








生物技术通报 ›› 2025, Vol. 41 ›› Issue (9): 302-313.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0271
• 研究报告 • 上一篇
廉少杰1( ), 唐胜硕1, 康传利1,2(
), 唐胜硕1, 康传利1,2( ), 刘磊1, 郑德强1,2, 杜帅1, 汤丽伟1, 张美霞1, 刘蔷1
), 刘磊1, 郑德强1,2, 杜帅1, 汤丽伟1, 张美霞1, 刘蔷1
收稿日期:2025-03-13
出版日期:2025-09-26
发布日期:2025-09-24
通讯作者:
康传利,男,硕士,高级工程师,研究方向 :发酵工程;E-mail: kangchuanli@focusfreda.com作者简介:廉少杰,男,硕士,高级工程师,研究方向 :发酵工程;E-mail: 17705371190@163.com
LIAN Shao-jie1( ), TANG Sheng-shuo1, KANG Chuan-li1,2(
), TANG Sheng-shuo1, KANG Chuan-li1,2( ), LIU Lei1, ZHENG De-qiang1,2, DU Shuai1, TANG Li-wei1, ZHANG Mei-xia1, LIU Qiang1
), LIU Lei1, ZHENG De-qiang1,2, DU Shuai1, TANG Li-wei1, ZHANG Mei-xia1, LIU Qiang1
Received:2025-03-13
Published:2025-09-26
Online:2025-09-24
摘要:
目的 银耳多糖因具有高分子量、高黏度等特性限制了其生物活性的发挥与应用。筛选高产银耳多糖酶的菌株并优化其发酵条件,以实现酶的量产,从而通过酶降解法降低银耳多糖分子量。 方法 根据微生物产酶分解银耳多糖的特性,从腐烂银耳样本中分离出以银耳多糖为唯一碳源的菌株,结合银耳多糖降解率测定与3,5-二硝基水杨酸(DNS)酶活测定法筛选出高产银耳多糖菌株。采用单因素实验与响应面法优化高产银耳多糖酶菌株的发酵培养基及发酵条件,并对所产银耳多糖酶的催化特性进行分析。 结果 从腐烂银耳样本中分离出40株能够以银耳多糖为唯一碳源的菌株,其中10株具有较高降解银耳多糖能力,酶活最高的菌株为Y3522,经16S rRNA基因测序鉴定为属间芽胞杆菌(Mesobacillus)。Y3522优化后的最佳发酵培养基组分为:银耳多糖(分子量为1 250 kD)8.07 g/L,酪蛋白胨25.47 g/L,K2HPO4 7.11 g/L,NaCl 2.0 g/L,MgSO4·7H2O 1.0 g/L;最佳发酵条件为温度35 ℃、pH 7.5、接种量3%、转速250 r/min、发酵时间18-24 h。优化后酶活力提升60.1%。Y3522来源的银耳多糖酶的最适催化条件为pH 7.5、温度35 ℃。此外,20%(V/V)粗酶液可将3 000 kD银耳多糖(0.5%,m/V)在30、60、90、120 min分别降解至922、308、85、18 kD。 结论 分离并鉴定了高产银耳多糖酶菌株为属间芽胞杆菌Y3522,并优化了其产酶发酵体系,所产酶展现出对银耳多糖分子量高效的降解能力。
廉少杰, 唐胜硕, 康传利, 刘磊, 郑德强, 杜帅, 汤丽伟, 张美霞, 刘蔷. 高产银耳多糖酶菌株的分离、鉴定、发酵条件优化及其酶的特性分析[J]. 生物技术通报, 2025, 41(9): 302-313.
LIAN Shao-jie, TANG Sheng-shuo, KANG Chuan-li, LIU Lei, ZHENG De-qiang, DU Shuai, TANG Li-wei, ZHANG Mei-xia, LIU Qiang. Isolation, Identification, Optimization of Fermentation Conditions of High-yield Tremella fuciformis Polysaccharides Enzyme-producing Strain and Its Enzyme Characteristics Analysis[J]. Biotechnology Bulletin, 2025, 41(9): 302-313.
水平 Level | 因素 Factor | ||
|---|---|---|---|
A 银耳多糖浓度 TFPs concentration (g/L) | B 酪蛋白胨浓度 Casein tryptone concentration (g/L) | C K2HPO4浓度 K2HPO4 concentration (g/L) | |
| -1 | 7.5 | 22.5 | 6.0 |
| 0 | 8.0 | 25.0 | 7.0 |
| 1 | 8.5 | 27.5 | 8.0 |
表1 发酵培养基优化响应面试验设计
Table 1 Response surface design for the optimization of fermentation medium
水平 Level | 因素 Factor | ||
|---|---|---|---|
A 银耳多糖浓度 TFPs concentration (g/L) | B 酪蛋白胨浓度 Casein tryptone concentration (g/L) | C K2HPO4浓度 K2HPO4 concentration (g/L) | |
| -1 | 7.5 | 22.5 | 6.0 |
| 0 | 8.0 | 25.0 | 7.0 |
| 1 | 8.5 | 27.5 | 8.0 |
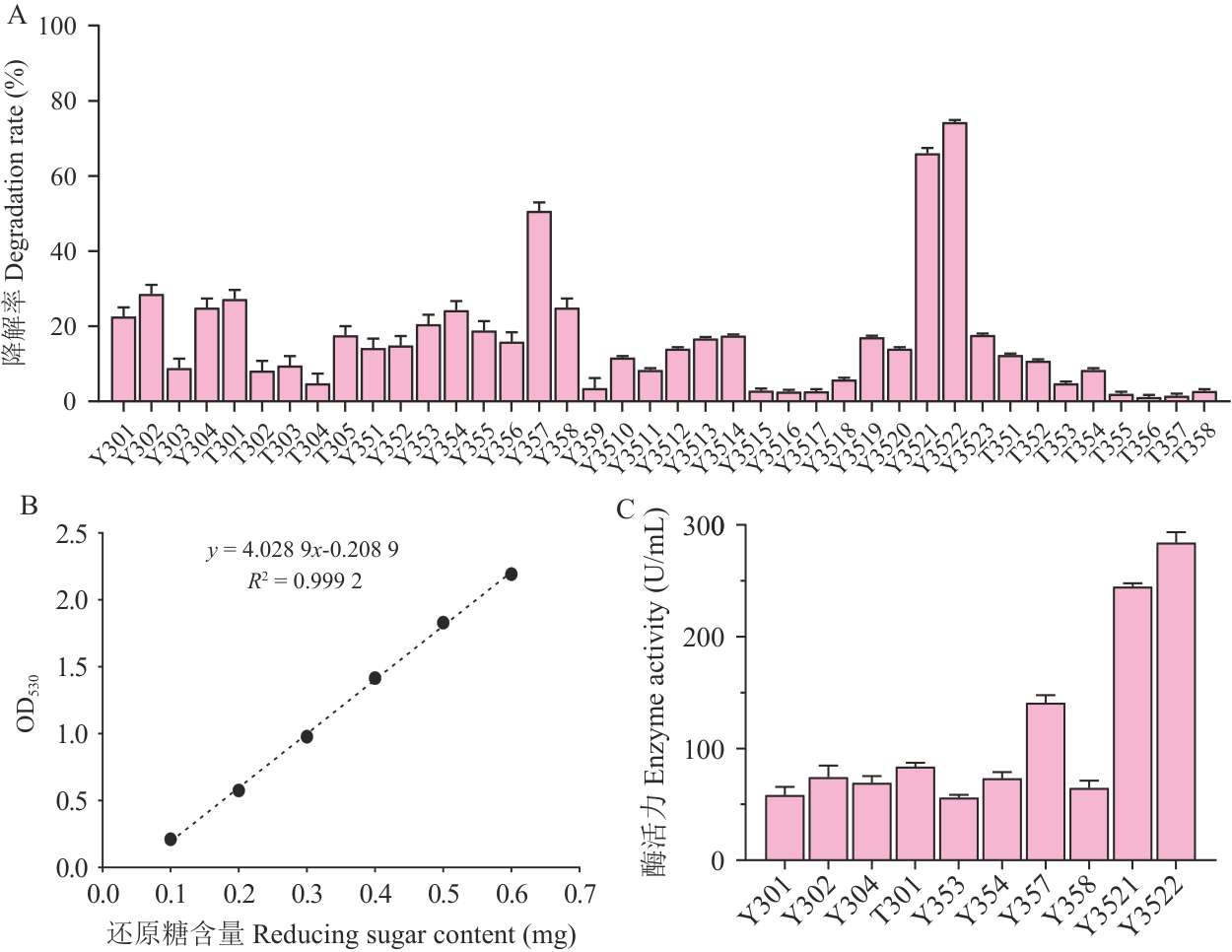
图1 高产银耳多糖酶菌株的筛选A: 银耳多糖降解实验结果; B: 葡萄糖醛酸标准曲线; C: 酶活测定结果
Fig. 1 Screening of strains producing high-yield TFPs enzymeA: Experimental results of TFPs degradation. B: Standard curve of glucuronic acid. C: Enzyme activity assay results

图2 菌株Y3522的形态学与分子生物学鉴定A: 菌落形态;B: 菌体形态;C: 基于16S rRNA基因的系统发育分析
Fig. 2 Morphological and molecular biology identification of strain Y3522A: Morphology of colony; B: morphology of cell; C: phylogenetic analysis based on the 16S rRNA gene
序号 Test No. | A 银耳多糖 TFPs | B酪蛋白胨 Casein peptone | C K2HPO4 | Y 银耳多糖酶活 Enzyme activity of TFPs(U/mL) |
|---|---|---|---|---|
| 1 | 7.5 | 22.5 | 7.0 | 332.47 |
| 2 | 8.5 | 22.5 | 7.0 | 333.33 |
| 3 | 7.5 | 27.5 | 7.0 | 333.36 |
| 4 | 8.5 | 27.5 | 7.0 | 348.39 |
| 5 | 7.5 | 25.0 | 6.0 | 334.14 |
| 6 | 8.5 | 25.0 | 6.0 | 333.28 |
| 7 | 7.5 | 25.0 | 8.0 | 327.40 |
| 8 | 8.5 | 25.0 | 8.0 | 349.77 |
| 9 | 8.0 | 22.5 | 6.0 | 340.28 |
| 10 | 8.0 | 27.5 | 6.0 | 348.17 |
| 11 | 8.0 | 22.5 | 8.0 | 343.75 |
| 12 | 8.0 | 27.5 | 8.0 | 349.75 |
| 13 | 8.0 | 25.0 | 7.0 | 362.98 |
| 14 | 8.0 | 25.0 | 7.0 | 372.05 |
| 15 | 8.0 | 25.0 | 7.0 | 370.73 |
| 16 | 8.0 | 25.0 | 7.0 | 373.57 |
| 17 | 8.0 | 25.0 | 7.0 | 364.28 |
表2 响应面试验设计及结果
Table 2 Response surface design and test results
序号 Test No. | A 银耳多糖 TFPs | B酪蛋白胨 Casein peptone | C K2HPO4 | Y 银耳多糖酶活 Enzyme activity of TFPs(U/mL) |
|---|---|---|---|---|
| 1 | 7.5 | 22.5 | 7.0 | 332.47 |
| 2 | 8.5 | 22.5 | 7.0 | 333.33 |
| 3 | 7.5 | 27.5 | 7.0 | 333.36 |
| 4 | 8.5 | 27.5 | 7.0 | 348.39 |
| 5 | 7.5 | 25.0 | 6.0 | 334.14 |
| 6 | 8.5 | 25.0 | 6.0 | 333.28 |
| 7 | 7.5 | 25.0 | 8.0 | 327.40 |
| 8 | 8.5 | 25.0 | 8.0 | 349.77 |
| 9 | 8.0 | 22.5 | 6.0 | 340.28 |
| 10 | 8.0 | 27.5 | 6.0 | 348.17 |
| 11 | 8.0 | 22.5 | 8.0 | 343.75 |
| 12 | 8.0 | 27.5 | 8.0 | 349.75 |
| 13 | 8.0 | 25.0 | 7.0 | 362.98 |
| 14 | 8.0 | 25.0 | 7.0 | 372.05 |
| 15 | 8.0 | 25.0 | 7.0 | 370.73 |
| 16 | 8.0 | 25.0 | 7.0 | 373.57 |
| 17 | 8.0 | 25.0 | 7.0 | 364.28 |
来源 Source | 平方和 Sum of square | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型 Model | 3 727.49 | 9 | 414.17 | 29.42 | < 0.001 | *** |
| A | 174.85 | 1 | 174.85 | 12.42 | 0.010 | ** |
| B | 111.30 | 1 | 111.30 | 7.91 | 0.026 | * |
| C | 27.38 | 1 | 27.38 | 1.94 | 0.206 | |
| AB | 50.20 | 1 | 50.20 | 3.57 | 0.101 | |
| AC | 134.91 | 1 | 134.91 | 9.58 | 0.017 | * |
| BC | 0.893 0 | 1 | 0.893 0 | 0.063 4 | 0.808 | |
| A2 | 1 784.57 | 1 | 1 784.57 | 126.75 | < 0.001 | *** |
| B2 | 532.63 | 1 | 532.63 | 37.83 | < 0.001 | *** |
| C2 | 605.03 | 1 | 605.03 | 42.97 | < 0.001 | *** |
| 残差 Residual | 98.55 | 7 | 14.08 | |||
| 失拟向 Lack of fit | 7.24 | 3 | 2.41 | 0.105 7 | 0.952 | Not significant |
| 纯误差 Pure error | 91.31 | 4 | 22.83 | |||
| 总差 Total error | 3 826.0 | 16 |
表3 回归模型方差分析结果
Table 3 Results of variance analysis by regression model
来源 Source | 平方和 Sum of square | 自由度 Freedom | 均方 Mean | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型 Model | 3 727.49 | 9 | 414.17 | 29.42 | < 0.001 | *** |
| A | 174.85 | 1 | 174.85 | 12.42 | 0.010 | ** |
| B | 111.30 | 1 | 111.30 | 7.91 | 0.026 | * |
| C | 27.38 | 1 | 27.38 | 1.94 | 0.206 | |
| AB | 50.20 | 1 | 50.20 | 3.57 | 0.101 | |
| AC | 134.91 | 1 | 134.91 | 9.58 | 0.017 | * |
| BC | 0.893 0 | 1 | 0.893 0 | 0.063 4 | 0.808 | |
| A2 | 1 784.57 | 1 | 1 784.57 | 126.75 | < 0.001 | *** |
| B2 | 532.63 | 1 | 532.63 | 37.83 | < 0.001 | *** |
| C2 | 605.03 | 1 | 605.03 | 42.97 | < 0.001 | *** |
| 残差 Residual | 98.55 | 7 | 14.08 | |||
| 失拟向 Lack of fit | 7.24 | 3 | 2.41 | 0.105 7 | 0.952 | Not significant |
| 纯误差 Pure error | 91.31 | 4 | 22.83 | |||
| 总差 Total error | 3 826.0 | 16 |
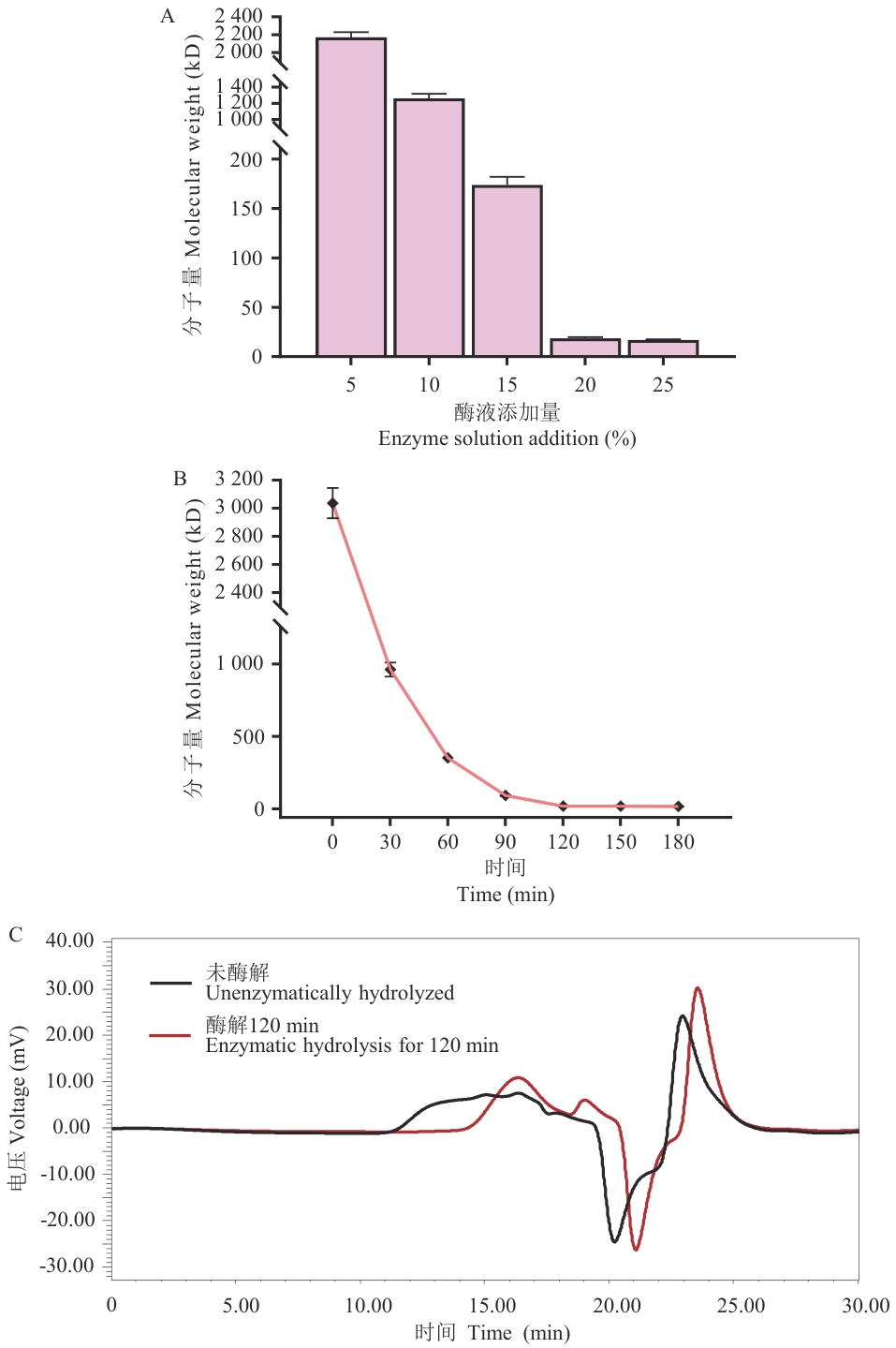
图7 不同条件下酶解液的主成分分子量的变化及酶解前后银耳多糖的GPC图谱
Fig. 7 Variations in the molecular weights of the main components of enzymatic hydrolysate under different conditions and GPC spectra of TFPs before and after enzymatic hydrolysis
| [1] | 叶俊博, 杨德红, 王坤, 等. 菌中之冠——银耳 [J]. 大学化学, 2023, 38(7): 206-211. |
| Ye JB, Yang DH, Wang K, et al. Crown of edible fungi: Tremella [J]. Univ Chem, 2023, 38(7): 206-211. | |
| [2] | Lin BB, Huang GL. Extraction, isolation, purification, derivatization, bioactivity, structure-activity relationship, and application of polysaccharides from White jellyfungus [J]. Biotechnol Bioeng, 2022, 119(6): 1359-1379. |
| [3] | Fu H, You SQ, Zhao D, et al. Tremella fuciformis polysaccharides inhibit UVA-induced photodamage of human dermal fibroblast cells by activating up-regulating Nrf2/Keap1 pathways [J]. J Cosmet Dermatol, 2021, 20(12): 4052-4059. |
| [4] | Khan TJ, Xu XF, Xie XL, et al. Tremella fuciformis crude polysaccharides attenuates steatosis and suppresses inflammation in diet-induced NAFLD mice [J]. Curr Issues Mol Biol, 2022, 44(3): 1224-1234. |
| [5] | Qin LL, Su GQ, Wu C, et al. Effects of Tremella fuciformis extract on growth performance, biochemical and immunological parameters of weaned piglets challenged with lipopolysaccharide [J]. Animal Production Science, 2022, 62(5): 462-469. |
| [6] | Ma X, Yang M, He Y, et al. A review on the production, structure, bioactivities and applications of Tremella polysaccharides [J]. Int J Immunopathol Pharmacol, 2021, 35: 20587384211000541. |
| [7] | 许臻军. 小分子量银耳多糖的制备及其益生作用研究 [D]. 福州: 福建农林大学, 2024. |
| Xu ZJ. Study on preparation and probiotics of small molecular weight Tremella polysaccharide [D]. Fuzhou: Fujian Agriculture and Forestry University, 2024. | |
| [8] | Chen B. Optimization of extraction of Tremella fuciformis polysaccharides and its antioxidant and antitumour activities in vitro [J]. Carbohydr Polym, 2010, 81(2): 420-424. |
| [9] | 吴琼, 代永刚, 高长城, 等. 酸降解水溶性银耳多糖及抗氧化作用研究 [J]. 食品科学, 2009, 30(13): 93-96. |
| Wu Q, Dai YG, Gao CC, et al. Antioxidations of acid-degrade water-soluble polysaccharides from Tremella fuciformis [J]. Food Sci, 2009, 30(13): 93-96. | |
| [10] | 何荣军, 刘高丹, 孙培龙. 一种银耳低聚糖的制备方法及其应用: 中国, CN110590968B [P]. 2021-09-28. |
| He RJ, Liu GD, Sun PL. A preparation method and application of Tremella fuciformis oligosaccharides: China, CN110590968B [P]. 2021-09-28. | |
| [11] | 吕国军, 陈立, 耿少特. 一种低分子量银耳多糖的制备方法: 中国, CN114671959A [P]. 2022-06-28. |
| Lyu GJ, Chen L, Geng ST. A preparation method of low molecular weight Tremella fuciformis polysaccharides: China, CN114671959A [P]. 2022-06-28. | |
| [12] | 石清东, 王姣. 一种制备低分子量多糖的方法: 中国, CN106191914A [P]. 2018-10-02. |
| Shi QD, Wang J. A method for preparing low molecular weight polysaccharides: China, CN106191914A [P]. 2018-10-02. | |
| [13] | 张天萌, 郭文逸, 孙劭靖, 等. 一种低分子量银耳多糖的生产方法: CN107459585B [P]. 2019-11-19. |
| Zhang TM, Guo WY, Sun SJ, et al. Production method of low-molecular weight tremella polysaccharide: China, CN107459585B [P]. 2019-11-19. | |
| [14] | 陈杰烽, 何衍建, 谢明容, 等. 一种低分子量银耳多糖及其制备方法与应用: CN106117387B [P]. 2019-03-12. |
| Chen JF, He YJ, Xie MR, et al. A low-molecular-weight Tremella fuciformis polysaccharide and its preparation method and application: China, CN106117387B [P]. 2019-03-12. | |
| [15] | 雷曦, 张蕊, 黄遵锡, 等. 透明质酸酶的研究进展 [J]. 微生物学通报, 2021, 48(3): 882-895. |
| Lei X, Zhang R, Huang ZX, et al. Research progress of hyaluronidases [J]. Microbiol China, 2021, 48(3): 882-895. | |
| [16] | 雷曦, 周峻沛, 黄遵锡, 等. 糖胺聚糖降解菌株的筛选及鉴定 [J]. 微生物学杂志, 2020, 40(3): 22-27. |
| Lei X, Zhou JP, Huang ZX, et al. Screening and identification of glycosaminoglycan degradation strains [J]. J Microbiol, 2020, 40(3): 22-27. | |
| [17] | Ingham E, Holland KT, Gowland G, et al. Purification and partial characterization of hyaluronate lyase (EC 4.2.2.1) from Propionibacterium acnes [J]. J Gen Microbiol, 1979, 115(2): 411-418. |
| [18] | Sting R, Schaufuss P, Blobel H. Isolation and Characterization of Hyaluronidases from Streptococcus dysgalactiae, S. zooepidemicus and S. equi [J]. Zentralbl Für Bakteriologie, 1990, 272(3): 276-282. |
| [19] | Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview [J]. Life Sci, 2007, 80(21): 1921-1943. |
| [20] | Sun XQ, Wang Z, Bi YL, et al. Genetic and functional characterization of the hyaluronate lyase HylB and the beta-N-acetylglucosaminidase HylZ in Streptococcus zooepidemicus [J]. Curr Microbiol, 2015, 70(1): 35-42. |
| [21] | 王聪聪, 周剑丽, 顾秋亚, 等. 产岩藻多糖酶菌株的筛选及其酶解制备低分子质量岩藻多糖的研究 [J]. 食品与发酵工业, 2022, 48(23):49-56. |
| Wang CC, Zhou JL, Gu QY, et al. Screening of fucoidanase-producing strains and preparation of low molecular weight fucoidan by enzymatic hydrolysis [J]. Food and Fermentation Industries, 2022, 48(23): 49-56. | |
| [22] | 汪乐盛, 李科, 方莎莎, 等. 丁酸梭菌高密度发酵及产孢条件优化 [J]. 食品与发酵工业, 2024, 50(24): 107-113. |
| Wang LS, Li K, Fang SS, et al. Optimization of high cell density fermentation conditions and sporulation of Clostridium butyricum [J]. Food and Fermentation Industries, 2024, 50(24): 107-113. | |
| [23] | 孔蒙蒙, 金静静, 卢鹏, 等. 高产纤维素酶工程菌株产酶条件优化 [J]. 生物技术进展, 2024, 14(6): 1032-1041. |
| Kong MM, Jin J, Lu P, et al. Optimization of enzyme production conditions of high-yielding cellulase engineering strains [J]. Current Biotechnology, 2024, 14(6): 1032-1041. | |
| [24] | 李鑫, 李志刚, 史仲平. 原料碳氮比对丁醇发酵两阶段发酵性能的影响 [J]. 食品与生物技术学报, 2014, 33(11): 1168-1175. |
| Li X, Li ZG, Shi ZP. Effect of carbon/nitrogen ratio on butanol fermentation performances in two periods [J]. J Food Sci Biotechnol, 2014, 33(11): 1168-1175. | |
| [25] | 刘思琪, 郭玉娟, 刘彤, 等. β-半乳糖苷酶的筛选及低聚半乳糖制备工艺优化研究 [J]. 中国乳品工业, 2025, 53(2): 51-58. |
| Liu SQ, Guo YJ, Liu T, et al. Screening of β-galactosidase and optimization of galactooligosaccharides preparation process [J]. China Dairy Industry, 2025, 53(2): 51-58. | |
| [26] | Hartwig A. Role of magnesium in genomic stability [J]. Mutat Res Mol Mech Mutagen, 2001, 475(1/2): 113-121. |
| [27] | 陈菲. 一株蛋白酶产生菌的鉴定、酶性质研究和表达载体构建 [D]. 郑州: 河南工业大学, 2018. |
| Chen F. Identification, characterization and expression vector construction of a protease-producing strain [D]. Zhengzhou: Henan University of Technology, 2018. | |
| [28] | Garcia-Ochoa F, Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview [J]. Biotechnol Adv, 2009, 27(2): 153-176. |
| [29] | 刘爽, 江洲, 赵帅, 等. 一株产蛋白酶细菌的筛选、鉴定及发酵工艺优化 [J]. 生物技术通报, 2025, 41(4): 335-344. |
| Liu S, Jiang Z, Zhao S, et al. Screening, identification, and fermentation optimization of a protease producing bacterial strain [J]. Biotechnol Bull, 2025, 41(4): 335-344. | |
| [30] | 韦燕琪, 宁思敏, 韦昌浩, 等. 高产纤维素酶产生菌的筛选、鉴定及发酵条件优化 [J]. 中国饲料, 2024(17): 46-54. |
| Wei YQ, Ning SM, Wei CH, et al. Screening and identification of cellulase-producing strain and optimization of the fermentation conditions [J]. China Feed, 2024(17): 46-54. | |
| [31] | 温冬灼. 产纤维素酶菌种筛选及产酶工艺与酶学性质研究 [D]. 哈尔滨: 东北林业大学, 2022. |
| Wen DZ. Screening of cellulase-producing strains and research on enzyme production and enzymatic properties [D]. Harbin: Northeast Forestry University, 2022. |
| [1] | 苏秀敏, 韩文清, 王佼, 李鹏, 王秋兰, 李万星, 曹晋军. 哈茨木霉M408的分离鉴定、生物学特性及对番茄早疫病的生防效果[J]. 生物技术通报, 2025, 41(9): 277-288. |
| [2] | 张茹, 李一鸣, 张桐溪, 孙占斌, 任清, 潘寒姁. 厚朴中1株高产厚朴酚与和厚朴酚菌株的分离鉴定及其“发汗”工艺优化[J]. 生物技术通报, 2025, 41(8): 322-334. |
| [3] | 项波卡, 周钻钻, 冯佳卉, 夏琛, 李奇, 陈春. 一株烟叶霉变真菌的分离鉴定及其致霉因素研究[J]. 生物技术通报, 2025, 41(2): 321-330. |
| [4] | 裴旭娟, 狄靖宜, 刘浩, 高伟霞. 基于转录组分析挖掘兽疫链球菌透明质酸分子量调控元件[J]. 生物技术通报, 2025, 41(1): 347-356. |
| [5] | 张亚亚, 李盼盼, 高惠惠, 贾晨波, 徐春燕. 基于根表真菌群落与病原菌鉴定探究‘宁杞5号’枸杞根腐病的发生机制[J]. 生物技术通报, 2024, 40(9): 238-248. |
| [6] | 王芳, 于璐, 齐泽铮, 周长军, 于吉东. 大豆镰刀菌根腐病拮抗菌的筛选及生防效果[J]. 生物技术通报, 2024, 40(7): 216-225. |
| [7] | 王璐, 刘梦雨, 张富源, 纪守坤, 王云, 张英杰, 段春辉, 刘月琴, 严慧. 瘤胃源粪臭素降解菌的分离鉴定及其降解特性研究[J]. 生物技术通报, 2024, 40(3): 305-311. |
| [8] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [9] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [10] | 车永梅, 郭艳苹, 刘广超, 叶青, 李雅华, 赵方贵, 刘新. 菌株C8和B4的分离鉴定及其耐盐促生效果和机制[J]. 生物技术通报, 2023, 39(5): 276-285. |
| [11] | 王凤婷, 王岩, 孙颖, 崔文婧, 乔凯彬, 潘洪玉, 刘金亮. 耐盐碱土曲霉SYAT-1的分离鉴定及抑制植物病原真菌特性研究[J]. 生物技术通报, 2023, 39(2): 203-210. |
| [12] | 李莹, 宋新颖, 何康, 郭志青, 于静, 张霞. 贝莱斯芽孢杆菌ZHX-7的分离鉴定及抑菌促生效果[J]. 生物技术通报, 2023, 39(12): 229-236. |
| [13] | 董怡华, 王凌潇, 任涵雪, 陈峰. 一株耐低温异养硝化-好氧反硝化菌的分离鉴定及其脱氮特性[J]. 生物技术通报, 2023, 39(12): 237-249. |
| [14] | 祖雪, 周瑚, 朱华珺, 任佐华, 刘二明. 枯草芽孢杆菌K-268的分离鉴定及对水稻稻瘟病的防病效果[J]. 生物技术通报, 2022, 38(6): 136-146. |
| [15] | 王新光, 田磊, 王恩泽, 钟成, 田春杰. 玉米秸秆高效降解微生物复合菌系的构建及降解效果评价[J]. 生物技术通报, 2022, 38(4): 217-229. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||