








生物技术通报 ›› 2021, Vol. 37 ›› Issue (11): 225-236.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0288
罗亚军1( ), 孙红敏2, 何宁2, 袁丽杰1(
), 孙红敏2, 何宁2, 袁丽杰1( ), 解云英2(
), 解云英2( )
)
收稿日期:2021-03-11
出版日期:2021-11-26
发布日期:2021-12-03
作者简介:罗亚军,男,硕士,研究方向:微生物的分离鉴定、抗菌活性及天然产物挖掘;E-mail: 基金资助:
LUO Ya-jun1( ), SUN Hong-min2, HE Ning2, YUAN Li-jie1(
), SUN Hong-min2, HE Ning2, YUAN Li-jie1( ), XIE Yun-ying2(
), XIE Yun-ying2( )
)
Received:2021-03-11
Published:2021-11-26
Online:2021-12-03
摘要:
探索西藏林芝与山南地区沙棘根瘤及其根际土壤样品中放线菌的多样性及抗菌活性,为天然产物药物研发提供新的微生物资源。采用稀释涂布法对10份样品中可培养放线菌进行分离,后经菌株16S rRNA序列测序,以邻接法(neighbor-joining method,N-J)构建系统发育树分析放线菌种属多样性;利用微孔板法对代表性菌株进行发酵培养及抗菌活性评价;进一步利用超高效液相色谱-质谱联用(UPLC-MS)考察活性菌株代谢产物情况。从10份样品中分离到112株放线菌,分属于7个目12科23个属,优势菌属为链霉菌属(Streptomyces);抗菌活性结果表明有17株放线菌至少对一种检定菌有抗菌活性,阳性抑菌率为36.9%。有16株放线菌对临床常见的耐甲氧西林金黄色葡萄球菌(methicillin-resistant Staphylococcus aureus,MRSA)有较强抑制活性,其中13株放线菌对MRSA抑制率超过阳性对照药万古霉素;另外有7株放线菌对耐碳青霉烯鲍曼不动杆菌(carbapenem-resistant Acinetobacter baumannii,CRAB)有抑制活性,其中Streptomyces sp. XZ19-363对CRAB抑菌作用最强,抑制率为91.3%,UPLC-MS分析显示,具有广谱抗菌活性的菌株在不同培养基中产生了较为丰富的次级代谢产物。西藏林芝与山南地区沙棘根瘤及其根际土壤中可培养放线菌资源丰富多样,同时具有较好的抗菌活性且代谢产物丰富。
罗亚军, 孙红敏, 何宁, 袁丽杰, 解云英. 西藏沙棘根瘤及根际土壤放线菌分离及抗菌活性研究[J]. 生物技术通报, 2021, 37(11): 225-236.
LUO Ya-jun, SUN Hong-min, HE Ning, YUAN Li-jie, XIE Yun-ying. Isolation and Antibacterial Activity of Actinomycetes from the Nodules and Rhizosphere Soil of Hippophae rhamnoides in Tibet[J]. Biotechnology Bulletin, 2021, 37(11): 225-236.
| 采样地点 Sampling location | 样品种类 Sample type | 编号 No. | 海拔 Altitude/m | 采样时间 Sampling time | 坐标 Coordinates |
|---|---|---|---|---|---|
| 林芝南伊沟区 | 沙棘根瘤 | P1 | 2 999 | 2019. 09. 21 | 29o08’25.15” N,94o12’58.71” E |
| 根际土壤 | E1 | 2 999 | 2019. 09. 21 | 29o08’25.15” N,94o12’58.71” E | |
| 山南市错那县 | 大沙棘根瘤 | P2 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E |
| 根际土壤 | E2 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E | |
| 山南市错那县 | 小沙棘根瘤 | P3 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E |
| 根际土壤 | E3 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E | |
| 山南市错那县 | 沙棘根瘤 | P4 | 4 255 | 2019. 09. 23 | 28o15’18.76” N,91o45’47.52” E |
| 根际土壤 | E4 | 4 255 | 2019. 09. 23 | 28o15’18.76” N,91o45’47.52” E | |
| 山南市隆子县 | 沙棘根瘤 | P5 | 3 915 | 2019. 09. 23 | 28o25’12.01” N,92o23’48.99” E |
| 根际土壤 | E5 | 3 915 | 2019. 09. 23 | 28o25’12.01” N,92o23’48.99” E |
表1 样品采集信息
Table 1 Sample collection information
| 采样地点 Sampling location | 样品种类 Sample type | 编号 No. | 海拔 Altitude/m | 采样时间 Sampling time | 坐标 Coordinates |
|---|---|---|---|---|---|
| 林芝南伊沟区 | 沙棘根瘤 | P1 | 2 999 | 2019. 09. 21 | 29o08’25.15” N,94o12’58.71” E |
| 根际土壤 | E1 | 2 999 | 2019. 09. 21 | 29o08’25.15” N,94o12’58.71” E | |
| 山南市错那县 | 大沙棘根瘤 | P2 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E |
| 根际土壤 | E2 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E | |
| 山南市错那县 | 小沙棘根瘤 | P3 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E |
| 根际土壤 | E3 | 4 515 | 2019. 09. 23 | 28o18’48.24” N,91o49’06.59” E | |
| 山南市错那县 | 沙棘根瘤 | P4 | 4 255 | 2019. 09. 23 | 28o15’18.76” N,91o45’47.52” E |
| 根际土壤 | E4 | 4 255 | 2019. 09. 23 | 28o15’18.76” N,91o45’47.52” E | |
| 山南市隆子县 | 沙棘根瘤 | P5 | 3 915 | 2019. 09. 23 | 28o25’12.01” N,92o23’48.99” E |
| 根际土壤 | E5 | 3 915 | 2019. 09. 23 | 28o25’12.01” N,92o23’48.99” E |
| 培养基Culture medium | 培养基成分Medium composition |
|---|---|
| PDB | 成品,购于北京奥博星生物技术公司,pH 7.2 |
| ISP 2 | 酵母膏4 g,麦芽糖10 g,葡萄糖4 g,pH 7.2 |
| FM3 | 可溶性淀粉20 g,甘油5 g,脱脂小麦胚芽10 g,肉提取物3 g,干酵母3 g,CaCO3 3 g,pH 7.0 |
| FM4 | 半乳糖3.3 g,水化糊精3.3 g,甘油1.7 g,豆胨1.7 g,玉米浆0.83 g,CaCO3 2 g,(NH4)2SO4 0.33 g,pH 7.0 |
| FM5 | 葡萄糖10 g,牛肉膏10 g,蛋白胨1 g,NaCl 5 g,pH 7.0 |
| M331 | 葡萄糖20 g,普通淀粉5 g,蛋白胨 10 g,(NH4)2SO4 7 g,CaCO3 2 g,pH自然 |
| F1 | 普通淀粉20 g,葡萄糖20 g,蛋白胨3 g,牛肉膏3 g,微量元素1 mL,CaCO3 2 g,花生饼粉10 g,pH 7.2 |
| M2 | 甘露醇40 g,麦芽浸粉40 g,酵母浸膏粉10 g,K2HPO4 2 g,MgSO4·7H2O 0.5 g,FeSO4·7H2O 0.01 g,pH 7.2 |
| Cazpeck | K2HPO4 1 g,NaNO3 0.3 g,KCl 0.005 g,MgSO4·7H2O 0.005 g,FeSO4 0.001 g,蔗糖30 g,pH 7.0 |
| FM10 | 甘油20 g,糖蜜10 g,酪蛋白5 g,蛋白胨1 g,CaCO3 4 g,pH 7.2 |
| FM11 | 葡萄糖20 g,麦芽浸粉40 g,酵母浸粉4 g,K2HPO4 5 g,NaCl 2.5 g,CaCO3 0.4 g,ZnSO4 0.04 g,pH 6.0 |
| YMS | 酵母浸粉4 g,麦芽浸粉10 g,可溶性淀粉4 g,pH 自然 |
表2 12种发酵培养基种类
Table 2 12 kinds of fermentation medium
| 培养基Culture medium | 培养基成分Medium composition |
|---|---|
| PDB | 成品,购于北京奥博星生物技术公司,pH 7.2 |
| ISP 2 | 酵母膏4 g,麦芽糖10 g,葡萄糖4 g,pH 7.2 |
| FM3 | 可溶性淀粉20 g,甘油5 g,脱脂小麦胚芽10 g,肉提取物3 g,干酵母3 g,CaCO3 3 g,pH 7.0 |
| FM4 | 半乳糖3.3 g,水化糊精3.3 g,甘油1.7 g,豆胨1.7 g,玉米浆0.83 g,CaCO3 2 g,(NH4)2SO4 0.33 g,pH 7.0 |
| FM5 | 葡萄糖10 g,牛肉膏10 g,蛋白胨1 g,NaCl 5 g,pH 7.0 |
| M331 | 葡萄糖20 g,普通淀粉5 g,蛋白胨 10 g,(NH4)2SO4 7 g,CaCO3 2 g,pH自然 |
| F1 | 普通淀粉20 g,葡萄糖20 g,蛋白胨3 g,牛肉膏3 g,微量元素1 mL,CaCO3 2 g,花生饼粉10 g,pH 7.2 |
| M2 | 甘露醇40 g,麦芽浸粉40 g,酵母浸膏粉10 g,K2HPO4 2 g,MgSO4·7H2O 0.5 g,FeSO4·7H2O 0.01 g,pH 7.2 |
| Cazpeck | K2HPO4 1 g,NaNO3 0.3 g,KCl 0.005 g,MgSO4·7H2O 0.005 g,FeSO4 0.001 g,蔗糖30 g,pH 7.0 |
| FM10 | 甘油20 g,糖蜜10 g,酪蛋白5 g,蛋白胨1 g,CaCO3 4 g,pH 7.2 |
| FM11 | 葡萄糖20 g,麦芽浸粉40 g,酵母浸粉4 g,K2HPO4 5 g,NaCl 2.5 g,CaCO3 0.4 g,ZnSO4 0.04 g,pH 6.0 |
| YMS | 酵母浸粉4 g,麦芽浸粉10 g,可溶性淀粉4 g,pH 自然 |
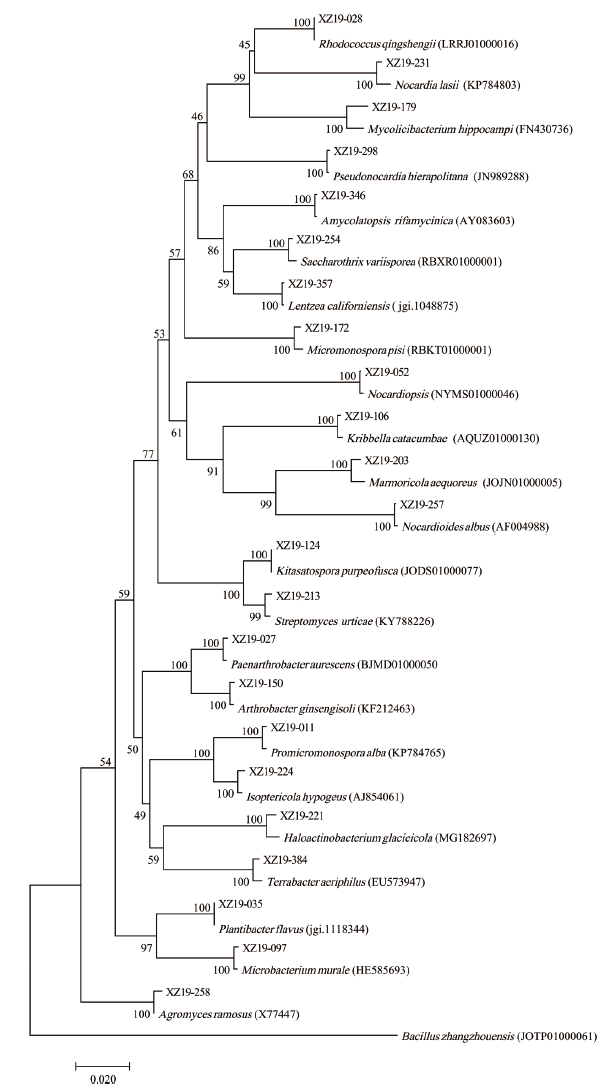
图1 基于16S rRNA各属代表菌株基因序列构建的菌株N-J系统进化树 分支点上的数值为1 000次自展值分析所得,标尺0.020进化距离
Fig. 1 Phylogenetic tree of strain N-J based on gene sequences of representative strains of 16S rRNA genera The value at the bifurcation point is obtained by 1 000 times of self-xpanding analysis. Scale 0.020 evolution distance
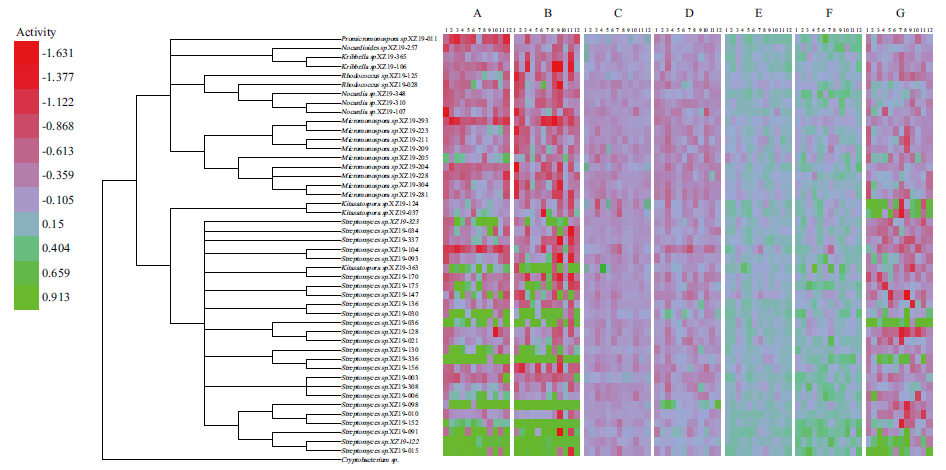
图4 46株代表性菌株发酵产物抗菌活性结果 A:金黄色葡萄球菌(ATCC 29213);B:耐甲氧西林金黄色葡萄球菌(MRSA)181223;C:铜绿假单胞菌(ATCC 27853);D:大肠埃希菌(ATCC 25922);E:肺炎克雷伯菌(ATCC 700603);F:耐碳青霉烯鲍曼不动杆菌(CRAB)181236;G:白色念珠菌(ATCC 10231)。热图(Heatmap):在7种检定菌96孔菌液板中,每孔分别加入在12种培养基发酵后的菌液,生长24 h的抑菌效果。图中绿色表示抗菌活性较好,红色表示几乎没有抗菌活性,由红色向绿色过渡为浅紫绿色,表示发酵液对检定菌活性较小或没有活性。1-12的培养基分别为PDB、ISP 2、FM3、FM4、FM5、M331、F1、M2、Cazpeck、FM10、FM11、YMS
Fig. 4 Antibacterial activities of fermentation products of 46 representative strains A:Staphylococcus aureus(ATCC 29213). B:Methicillin resistant S. aureus(MRSA)181223. C:Pseudomonas aeruginosa(ATCC 27853). D:Escherichia coli(ATCC 25922). E:Klebsiella pneumoniae(ATCC 700603). F:Carbapenem resistant Acinetobacter baumannii(crab)181236. G:Candida albicans(ATCC 10231). Heatmap:In the 96 well plates of 7 kinds of bacteria,the bacteria liquid fermented in 12 kinds of media was added to each well,and the growth time was 24 h. In the figure,green indicates good antibacterial activity,red indicates almost no antibacterial activity,and the transition from red to green to light purple green indicates that the fermentation broth has little or no antibacterial activity against the tested bacteria. The medium of 1-12 is PDB,ISP 2,FM3,FM4,FM5,M331,F1,M2,Cazpeck,FM10,FM11,and YMS
| 发酵菌株 Fermentation strain | 菌株发酵液对检定菌最大抑制率及发酵培养基 The maximum inhibition rate of fermentation broth to the tested bacteria and fermentation medium | ||||||
|---|---|---|---|---|---|---|---|
| 金黄色葡萄球菌ATCC 29213 | 耐甲氧西林金黄色葡萄球菌MRSA 181223 | 铜绿假单胞菌 ATCC 27853 | 大肠埃希菌ATCC 25922 | 肺炎克雷伯菌ATCC 700603 | 耐碳青霉烯鲍曼不动杆菌 CRAB 181236 | 白色念珠菌ATCC 10231 | |
| Streptomyces sp. XZ19-363 | 87.4%(FM4) | 82.7%(FM4) | 87.1%(FM4) | < 50% | 50.8%(FM4) | 91.3%(FM4) | < 50% |
| Streptomyces sp. XZ19-323 | 86.9%(FM3) | 79.6%(FM3) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-034 | 84.6%(Cazpeck) | 73.1%(Cazpeck) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-175 | 86.2%(FM3) | 79.6%(FM3/FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-147 | 86.4%(FM4) | 81.0%(FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-130 | 88.8% (ISP 2) | 83.4%(ISP 2) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-336 | 88.1%(ISP 2) | 83.1%(ISP 2) | < 50% | < 50% | < 50% | < 50% | 78.9%(FM3) |
| Streptomyces sp. XZ19-098 | 88.3%(FM3) | 82.7% (ISP 2/FM4) | < 50% | 72.6%(YMS) | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-152 | 84.1%(PDB) | 82.7%(FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-091 | 88.1%(FM3) | 83.1%(FM4) | < 50% | < 50% | < 50% | < 50% | 83.8%(FM3) |
| Streptomyces sp. XZ19-122 | 88.3%(FM3) | 83.4%(ISP 2) | < 50% | < 50% | < 50% | 53.0%(F1) | 84.1%(FM3) |
| Streptomyces sp. XZ19-015 | 88.3% (ISP 2) | 83.7%(ISP 2) | < 50% | < 50% | < 50% | 64.4%(FM3) | 83.8%(FM4) |
| Streptomyces sp. XZ19-030 | 88.3% (ISP 2) | 82.0%(FM4) | < 50% | < 50% | < 50% | < 0.5% | 81.0%(M2) |
| Streptomyces sp. XZ19-036 | 87.4% (ISP 2) | 81.7%(ISP 2) | < 50% | < 50% | < 50% | < 0.5% | 85.0% (FM11) |
| Streptomyces sp. XZ19-093 | < 50% | < 50% | < 50% | < 50% | < 50% | 55.1%(M331) | < 50% |
| Streptomyces sp. XZ19-021 | < 50% | < 50% | < 50% | < 50% | < 50% | 55.7%(F1) | < 50% |
| Streptomyces sp. XZ19-006 | 77.3%(M2) | 71.3%(M2) | < 50% | < 50% | < 50% | 51.2%(M331) | < 50% |
| Streptomyces sp. XZ19-003 | 82.2%(YMS) | 65.5%(YMS) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Promicromonospora sp. XZ19-011 | < 50% | < 50% | < 50% | < 50% | < 50% | 73.9%(M331) | < 50% |
| Micromonospora sp. XZ19-205 | 59.4%(YMS) | < 50% | < 50% | < 50% | < 50% | < 50% | < 50% |
| 美罗培南 | 87.1% | 9.50% | 91. 5% | 91.6% | 93.5% | 42.2% | - |
| 万古霉素 | 83.3% | 74.1% | -1.2% | 37.8% | 15.1% | 40.0% | - |
| 氟康唑 | - | - | - | - | - | - | 66.1% |
| 伊曲康唑 | - | - | - | - | - | - | 44.5% |
| 两性霉素B | - | - | - | - | - | - | 64.6% |
表3 部分菌株发酵提取物对检定菌抑制率
Table 3 Inhibition rate of fermentation extracts of some strains to the tested strains
| 发酵菌株 Fermentation strain | 菌株发酵液对检定菌最大抑制率及发酵培养基 The maximum inhibition rate of fermentation broth to the tested bacteria and fermentation medium | ||||||
|---|---|---|---|---|---|---|---|
| 金黄色葡萄球菌ATCC 29213 | 耐甲氧西林金黄色葡萄球菌MRSA 181223 | 铜绿假单胞菌 ATCC 27853 | 大肠埃希菌ATCC 25922 | 肺炎克雷伯菌ATCC 700603 | 耐碳青霉烯鲍曼不动杆菌 CRAB 181236 | 白色念珠菌ATCC 10231 | |
| Streptomyces sp. XZ19-363 | 87.4%(FM4) | 82.7%(FM4) | 87.1%(FM4) | < 50% | 50.8%(FM4) | 91.3%(FM4) | < 50% |
| Streptomyces sp. XZ19-323 | 86.9%(FM3) | 79.6%(FM3) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-034 | 84.6%(Cazpeck) | 73.1%(Cazpeck) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-175 | 86.2%(FM3) | 79.6%(FM3/FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-147 | 86.4%(FM4) | 81.0%(FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-130 | 88.8% (ISP 2) | 83.4%(ISP 2) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-336 | 88.1%(ISP 2) | 83.1%(ISP 2) | < 50% | < 50% | < 50% | < 50% | 78.9%(FM3) |
| Streptomyces sp. XZ19-098 | 88.3%(FM3) | 82.7% (ISP 2/FM4) | < 50% | 72.6%(YMS) | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-152 | 84.1%(PDB) | 82.7%(FM4) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Streptomyces sp. XZ19-091 | 88.1%(FM3) | 83.1%(FM4) | < 50% | < 50% | < 50% | < 50% | 83.8%(FM3) |
| Streptomyces sp. XZ19-122 | 88.3%(FM3) | 83.4%(ISP 2) | < 50% | < 50% | < 50% | 53.0%(F1) | 84.1%(FM3) |
| Streptomyces sp. XZ19-015 | 88.3% (ISP 2) | 83.7%(ISP 2) | < 50% | < 50% | < 50% | 64.4%(FM3) | 83.8%(FM4) |
| Streptomyces sp. XZ19-030 | 88.3% (ISP 2) | 82.0%(FM4) | < 50% | < 50% | < 50% | < 0.5% | 81.0%(M2) |
| Streptomyces sp. XZ19-036 | 87.4% (ISP 2) | 81.7%(ISP 2) | < 50% | < 50% | < 50% | < 0.5% | 85.0% (FM11) |
| Streptomyces sp. XZ19-093 | < 50% | < 50% | < 50% | < 50% | < 50% | 55.1%(M331) | < 50% |
| Streptomyces sp. XZ19-021 | < 50% | < 50% | < 50% | < 50% | < 50% | 55.7%(F1) | < 50% |
| Streptomyces sp. XZ19-006 | 77.3%(M2) | 71.3%(M2) | < 50% | < 50% | < 50% | 51.2%(M331) | < 50% |
| Streptomyces sp. XZ19-003 | 82.2%(YMS) | 65.5%(YMS) | < 50% | < 50% | < 50% | < 50% | < 50% |
| Promicromonospora sp. XZ19-011 | < 50% | < 50% | < 50% | < 50% | < 50% | 73.9%(M331) | < 50% |
| Micromonospora sp. XZ19-205 | 59.4%(YMS) | < 50% | < 50% | < 50% | < 50% | < 50% | < 50% |
| 美罗培南 | 87.1% | 9.50% | 91. 5% | 91.6% | 93.5% | 42.2% | - |
| 万古霉素 | 83.3% | 74.1% | -1.2% | 37.8% | 15.1% | 40.0% | - |
| 氟康唑 | - | - | - | - | - | - | 66.1% |
| 伊曲康唑 | - | - | - | - | - | - | 44.5% |
| 两性霉素B | - | - | - | - | - | - | 64.6% |
| [1] | Frieri M, Kumar K, Boutin A. Antibiotic resistance[J]. J Infect Public Heal, 2017, 10(4): 369-378. |
| [2] |
Xie CL, Xia JM, et al. Metabolomic investigations on Nesterenkonia flava revealed significant differences between marine and terrestrial actinomycetes[J]. Mar Drugs, 2018, 16(10): 356.
doi: 10.3390/md16100356 URL |
| [3] | 任建委, 杜宝中. 放线菌资源及主要次级代谢产物活性概述[J]. 西藏科技, 2020(4): 15-18, 28. |
| Ren JW, Du BZ. Summary of actinomycetes resources and activities of main secondary metabolites[J]. Tibet Sci Technol, 2020(4): 15-18, 28. | |
| [4] | 徐志勇, 冯昭, 徐静. 红树林微生物抗菌活性成分研究进展[J]. 中国抗生素杂志, 2017, 42(4): 241-254. |
| Xu ZY, Feng Z, Xu J. Research advances on antimicrobial activities of microbes derived from mangrove[J]. Chin J Antibiot, 2017, 42(4): 241-254. | |
| [5] |
Clardy J, et al. New antibiotics from bacterial natural products[J]. Nat Biotechnol, 2006, 24(12): 1541-1550.
pmid: 17160060 |
| [6] |
Chen P, Zhang C, Ju X, et al. Community composition and metabolic potential of endophytic actinobacteria from coastal salt marsh plants in Jiangsu, China[J]. Front Microbiol, 2019, 10: 1063.
doi: 10.3389/fmicb.2019.01063 pmid: 31139174 |
| [7] | 张万芹, 等. 贵州兴义喀斯特洞穴可培养放线菌多样性及抗菌活性初筛[J]. 微生物学报, 2020, 60(6): 1063-1073. |
| Zhang WQ, Fang BZ, Han MX, et al. Diversity and antibacterial activity of culturable actinobacteria in Karst cave soil in Xingyi, Guizhou[J]. Acta Microbiol Sin, 2020, 60(6): 1063-1073. | |
| [8] | 祖健, 刘少伟, 庹利, 等. 湖北利川喀斯特洞穴放线菌多样性及抗菌活性研究[J]. 中国抗生素杂志, 2016, 41(3): 186-195. |
| Zu J, Liu SW, Tuo L, et al. Studies on diversity and anti-microbial activity of actinobacteria isolated from Karst caves in Lichuan, Hubei[J]. Chin J Antibiot, 2016, 41(3): 186-195. | |
| [9] | 张爱梅, 韩雪英, 孙坤, 等. 高通量测序分析中国沙棘根瘤与根际土壤细菌多样性[J]. 草原与草坪, 2018, 38(2): 49-55. |
| Zhang AM, Han XY, Sun K, et al. Root nodules endophytic and rhizosphere soil bacteria diversityof Hippophae rhamnoidoes subsp. sinensis based on high-throughput sequencing[J]. Grassland Turf, 2018, 38(2): 49-55. | |
| [10] | 黄路枝, 胡兆农, 郭正彦, 等. 土壤稀有放线菌的选择性分离及其抗菌活性研究[J]. 农药学学报, 2007, 9(1): 59-65. |
| Huang LZ, Hu ZN, Guo ZY, et al. Study on selective isolation and antibiotic activity of rare actinomycetes from soil[J]. Chin J Pestic Sci, 2007, 9(1): 59-65. | |
| [11] | 彭云霞, 姜怡, 段淑蓉, 等. 稀有放线菌的选择性分离方法[J]. 云南大学学报:自然科学版, 2007, 29(1): 86-89. |
| Peng YX, Jiang Y, et al. Selective isolation methods of rare actinomycetes[J]. J Yunnan Univ:Nat Sci Ed, 2007, 29(1): 86-89. | |
| [12] | 李利坤. 沙棘根瘤菌的分离鉴定及根瘤菌对植株生长发育的影响[D]. 长春:吉林农业大学, 2018. |
| Li LK. Isolation and identification of Rhizobium from seabuckthorn and effects of Rhizobium on the growth and development of plants[D]. Changchun:Jilin Agricultural University, 2018. | |
| [13] | 张情. 沙棘根瘤形态结构及根瘤细菌的分离鉴定[D]. 杨凌:西北农林科技大学, 2019. |
| Zhang Q. Morphology and structure of root nodules of Hippophae rhamnides and isolation and identification of bacteria in the nodules[D]. Yangling:Northwest A & F University, 2019. | |
| [14] |
Bauermeister A, Calil FA, das C L Pinto F, et al. Pradimicin-IRD from Amycolatopsis sp. IRD-009 and its antimicrobial and cytotoxic activities[J]. Nat Prod Res, 2019, 33(12): 1713-1720.
doi: 10.1080/14786419.2018.1434639 pmid: 29451013 |
| [15] |
Nakashima T, Kimura T, et al. Nanaomycin H:a new nanaomycin analog[J]. J Biosci Bioeng, 2017, 123(6): 765-770.
doi: S1389-1723(16)30650-8 pmid: 28202308 |
| [16] |
Hashizume H, Sawa R, Yamashita K, et al. Structure and antibacterial activities of new cyclic peptide antibiotics, pargamicins B, C and D, from Amycolatopsis sp. ML1-hF4[J]. J Antibiot, 2017, 70(5): 699-704.
doi: 10.1038/ja.2017.34 pmid: 28293037 |
| [17] |
Serrill JD, Tan M, Fotso S, et al. Apoptolidins A and C activate AMPK in metabolically sensitive cell types and are mechanistically distinct from oligomycin A[J]. Biochem Pharmacol, 2015, 93(3): 251-265.
doi: 10.1016/j.bcp.2014.11.015 URL |
| [18] |
Dasari VRRK, Muthyala MKK, Nikku MY, et al. Novel Pyridinium compound from marine actinomycete, Amycolatopsis alba var. nov. DVR D4 showing antimicrobial and cytotoxic activities in vitro[J]. Microbiol Res, 2012, 167(6): 346-351.
doi: 10.1016/j.micres.2011.12.003 URL |
| [19] | 周双清, 黄小龙, 等. Chelex-100快速提取放线菌DNA作为PCR扩增模板[J]. 生物技术通报, 2010(2): 123-125. |
| Zhou SQ, Huang XL, Huang DY, et al. A rapid method for extracting DNA from actinomycetes by chelex-100[J]. Biotechnol Bull, 2010(2): 123-125. | |
| [20] | 张玉琴, 李文均, 等. PCR法快速识别actinobacteria的五种模板制备方法的比较[J]. 生物技术, 2004, 14(5): 37-39. |
| Zhang YQ, Li WJ, Chen GZ, et al. Comparison of five PCR template preparation methods for fast identification of actinobacteria[J]. Biotechnology, 2004, 14(5): 37-39. | |
| [21] |
Tamura K, Peterson D, Peterson N, et al. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods[J]. Mol Biol Evol, 2011, 28(10): 2731-2739.
doi: 10.1093/molbev/msr121 URL |
| [22] |
Saitou N, Nei M. The neighbor-joining method:a new method for reconstructing phylogenetic trees[J]. Mol Biol Evol, 1987, 4(4): 406-425.
pmid: 3447015 |
| [23] | 周子雄, 黄庆华, 朱爽, 等. 酶标仪快速测定抗菌物质抑菌活性方法的建立[J]. 微生物前沿, 2014, 3(2): 29-35. |
|
Zhou ZX, Huang QH, Zhu S, et al. Establishment of rapid determining method for antibacterial activity by microplate reader[J]. Advances in Microbiology, 2014, 3(2): 29-35.
doi: 10.12677/AMB.2014.32004 URL |
|
| [24] |
Omata K, Watanabe Y, Umegaki T, et al. Catalyst development for methanol synjournal using parallel reactors for high-throughput screening based on a 96 well microplate system[J]. J Japan Petrol Inst, 2003, 46(5): 328-334.
doi: 10.1627/jpi.46.328 URL |
| [25] |
Li YH, He N, Luo MN, et al. Application of untargeted tandem mass spectrometry with molecular networking for detection of enniatins and beauvericins from complex samples[J]. J Chromatogr A, 2020, 1634: 461626.
doi: 10.1016/j.chroma.2020.461626 URL |
| [26] |
Schulz B, Boyle C, Draeger S, et al. Endophytic fungi:a source of novel biologically active secondary metabolites[J]. Mycol Res, 2002, 106(9): 996-1004.
doi: 10.1017/S0953756202006342 URL |
| [27] | 张爱梅. 沙棘属植物根际可培养微生物的初步研究[D]. 兰州:西北师范大学, 2008. |
| Zhang AM. Study on the cultivable microorganism of Hippophae rhizosphere[D]. Lanzhou:Northwest Normal University, 2008. | |
| [28] | 全国细菌耐药监测网. 全国细菌耐药监测网2014—2019年老年患者常见临床分离细菌耐药性监测报告[J]. 中国感染控制杂志, 2021, 20(2): 112-123. |
| System CARS. Antimicrobial resistance of clinically isolated bacteria from elderly patients:surveillance report from China antimicrobial resistance surveillance system in 2014-2019[J]. Chin J Infect Control, 2021, 20(2): 112-123. | |
| [29] | Lee CR, et al. Biology of Acinetobacter baumannii:pathogenesis, antibiotic resistance mechanisms, and prospective treatment options[J]. Front Cell Infect Microbiol, 2017, 7: 55. |
| [1] | 谢田朋, 张佳宁, 董永骏, 张建, 景明. 早期抽薹对当归根际土壤微环境的影响[J]. 生物技术通报, 2023, 39(7): 206-218. |
| [2] | 申云鑫, 施竹凤, 周旭东, 李铭刚, 张庆, 冯路遥, 陈齐斌, 杨佩文. 三株具生防功能芽孢杆菌的分离鉴定及其生物活性研究[J]. 生物技术通报, 2023, 39(3): 267-277. |
| [3] | 刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105. |
| [4] | 谢田朋, 柳娜, 刘越敏, 曲馨, 薄双琴, 景明. 化肥减量配施中药源植物生长调节剂对当归质量和根际土壤细菌群落的影响[J]. 生物技术通报, 2022, 38(3): 79-91. |
| [5] | 龚晓惠, 杨敏, 李舒婷, 林晟豪, 许文涛. 银纳米簇抗菌机理、活性及其应用的研究进展[J]. 生物技术通报, 2021, 37(5): 212-220. |
| [6] | 张雅静, 宋美燕, 张怡静, 房庆, 杨俊, 彭德良, 黄文坤, 彭焕, 朱英波, 孔令安. 兼防黄瓜根腐病和根结线虫病的淡紫拟青霉和哈茨木霉的筛选[J]. 生物技术通报, 2021, 37(2): 40-50. |
| [7] | 张淼, 陈裕凤, 陈龙, 黄飘玲, 韦露玲. 不同地区药用植物两面针根际土壤真菌种群多样性差异分析[J]. 生物技术通报, 2020, 36(9): 167-179. |
| [8] | 潘文娟, 林家富, 王小桃, 郭义东, 褚以文, 刘超兰. 西藏湖泊放线菌的分离鉴定及抗菌活性测定[J]. 生物技术通报, 2020, 36(7): 97-103. |
| [9] | 许广, 王梦姣, 邓百万, 郭苗苗. 不同植茶年限茶树根际土壤细菌多样性及群落结构研究[J]. 生物技术通报, 2020, 36(3): 124-132. |
| [10] | 张鸿雁, 高擎, 张琳园, 林国莉, 李如莲. 大豆疫病拮抗菌的筛选及促生抗病作用研究[J]. 生物技术通报, 2020, 36(10): 25-31. |
| [11] | 李林超, 张超, 董庆, 郭成, 周波, 高峥. 堆肥过程中纤维素降解菌的分离与鉴定[J]. 生物技术通报, 2019, 35(9): 165-171. |
| [12] | 王雪寒, 马强, 田媛, 胡靖, 刘惠荣. 内蒙古呼伦贝尔地区的可培养黏细菌及其抗菌活性[J]. 生物技术通报, 2019, 35(9): 224-233. |
| [13] | 张圣良, 楚肖肖, 赵友兴, 孔凡栋, 黄小龙. 海洋生物共附生真菌的分离、鉴定及抗菌活性分析[J]. 生物技术通报, 2019, 35(3): 59-64. |
| [14] | 朱荣贵, 关统伟, 姜秀娟. 塔里木盆地5个生态小区稀有放线菌分离及合成抗生素基因分布[J]. 生物技术通报, 2018, 34(9): 230-236. |
| [15] | 罗国聪, 黄蕴怡, 柴慧子, 雷晓凌, 聂芳红. 海鞘真菌的形态鉴定及其代谢产物抗菌活性研究[J]. 生物技术通报, 2018, 34(9): 244-248. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||