








生物技术通报 ›› 2021, Vol. 37 ›› Issue (9): 114-124.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1482
孙瑞芬1( ), 张艳芳2, 牛素清1, 郭树春1, 李素萍1, 于海峰1, 聂惠1, 牟英男1
), 张艳芳2, 牛素清1, 郭树春1, 李素萍1, 于海峰1, 聂惠1, 牟英男1
收稿日期:2020-12-07
出版日期:2021-09-26
发布日期:2021-10-25
作者简介:孙瑞芬,女,硕士,研究员,研究方向:向日葵抗逆分子;E-mail: 基金资助:
SUN Rui-fen1( ), ZHANG Yan-fang2, NIU Su-qing1, GUO Shu-chun1, LI Su-ping1, YU Hai-feng1, NIE Hui1, MOU Ying-nan1
), ZHANG Yan-fang2, NIU Su-qing1, GUO Shu-chun1, LI Su-ping1, YU Hai-feng1, NIE Hui1, MOU Ying-nan1
Received:2020-12-07
Published:2021-09-26
Online:2021-10-25
摘要:
为加强对向日葵ACC氧化酶基因的利用,以前期从盐诱导的向日葵中克隆的ACC氧化酶基因HaACO1(GenBank accession number. KP966508)为对象,进行了该基因在不同胁迫条件下的表达分析及在烟草中的超表达研究。结果表明,该基因受病原菌、机械损伤、低温、NaCl和水杨酸胁迫诱导表达,且在不同的胁迫下表现出不同的表达模式;HaACO1在向日葵根、下胚轴和叶中均有表达,但在叶中的表达量最高,在根中的表达量最低。利用瞬时表达载体进行亚细胞定位分析,发现HaACO1-GFP在洋葱表皮细胞的细胞质中有表达。构建HaACO1植物表达载体进行过表达分析,表明在含有NaCl的分化培养基上,转基因烟草叶色失绿程度较野生型的轻,分化能力较野生型的高;低温、干旱和NaCl胁迫下,转基因烟草的HaACO1相对表达量高于野生型;NaCl胁迫下,转基因烟草的可溶性蛋白(soluble protein)、脯氨酸(proline,PRO)和叶绿素(chlorophyll)含量及过氧化物酶(peroxidase,POD)和超氧化物歧化酶(superoxide dismutase,SOD)活性提高;脯氨酸合成关键酶基因P5CS及抗氧化相关基因POD、MnSOD和GuZnSOD表达上调。HaACO1过表达提高了烟草的耐盐性,这将为进一步理解向日葵耐盐分子机制以及利用该基因进行作物抗逆性状改良奠定基础。
孙瑞芬, 张艳芳, 牛素清, 郭树春, 李素萍, 于海峰, 聂惠, 牟英男. 向日葵HaACO1基因的表达分析及功能验证[J]. 生物技术通报, 2021, 37(9): 114-124.
SUN Rui-fen, ZHANG Yan-fang, NIU Su-qing, GUO Shu-chun, LI Su-ping, YU Hai-feng, NIE Hui, MOU Ying-nan. Expression Analysis and Functional Verification of the HaACO1 Gene in Sunflower[J]. Biotechnology Bulletin, 2021, 37(9): 114-124.
| 名称 Name | 编号 No. | 序列 Sequence(5'-3') | 用途 Application |
|---|---|---|---|
| qHaACO1 | F1 | GCTTCAAAGAAATGGTGGCT | HaACO1的qRT-PCR qRT-PCR of HaACO1 |
| R1 | GGGAGATGGCGGAGATAGA | ||
| 18S rRNA | F2 | AGAAACGGCTACCACATCCA | 向日葵内参基因18S rRNA Reference gene 18S rRNA in sunflower |
| R2 | TTGTTATTTATTGTCACTACCTCCC | ||
| sHaACO1 | F3 | CGGGGTACCATGGAGGAGGAGACATTTCCAG Kpn I | HaACO1编码区扩增(引入KpnⅠ/BamⅠ位点) Amplification of HaACO1 coding region(carrying KpnⅠ/BamH I sites) |
| R3 | CGGGATCCAGCAGTTGCAATGGGGTC Bam Ⅰ | ||
| zHaACO1 | F4 | CGGGATCCATGGAGGAGGAGACATTTCCAG Bam HI | HaACO1编码区扩增(引入Bam HⅠ/KpnⅠ位点) Amplification of HaACO1 coding region(carrying BamHⅠ/KpnⅠ sites) |
| R4 | CGGGGTACCTTAAGCAGTTGCAATGGGGTC Kpn I | ||
| CaMV35S | p-F | AGACGTTCCAACCACGTCTTCA | 转基因烟草中HaACO1基因检测(CaMV 35S启动子近3'端序列) HaACO1 detection in transgenic tobacco(near 3' terminal sequence of CaMV35S promoter) |
| NtEF-1α | F5 | TGAGATGCACCACGAAGCTC | 烟草内参基因EF-1α Reference gene EF-1α in tobacco |
| R5 | CCAACATTGTCACCAGGAAGTG | ||
| P5CS | F6 | TGGTCGTCAGCGGCTTAGAT | 烟草P5CS的qRT-PCR qRT-PCR of P5CS in tobacco |
| R6 | TGCCAAACTGTCATTGTCCC | ||
| POD | F7 | CCCTGGTGTTGTTTCTTGTG | 烟草POD的qRT-PCR qRT-PCR of POD in tobacco |
| R7 | CCTGAGCCTGAACTTCTTGG | ||
| MnSOD | F8 | GCAGACGGACCTTAGCAACA | 烟草MnSOD的qRT-PCR qRT-PCR of MnSOD in tobacco |
| R8 | GGGAGCCAAAGTTAGTGTCG | ||
| GuZnSOD | F9 | CGGGACCACATTACAATCCT | 烟草GuZnSOD的qRT-PCR qRT-PCR of GuZnSOD in tobacco |
| R9 | ATCAGCGTGAACAACCACAG |
表1 引物信息
Table 1 Primer information
| 名称 Name | 编号 No. | 序列 Sequence(5'-3') | 用途 Application |
|---|---|---|---|
| qHaACO1 | F1 | GCTTCAAAGAAATGGTGGCT | HaACO1的qRT-PCR qRT-PCR of HaACO1 |
| R1 | GGGAGATGGCGGAGATAGA | ||
| 18S rRNA | F2 | AGAAACGGCTACCACATCCA | 向日葵内参基因18S rRNA Reference gene 18S rRNA in sunflower |
| R2 | TTGTTATTTATTGTCACTACCTCCC | ||
| sHaACO1 | F3 | CGGGGTACCATGGAGGAGGAGACATTTCCAG Kpn I | HaACO1编码区扩增(引入KpnⅠ/BamⅠ位点) Amplification of HaACO1 coding region(carrying KpnⅠ/BamH I sites) |
| R3 | CGGGATCCAGCAGTTGCAATGGGGTC Bam Ⅰ | ||
| zHaACO1 | F4 | CGGGATCCATGGAGGAGGAGACATTTCCAG Bam HI | HaACO1编码区扩增(引入Bam HⅠ/KpnⅠ位点) Amplification of HaACO1 coding region(carrying BamHⅠ/KpnⅠ sites) |
| R4 | CGGGGTACCTTAAGCAGTTGCAATGGGGTC Kpn I | ||
| CaMV35S | p-F | AGACGTTCCAACCACGTCTTCA | 转基因烟草中HaACO1基因检测(CaMV 35S启动子近3'端序列) HaACO1 detection in transgenic tobacco(near 3' terminal sequence of CaMV35S promoter) |
| NtEF-1α | F5 | TGAGATGCACCACGAAGCTC | 烟草内参基因EF-1α Reference gene EF-1α in tobacco |
| R5 | CCAACATTGTCACCAGGAAGTG | ||
| P5CS | F6 | TGGTCGTCAGCGGCTTAGAT | 烟草P5CS的qRT-PCR qRT-PCR of P5CS in tobacco |
| R6 | TGCCAAACTGTCATTGTCCC | ||
| POD | F7 | CCCTGGTGTTGTTTCTTGTG | 烟草POD的qRT-PCR qRT-PCR of POD in tobacco |
| R7 | CCTGAGCCTGAACTTCTTGG | ||
| MnSOD | F8 | GCAGACGGACCTTAGCAACA | 烟草MnSOD的qRT-PCR qRT-PCR of MnSOD in tobacco |
| R8 | GGGAGCCAAAGTTAGTGTCG | ||
| GuZnSOD | F9 | CGGGACCACATTACAATCCT | 烟草GuZnSOD的qRT-PCR qRT-PCR of GuZnSOD in tobacco |
| R9 | ATCAGCGTGAACAACCACAG |
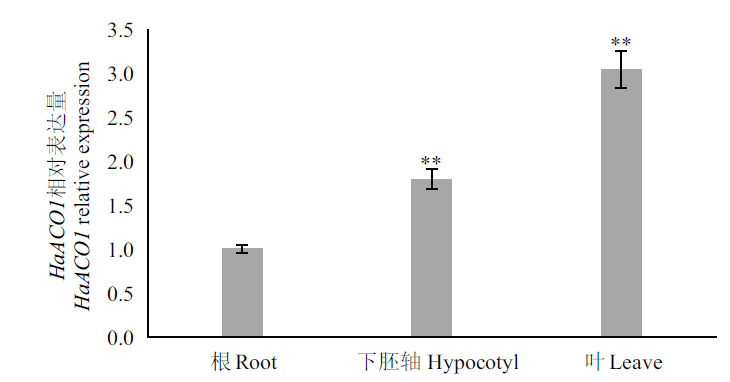
图1 HaACO1在向日葵不同器官的表达分析 **:相对于根差异极显著(P<0.01)
Fig.1 Expression of HaACO1 in different organs of sunflower **:Extremely significant difference compared with root(P<0.01)

图2 HaACO1在黄萎病病原菌V21诱导下的表达分析 **:相对于0 d差异极显著(P<0.01)
Fig.2 HaACO1 expression under the induction of Verticillium wilt pathogen V21 **:Extremely significant difference compared with 0 d(P<0.01)
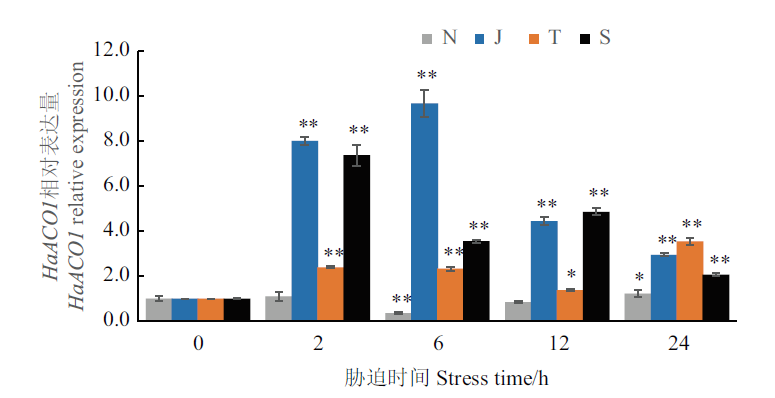
图3 非生物胁迫的不同时间点HaACO1相对表达分析 N:NaCl胁迫;J:机械损伤胁迫;T:低温胁迫;S:水杨酸胁迫;*,**:分别为相对于0 d差异显著(P<0.05)和极显著(P<0.01)
Fig.3 HaACO1 expression at different time under different abiotic stresses N:NaCl stress. J:Mechanical damage stress. T:Low temperature stress. S:Salicylic acid stress. *,**:Significant difference(P<0.05)and extremely significant difference(P<0.01),respectively,compared with 0 d
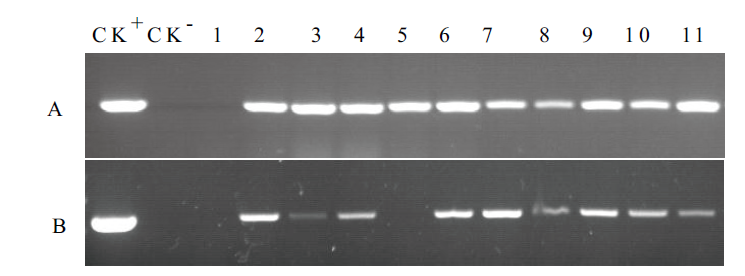
图5 抗性植株的分子检测 A:PCR;B:RT-PCR. CK+:重组质粒pPZP221-HaACO1;CK-:WT;1-11:转化植株
Fig.5 Molecular test of resistant plants with PCR and RT-PCR CK+:Recombinant plasmid pPZP221-HaACO1. CK-:Wild type(WT). 1-11:Transformed plants

图7 非生物胁迫条件下转基因烟草中HaACO1的表达分析 CK:未处理;DW:4℃低温处理;PEG:20% PEG6000处理;NaCl:120 mmol/L处理;**:胁迫条件下转基因株系T-10相对于WT差异极显著(P<0.01)
Fig. 7 HaACO1 expression analysis in transgenic plants under abiotic stresses CK: Untreatment; DW: lower temperature treatment under 4℃; PEG: the treatment with 20% PEG6000; NaCl: the treatment with 120 mol/L NaCl; **: extremely significant difference of transgenetic line T-10 compared with WT under stress (P<0.01)
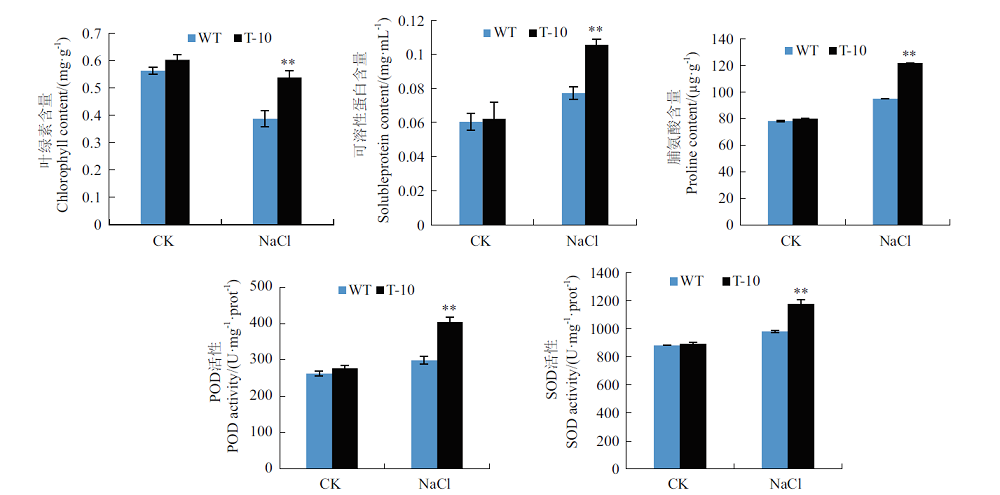
图8 转基因烟草中抗逆相关生理生化指标分析 **:NaCl胁迫下转基因株系T-10相对于WT差异极显著(P<0.01),下同
Fig.8 Analyses of physiological parameters related to stress tolerance in transgenic tobacco **:Extremely significant difference of transgenic line T-11 compared with WT under stress(P<0.01). The same below
| [1] | 陈银华, 黄伟, 王海. ACC氧化酶基因研究进展[J]. 海南大学学报:自然科学版, 2006, 24(2):194-200. |
| Chen YH, Huang W, Wang H. Review on researching advance in ACC oxidase genes[J]. Natural Science Journal of Hainan University, 2006, 24(2):194-200. | |
| [2] | Ismawanto S. Functional analysis of Arabidopsis thaliana 1-aminocyclopropane-1-carboxylic acid oxidase gene in response to limited water[D]. Malaysia:Universiti Putra Malaysia, 2013. |
| [3] | Yuan H, Yue PT, Bu HD, et al. Genome-wide analysis of ACO and ACS genes in pear(Pyrus ussuriensis)[J]. In Vitro Cellular & Developmental Biology - Plant, 2020, 56(2):193-199. |
| [4] |
Jakubowicz M, Nowak W, Gałgański Ł, et al. Expression profiling of the genes encoding ABA route components and the ACC oxidase isozymes in the senescing leaves of Populus tremula[J]. Journal of Plant Physiology, 2020, 248:153143.
doi: 10.1016/j.jplph.2020.153143 URL |
| [5] |
Sornchai P, Doorn WG, Imsabai W, et al. Dendrobium orchids carrying antisense ACC oxidase:small changes in flower morphology and a delay of bud abortion, flower senescence, and abscission of flowers[J]. Transgenic Research, 2020, 29(4):429-442.
doi: 10.1007/s11248-020-00209-8 URL |
| [6] | 孙申申, 温秀萍, 陈晓静. ‘云香’水仙ACC氧化酶基因的克隆表达分析及其遗传转化与鉴定[J]. 西北植物学报, 2017, 37(9):1685-1692. |
| Sun SS, Wen XP, Chen XJ. Clone, Expression Analysis, Genetic transformation and identification of ACC Oxidase Gene in Narcissus tazetta var. ‘Yunxiang’[J]. Acta Botanica Boreali-Occidentalia Sinica, 2017, 37(9):1685-1692. | |
| [7] | 吴建阳, 李彩琴, 陆旺金, 等. 荔枝ACO1基因克隆及其与幼果落果的关系[J]. 果树学报, 2013, 30(2):207-213. |
| Wu JY, Li CQ, Lu WJ, et al. Cloning of Lc -ACO1 and its expression related to fruitlet abscission in Litchi[J]. Journal of Fruit Science, 2013, 30(2):207-213. | |
| [8] | 薛丽君, 周精华, 邢虎成. 苎麻ACC氧化酶基因(BnACO1)的克隆及表达[J]. 中国农业科学, 2013, 46(11):2377-2385. |
| Xue LJ, Zhou JH, Xing HC. Cloning and characterization of ACC oxidase gene(BnACO1)from ramie(Boehmeria nivea)[J]. Scientia Agricultura Sinica, 2013, 46(11):2377-2385. | |
| [9] | 张萍, 王斐, 孙辉, 等. 过量表达棉花GhACO2基因增强拟南芥抗逆性研究[J]. 中国农学通报, 2011, 27(12):255-260. |
| Zhang P, Wang F, Sun H, et al. Overexpression of a cotton GhACO2 gene enhancing the stress tolerance of ababidopsis thaliana[J]. Chinese Agricultural Science Bulletin, 2011, 27(12):255-260. | |
| [10] | 孙瑞芬, 张艳芳, 郭树春, 等. 向日葵盐胁迫相关基因的cDNA-AFLP差异表达[J]. 中国生物工程杂志, 2015, 35(1):34-40. |
| Sun RF, Zhang YF, Guo SC, et al. Differentially expressed analysis on the responsive genes to salt stress in sunflower by cDNA- AFLP[J]. China Biotechnology, 2015, 35(1):34-40. | |
| [11] | 孙瑞芬, 张艳芳, 郭树春, 等. 向日葵ACC氧化酶基因(HaACO1)的克隆及表达分析[J]. 中国生物工程杂志, 2015, 35(9):21-27. |
| Sun RF, Zhang YF, Guo SC, et al. Cloning and expression analysis of ACC oxidase gene(HaACO1)from sunflower(Helianthus annuus L. )[J]. China Biotechnology, 2015, 35(9):21-27. | |
| [12] | 郭树春, 张艳芳, 孙瑞芬, 等. 定量蘸菌法鉴定向日葵品种黄萎病抗性[J]. 北方农业学报, 2017, 45(2):77-81. |
| Guo SC, Zhang YF, Sun RF, et al. Identification of sunflower Verticillium wilt resistant with a quantitative method for dipping bacteria solution[J]. Journal of Northern Agriculture, 2017, 45(2):77-81. | |
| [13] |
Wang FF, Cui XK, Sun Y, et al. Ethylene signaling and regulation in plant growth and stress responses[J]. Plant Cell Reports, 2013, 32(7):1099-1109.
doi: 10.1007/s00299-013-1421-6 URL |
| [14] |
Light KM, Wisniewski JA, Vinyard WA, et al. Perception of the plant hormone ethylene:known-knowns and known-unknowns[J]. Journal of Biological Inorganic Chemistry, 2016, 21(5/6):715-728.
doi: 10.1007/s00775-016-1378-3 URL |
| [15] |
Takahashi H, Shinkawa T, Nakai S, et al. Differential expression of ACC oxidase genes during low-pH-induced root hair formation in lettuce(Lactuca sativa L. )seedlings[J]. Plant Growth Regulation, 2010, 62(2):137-149.
doi: 10.1007/s10725-010-9499-0 URL |
| [16] |
Liu JH, Hwee Lee-Tamon S, Reid DM. Differential and wound-inducible expression of 1-aminocylopropane-1-carboxylate oxidase genes in sunflower seedlings[J]. Plant Molecular Biology, 1997, 34(6):923-933.
pmid: 9290644 |
| [17] |
Ouvrard O, Cellier F, Ferrare K, et al. Identification and expression of water stress- and abscisic acid-regulated genes in a drought-tolerant sunflower genotype[J]. Plant Molecular Biology, 1996, 31(4):819-829.
pmid: 8806412 |
| [18] |
Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries:does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits?[J]. Journal of Experimental Botany, 2005, 56(418):2037-2046.
pmid: 15955790 |
| [19] |
Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits[J]. Journal Experimental Botany, 2005, 56(418):2037-2046.
doi: 10.1093/jxb/eri202 URL |
| [20] | 潘刚. 桑树ACC氧化酶基因分离其及在生长发育和胁迫应答中的功能研究[D]. 杭州:浙江大学, 2007. |
| Pan G. Isolation of an ACC oxidase gene from mulberry(Morus alba L. )and analysis of the function of this gene in plant development and stresses response[D]. Hangzhou:Zhejiang University, 2007. | |
| [21] | 李立芹, 王西瑶, 鲁黎明. 马铃薯ACC氧化酶参与低钾胁迫的功能研究[C]. 2019年中国马铃薯大会论文集. 恩施, 2019:266-267. |
| Li LQ, Wang XY, Lu LM. Research on the function of ACC oxidase in potato under low potassium stress[C]. Proceeding of 2019 Chinese Potato Congress, 2019:266-267. | |
| [22] | Xiao GZ, Li LJ, Teng K, et al. Cloning and expression of the 1-aminocyclopropane-1-carboxylic oxidase gene from Agrostis stolonifera[J]. Genetics and Molecular Research, 2016, 15(4):1-12. |
| [23] |
Shi HY, Zhang YX. Pear ACO genes encoding putative 1-aminocyclopropane-1-carboxylate oxidase homologs are functionally expressed during fruit ripening and involved in response to salicylic acid[J]. Molecular Biology Reports, 2012, 39(10):9509-9519.
doi: 10.1007/s11033-012-1815-5 URL |
| [24] | 朱家红, 李超燕, 范鸿雁, 等. 菠萝ACC氧化酶基因AcACO1的克隆与表达分析[J]. 基因组学与应用生物学, 2018, 37(1):339-344. |
| Zhu JH, Li CY, Fan HY, et al. Cloning and expression analysis of ACC oxidase gene AcACO1 in pineapple(Ananas comosus L. Merr.)[J]. Genomics and Applied Biology, 2018, 37(1):339-344. | |
| [25] | 孙明明, 房磊, 郭悦, 等. 花生AhACO基因的克隆及功能验证[C]. 中国作物学会油料作物专业委员会第八次会员代表大会暨学术年会论文集. 青岛, 2018: 259. |
| Sun MM, Fang L, Guo Y, et al. Cloning and functional verification of AhACO gene in Peanut[C]. Summary of the 8th Member Representative Conference and Annual Conference of Oil Crops Committee of Chinese Crop Science Society, 2019: 259. | |
| [26] |
Ramadoss N, Gupta D, Vaidya BN, et al. Functional characterization of 1-aminocyclopropane-1-carboxylic acid oxidase gene in Arabidopsis thaliana and its potential in providing flood tolerance[J]. Biochemical and Biophysical Research Communications, 2018, 503(1):365-370.
doi: 10.1016/j.bbrc.2018.06.036 URL |
| [27] | 刘为远, 刘桂男, 陈双, 等. 苦荞转录因子基因FtDREB1和FtDREB2的克隆及其对非生物胁迫的应答[J]. 基因组学与应用生物学, 2018, 37(5):1985-1992. |
| Liu WY, Liu GN, Chen S, et al. Cloning of FtDREB1 and FtDREB2 transcription factor genes from Fagopyrum tataricum and their responses to abiotic stress[J]. Genomics and Applied Biology, 2018, 37(5):1985-1992. | |
| [28] |
Ban QY, Liu GF, Wang YC. A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco[J]. Journal of Plant Physiology, 2011, 168(5):449-458.
doi: 10.1016/j.jplph.2010.08.013 URL |
| [29] | 杨静, 毛笈华, 于永涛, 等. 低温对甜玉米种子氧化酶活性的影响及相关基因表达分析[J]. 核农学报, 2016, 30(9):1840-1847. |
| Yang J, Mao JH, Yu YT, et al. Effects of chilling on antioxidant enzyme activity and related gene expression levels during seed germination[J]. Journal of Nuclear Agricultural Sciences, 2016, 30(9):1840-1847. | |
| [30] |
Najafi S, Sorkheh K, Nasernakhaei F. Characterization of the APETALA2/Ethylene-responsive factor(AP2/ERF)transcription factor family in sunflower[J]. Scientific Reports, 2018, 8(1):11576.
doi: 10.1038/s41598-018-29526-z URL |
| [31] |
Shahbaz M, Ashraf M, Akram NA, et al. Salt-induced modulation in growth, photosynthetic capacity, proline content and ion accumulation in sunflower(Helianthus annuus L. )[J]. Acta Physiologiae Plantarum, 2011, 33(4):1113-1122.
doi: 10.1007/s11738-010-0639-y URL |
| [32] |
Liu J, Shi DC. Photosynjournal, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress[J]. Photosynthetica, 2010, 48(1):127-134.
doi: 10.1007/s11099-010-0017-4 URL |
| [33] |
Chen M, Xu ZS, Xia LQ, et al. Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean(Glycine max L. )[J]. Journal of Experimental Botany, 2009, 60(1):121-135.
doi: 10.1093/jxb/ern269 URL |
| [34] | Tang L, Tang H, Sangsoo K, et al. Improving potato plants oxidative stress and salt tolerance by gene transfer both of Cu/Zn superoxide dismutase and ascorbate peroxidase[J]. China Biotechnology, 2008, 28(3):25-31. |
| [1] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [2] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [3] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [4] | 刘媛媛, 魏传正, 谢永波, 仝宗军, 韩星, 甘炳成, 谢宝贵, 严俊杰. 金针菇II类过氧化物酶基因在子实体发育与胁迫应答过程的表达特征[J]. 生物技术通报, 2023, 39(11): 340-349. |
| [5] | 李建建, 贺宸靖, 黄小平, 向太和. 植物长链非编码RNA调控发育与胁迫应答的研究进展[J]. 生物技术通报, 2023, 39(1): 48-58. |
| [6] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [7] | 杨佳宝, 周至铭, 张展, 冯丽, 孙黎. 向日葵HaLACS1的克隆、表达及酵母功能互补鉴定[J]. 生物技术通报, 2022, 38(6): 147-156. |
| [8] | 林科运, 段钰晶, 王高升, 孙念礼, 方玉洁, 王幼平. 甘蓝型油菜BnNF-YA1的克隆和功能鉴定[J]. 生物技术通报, 2022, 38(4): 106-116. |
| [9] | 镐青青, 姚圣, 刘佳禾, 陈佩珍, 张梦洋, 季孔庶. 马尾松NAC转录因子基因PmNAC8的克隆及表达分析[J]. 生物技术通报, 2022, 38(4): 202-216. |
| [10] | 赵婷婷, 王俊刚, 王文治, 冯翠莲, 冯小艳, 张树珍. 甘蔗单糖转运蛋白基因ShSTP7序列分析及组织表达特征测定[J]. 生物技术通报, 2022, 38(4): 72-78. |
| [11] | 党瑗, 李维, 苗向, 修宇, 林善枝. 山杏油体蛋白基因PsOLE4克隆及其调控油脂累积功能分析[J]. 生物技术通报, 2022, 38(11): 151-161. |
| [12] | 许机分, 陈泓妃, 王娜, 刘晶. 真菌Hog1 MAPK信号通路研究进展[J]. 生物技术通报, 2022, 38(11): 32-40. |
| [13] | 骆鹰, 谭智, 王帆, 刘晓霞, 罗小芳, 何福林. 银杏GbR2R3-MYB1基因的克隆及非生物胁迫应答分析[J]. 生物技术通报, 2022, 38(10): 184-194. |
| [14] | 范亚朋, 芮存, 张悦新, 陈修贵, 陆许可, 王帅, 张红, 徐楠, 王晶, 陈超, 叶武威. 陆地棉耐碱基因GHZAT12的克隆、表达及生物信息学分析[J]. 生物技术通报, 2021, 37(8): 121-130. |
| [15] | 山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||