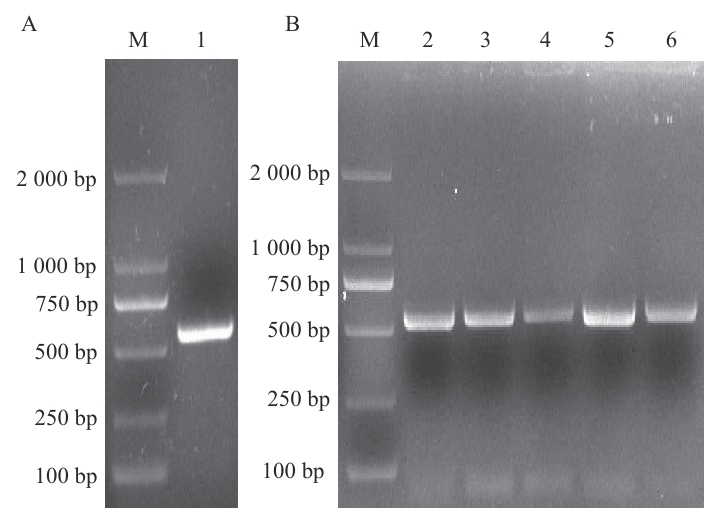
图1 CsGR-RBP3编码序列的PCR产物电泳图A:以黄瓜果皮cDNA为模板扩增的PCR产物;B:以重组质粒为模板扩增的PCR产物;M:DNA标准物;1‒6:PCR产物
Fig. 1 Electrophoresis images of PCR products of CsGR-RBP3 coding sequencesA: The PCR product amplified by cucumber cDNA as template. B: The PCR product amplified by recombinant plasmid as template. M: DNA marker; 1‒6: PCR products

图2 CsGR-RBP3与拟南芥AtGR-RBPs多序列比较(A)和RRM结构域保守性分析(B)蓝色方框内为RRM保守域序列
Fig. 2 Multi sequence comparison (A) and RRM domain conservation analysis (B) of CsGR-RBP3 and Arabidopsis AtGR-RBP proteinsRRM conserved domain is indicated in blue box

图4 抑制低温诱导的CsGR-RBP3表达对冷藏黄瓜冷害的影响A:黄瓜果实叶绿素荧光;B:叶绿素荧光参数Fv/Fm;C:叶绿素荧光参数Y(NO);D:相对电导率;*表示在P<0.05水平上差异显著。下同
Fig. 4 Effects of reducing cold-inducible CsGR-RBP3 expression on the chilling injury of cold-stored cucumber fruitA: Chlorophyll fluorescence of cucumber fruit. B: Chlorophyll fluorescence parameter, Fv/Fm. C: Chlorophyll fluorescence parameter, Y(NO). D: Relative electrical conductivity. * indicates significant difference at P<0.05 level. The same below

图5 抑制低温诱导的CsGR-RBP3表达对冷藏黄瓜低温响应基因表达的影响
Fig. 5 Effects of reducing cold-inducible CsGR-RBP3 expression on the expressions of cold-responsive genes in cold-stored cucumber fruit

图6 抑制低温诱导的CsGR-RBP3表达对冷藏黄瓜线粒体抗氧化酶活性和基因表达的影响
Fig. 6 Effects of reducing cold-inducible CsGR-RBP3 expressions on mitochondrial antioxidant enzyme activity and gene expression in cold-stored cucumber fruit

图7 抑制低温诱导的CsGR-RBP3表达引起的差异表达基因功能富集和表达模式分析A:差异表达基因的韦恩图;B:2个比较组重叠的差异表达基因的KEGG富集结果;C:苯丙氨酸代谢途径中差异表达基因的表达模式;D:植物与病原菌互作途径中差异表达基因的表达模式;E:苯丙烷素生物合成途径中差异表达基因的表达模式
Fig. 7 Functional enrichment and expression pattern analysis of differentially expressed genes caused by reducing cold-inducible CsGR-RBP3 expressionA: Venn diagram of differentially expressed genes (DEGs). B: KEGG enrichment of overlapped DEGs from two comparison groups. C: Expression patterns of DEGs in the phenylalanine metabolism pathway. D: Expression patterns of DEGs in the plant-pathogen interaction pathway. E: Expression patterns of DEGs in the phenylpropanoid biosynthesis pathway
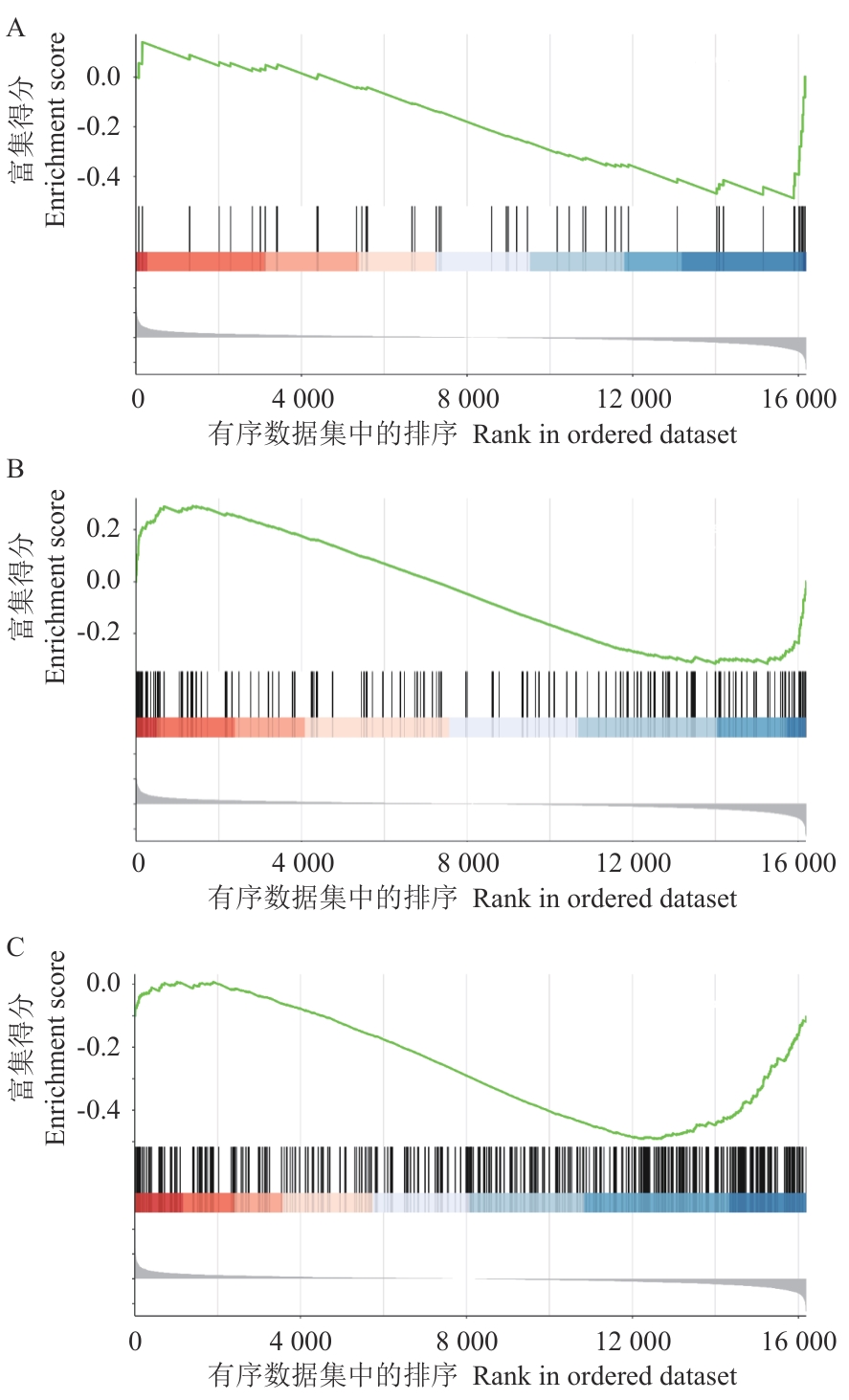
图8 抑制低温诱导的CsGR-RBP3表达引起的差异表达基因GSEA分析A:苯丙氨酸代谢途径;B:苯丙烷素生物合成途径;C:植物-病原菌互作途径
Fig. 8 GSEA analysis of differentially expressed genes caused by reducing cold-inducible CsGR-RBP3 expressionA: Phenylalanine metabolism pathway. B: Phenylpropanoid biosynthesis pathway. C: Plant-pathogen interaction pathway
| 1 | Franzoni G, Spadafora ND, Sirangelo TM, et al. Biochemical and molecular changes in peach fruit exposed to cold stress conditions [J]. Mol Hortic, 2023, 3(1): 24. |
| 2 | Rodrigues M, Ordoñez-Trejo EJ, Rasori A, et al. Dissecting postharvest chilling injuries in pome and stone fruit through integrated omics [J]. Front Plant Sci, 2024, 14: 1272986. |
| 3 | Zhang YP, Lin D, Yan RY, et al. Amelioration of chilling injury by fucoidan in cold-stored cucumber via membrane lipid metabolism regulation [J]. Foods, 2023, 12(2): 301. |
| 4 | 王斌, 杨盼迪, 王玉昆, 等. 采后黄瓜在冷驯化处理过程中的转录组变化 [J]. 西北农业学报, 2024, 33(2): 256-270. |
| Wang B, Yang PD, Wang YK, et al. Transcriptomic changes of postharvest cucumber during cold acclimation [J]. Acta Agric Boreali Occidentalis Sin, 2024, 33(2): 256-270. | |
| 5 | Wang JD, Zhao YQ, Ma ZQ, et al. Hydrogen sulfide treatment alleviates chilling injury in cucumber fruit by regulating antioxidant capacity, energy metabolism and proline metabolism [J]. Foods, 2022, 11(18): 2749. |
| 6 | 王斌, 袁晓, 蒋园园, 等. 采后黄瓜冷害及耐冷性调控研究进展 [J]. 江苏农业学报, 2023, 39(2): 596-608. |
| Wang B, Yuan X, Jiang YY, et al. Research advances in chilling injury and the regulation of chilling tolerance of postharvest cucumber fruit [J]. Jiangsu J Agric Sci, 2023, 39(2): 596-608. | |
| 7 | Shan YX, Zhang DD, Luo ZS, et al. Advances in chilling injury of postharvest fruit and vegetable: Extracellular ATP aspects [J]. Compr Rev Food Sci Food Saf, 2022, 21(5): 4251-4273. |
| 8 | Min DD, Li FJ, Ali M, et al. Application of methyl jasmonate to control chilling tolerance of postharvest fruit and vegetables: a meta-analysis and eliciting metabolism review [J]. Crit Rev Food Sci Nutr, 2024, 64(33): 12878-12891. |
| 9 | 张瑞平, 杨峰, 陈乐章, 等. 交替呼吸途径在油菜素内酯调控本氏烟响应高温胁迫中的作用研究 [J]. 生物技术通报, 2020, 36(10): 8-14. |
| Zhang RP, Yang F, Chen LZ, et al. Role of alternative respiratory pathway in brassinosteroids inducing heat stress response in Nicotiana benthamiana [J]. Biotechnol Bull, 2020, 36(10): 8-14. | |
| 10 | Zu XF, Luo LL, Wang Z, et al. A mitochondrial pentatricopeptide repeat protein enhances cold tolerance by modulating mitochondrial superoxide in rice [J]. Nat Commun, 2023, 14(1): 6789. |
| 11 | Erdal S, Genisel M, Turk H, et al. Modulation of alternative oxidase to enhance tolerance against cold stress of chickpea by chemical treatments [J]. J Plant Physiol, 2015, 175: 95-101. |
| 12 | Ma LQ, Cheng K, Li JY, et al. Roles of plant glycine-rich RNA-binding proteins in development and stress responses [J]. Int J Mol Sci, 2021, 22(11): 5849. |
| 13 | Kim JY, Park SJ, Jang B, et al. Functional characterization of a glycine-rich RNA-binding protein 2 in Arabidopsis thaliana under abiotic stress conditions [J]. Plant J, 2007, 50(3): 439-451. |
| 14 | Kim MK, Jung HJ, Kim DH, et al. Characterization of glycine-rich RNA-binding proteins in Brassica napus under stress conditions [J]. Physiol Plant, 2012, 146(3): 297-307. |
| 15 | 王斌, 武春爽, 汤冰琳, 等. 黄瓜果实CsMYB62克隆及其对CsGR-RBP3表达的调控 [J]. 核农学报, 2022, 36(5): 907-917. |
| Wang B, Wu CS, Tang BL, et al. Cloning of CsMYB62 and its regulations on CsGR-RBP3 expression in cucumber fruit [J]. J Nucl Agric Sci, 2022, 36(5): 907-917. | |
| 16 | 王斌, 黄泳谚, 易景怡, 等. 黄瓜GR-RBP3启动子克隆及低温对其活性的诱导 [J]. 山东农业科学, 2022, 54(7): 15-23. |
| Wang B, Huang YY, Yi JY, et al. Molecular cloning of cucumber GR-RBP3 promoter and induction of low temperature on its activity [J]. Shandong Agric Sci, 2022, 54(7): 15-23. | |
| 17 | Wang YK, Ye H, Lin W, et al. Cinnamic acid application inhibits the browning of cold-stored taro slices by maintaining membrane function, reducing flavonoid biosynthesis and enhancing glutathione metabolism [J]. Postharvest Biol Technol, 2024, 218: 113180. |
| 18 | Crooks GE, Hon G, Chandonia JM, et al. WebLogo: a sequence logo generator [J]. Genome Res, 2004, 14(6): 1188-1190. |
| 19 | Teufel F, Almagro Armenteros JJ, Johansen AR, et al. SignalP 6.0 predicts all five types of signal peptides using protein language models [J]. Nat Biotechnol, 2022, 40(7): 1023-1025. |
| 20 | Wang B, Wang G, Wang YK, et al. A cold-inducible MYB like transcription factor, CsHHO2, positively regulates chilling tolerance of cucumber fruit by enhancing CsGR-RBP3 expression [J]. Postharvest Biol Technol, 2024, 218: 113172. |
| 21 | Wang B, Wang G, Zhu SJ. DNA damage inducible protein 1 is involved in cold adaption of harvested cucumber fruit [J]. Front Plant Sci, 2020, 10: 1723. |
| 22 | 井广琴. 一氧化氮对冷藏肥城桃果实线粒体抗氧化防御和膜脂代谢的调控作用 [D]. 泰安: 山东农业大学, 2015. |
| Jing GQ. Effects of nitric oxide on mitochondrial antioxidant defense and membrane lipid metabolism of frozen Feicheng peach fruit [D]. Tai'an: Shandong Agricultural University, 2015. | |
| 23 | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method [J]. Nat Protoc, 2008, 3(6): 1101-1108. |
| 24 | Cheng K, Zhang CJ, Lu Y, et al. The Glycine-rich RNA-binding protein is a vital post-transcriptional regulator in crops [J]. Plants, 2023, 12(19): 3504. |
| 25 | Czolpinska M, Rurek M. Plant Glycine-rich proteins in stress response: an emerging, still prospective story [J]. Front Plant Sci, 2018, 9: 302. |
| 26 | 王斌, 林薇, 肖艳辉, 等. 植物富含甘氨酸蛋白家族功能研究进展 [J]. 生物技术通报, 2025, 41(2): 1-17. |
| Wang B, Lin W, Xiao YH, et al. Research progress in the function of glycine-rich protein family in plants [J]. Biotechnol Bull, 2025, 41(2): 1-17. | |
| 27 | Ciuzan O, Hancock J, Pamfil D, et al. The evolutionarily conserved multifunctional glycine-rich RNA-binding proteins play key roles in development and stress adaptation [J]. Physiol Plant, 2015, 153(1): 1-11. |
| 28 | Saha B, Borovskii G, Panda SK. Alternative oxidase and plant stress tolerance [J]. Plant Signal Behav, 2016, 11(12): e1256530. |
| 29 | Wang B, Wang G, Shen F, et al. A Glycine-rich RNA-binding protein, CsGR-RBP3, is involved in defense responses against cold stress in harvested cucumber (Cucumis sativus L.) fruit [J]. Front Plant Sci, 2018, 9: 540. |
| 30 | Zhang YJ, Mo YJ, Li JY, et al. Divergence in regulatory mechanisms of GR-RBP genes in different plants under abiotic stress [J]. Sci Rep, 2024, 14(1): 8743. |
| 31 | Shi XW, Germain A, Hanson MR, et al. RNA recognition motif-containing protein ORRM4 broadly affects mitochondrial RNA editing and impacts plant development and flowering [J]. Plant Physiol, 2016, 170(1): 294-309. |
| 32 | Shi XW, Castandet B, Germain A, et al. ORRM5, an RNA recognition motif-containing protein, has a unique effect on mitochondrial RNA editing [J]. J Exp Bot, 2017, 68(11): 2833-2847. |
| 33 | 刘传和, 贺涵, 何秀古, 等. 转录组与代谢组联合分析菠萝网纱覆盖防寒机制 [J]. 生物技术通报, 2022, 38(11): 58-69. |
| Liu CH, He H, He XG, et al. Unveiling the mechanisms of pineapple responding to anti-chilling by gauze covering in winter via transcriptome and metabolome profiling [J]. Biotechnol Bull, 2022, 38(11): 58-69. | |
| 34 | Wang B, Wu CS, Wang G, et al. Transcriptomic analysis reveals a role of phenylpropanoid pathway in the enhancement of chilling tolerance by pre-storage cold acclimation in cucumber fruit [J]. Sci Hortic, 2021, 288: 110282. |
| 35 | Gusain S, Joshi S, Joshi R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants [J]. Plant Physiol Biochem, 2023, 197: 107646. |
| 36 | Gao H, Wu FZ. Physiological and transcriptomic analysis of tomato in response to sub-optimal temperature stress [J]. Plant Signal Behav, 2024, 19(1): 2332018. |
| 37 | 黄泳谚, 易景怡, 王斌. 采后黄瓜响应低温胁迫的转录组学分析 [J]. 热带作物学报, 2023, 44(1): 35-48. |
| Huang YY, Yi JY, Wang B. Transcriptomic analysis of harvested cucumber in response to cold stress [J]. Chin J Trop Crops, 2023, 44(1): 35-48. |
| [1] | 范宗强, 冯靖涵, 郑丽雪, 王硕, 彭向前, 陈芳. 枯草芽孢杆菌B579对黄瓜枯萎病的防治及其诱导抗性研究[J]. 生物技术通报, 2024, 40(7): 226-234. |
| [2] | 胡永波, 雷雨田, 杨永森, 陈馨, 林黄昉, 林碧英, 刘爽, 毕格, 申宝营. 黄瓜和南瓜Bcl-2相关抗凋亡家族全基因组鉴定与表达模式分析[J]. 生物技术通报, 2024, 40(6): 219-237. |
| [3] | 肖雅茹, 贾婷婷, 罗丹, 武喆, 李丽霞. 黄瓜CsERF025L转录因子的克隆及表达分析[J]. 生物技术通报, 2024, 40(4): 159-166. |
| [4] | 褚睿, 李昭轩, 张学青, 杨东亚, 曹行行, 张雪艳. 黄瓜枯萎病拮抗芽孢杆菌的筛选、鉴定及其生防潜力[J]. 生物技术通报, 2023, 39(8): 262-271. |
| [5] | 谢洋, 邢雨蒙, 周国彦, 刘美妍, 银珊珊, 闫立英. 黄瓜二倍体及其同源四倍体果实转录组分析[J]. 生物技术通报, 2023, 39(3): 152-162. |
| [6] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [7] | 杨东亚, 祁瑞雪, 李昭轩, 林薇, 马慧, 张雪艳. 黄瓜茄病镰刀菌拮抗芽孢杆菌的筛选、鉴定及促生效果[J]. 生物技术通报, 2023, 39(2): 211-220. |
| [8] | 谢洋, 周国彦, 苏航, 邢雨蒙, 闫立英. PEG模拟干旱条件下黄瓜种子发芽前后转录组分析[J]. 生物技术通报, 2023, 39(12): 148-157. |
| [9] | 杨艳, 莫雨杏, 周祎, 陈惠明, 肖浪涛, 王若仲. 黄瓜内果皮汁液对种子萌发的影响[J]. 生物技术通报, 2023, 39(12): 158-168. |
| [10] | 张林林, 沈虎生, 杨冰, 何梦菡, 朴凤植, 申顺善. 生防细菌HK11-9对黄瓜棒孢叶斑病的防病能力及其鉴定[J]. 生物技术通报, 2023, 39(12): 209-218. |
| [11] | 李霁虹, 荆玉玲, 马桂珍, 郭荣君, 李世东. 无色杆菌77的基因组构成及其趋化和耐药特性[J]. 生物技术通报, 2022, 38(9): 136-146. |
| [12] | 陈宏艳, 李小二, 李忠光. 糖信号及其在植物响应逆境胁迫中的作用[J]. 生物技术通报, 2022, 38(7): 80-89. |
| [13] | 周国彦, 银珊珊, 高佳鑫, 武春成, 闫立英, 谢洋. 黄瓜AHP基因家族的鉴定及其非生物胁迫表达分析[J]. 生物技术通报, 2022, 38(6): 112-119. |
| [14] | 武杞蔓, 田诗涵, 李昀烨, 潘英杰, 张颖. 微生物菌肥对设施黄瓜生长、产量及品质的影响[J]. 生物技术通报, 2022, 38(1): 125-131. |
| [15] | 张美君, 吴庆, 尹翠, 王妮, 马晓庆, 马晓霞, 曹云娥. 尖镰孢黄瓜专化型枯萎病菌拮抗菌的筛选、鉴定及培养条件优化[J]. 生物技术通报, 2020, 36(9): 125-136. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||