








生物技术通报 ›› 2022, Vol. 38 ›› Issue (3): 276-284.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1138
兰欣悦1,2( ), 刘宁宁2,3, 朱龙佼2, 陈旭1,2, 褚华硕2, 李相阳4, 段诺5, 许文涛1,2(
), 刘宁宁2,3, 朱龙佼2, 陈旭1,2, 褚华硕2, 李相阳4, 段诺5, 许文涛1,2( )
)
收稿日期:2021-09-04
出版日期:2022-03-26
发布日期:2022-04-06
作者简介:兰欣悦,女,硕士研究生,研究方向:营养与食品安全;E-mail: 基金资助:
LAN Xin-yue1,2( ), LIU Ning-ning2,3, ZHU Long-jiao2, CHEN Xu1,2, CHU Hua-shuo2, LI Xiang-yang4, DUAN Nuo5, XU Wen-tao1,2(
), LIU Ning-ning2,3, ZHU Long-jiao2, CHEN Xu1,2, CHU Hua-shuo2, LI Xiang-yang4, DUAN Nuo5, XU Wen-tao1,2( )
)
Received:2021-09-04
Published:2022-03-26
Online:2022-04-06
摘要:
四环素(tetracycline,TC)作为广普性抗生素,在畜产养殖业中常因使用不当或滥用致使奶乳制品中出现抗生素残留,对人类健康造成严重威胁。传统检测方法如色谱,酶联免疫分析等,对仪器依赖性较强,且检测体系复杂、时间较长,不能很好地满足现场快速检测需求。因此,本研究设计了四环素双价适配体非酶标记传感器,以硫黄素T(ThT)荧光信号变化的形式响应检测结果,在10 nmol/L-1 μmol/L的四环素浓度范围内的对数值呈现良好的线性关系,R 2达0.99以上,检测限低至96 pmol/L,同时,还实现了在20 min内对牛奶样本中四环素的快速检测,且特异性良好。
兰欣悦, 刘宁宁, 朱龙佼, 陈旭, 褚华硕, 李相阳, 段诺, 许文涛. 四环素双价适配体非酶免标记传感器[J]. 生物技术通报, 2022, 38(3): 276-284.
LAN Xin-yue, LIU Ning-ning, ZHU Long-jiao, CHEN Xu, CHU Hua-shuo, LI Xiang-yang, DUAN Nuo, XU Wen-tao. Tetracycline Bivalent Aptamer Non-enzyme Label-free Sensor[J]. Biotechnology Bulletin, 2022, 38(3): 276-284.
| 名称Name | 序列Sequence(5'-3') | 碱基数Number of bases/nt |
|---|---|---|
| 四环素适配体 Tetracycline aptamer(TCA) | CGGTGGTG | 8 |
| 双价裁剪适配体 Bivalent shortened aptamer(TCSA) | CGGTGGTGCGGTGGTG | 16 |
| 间隔插入2T Intervening 2T(2T) | CGGTGGTGTTCGGTGGTG | 18 |
| 间隔插入2A Intervening 2A(2A) | CGGTGGTGAACGGTGGTG | 18 |
| 间隔插入2C Intervening 2C(2C) | 5CGGTGGTGCCCGGTGGTG | 18 |
| 间隔插入2G Intervening 2G(2G) | 5CGGTGGTGGGCGGTGGTG | 18 |
| 间隔插入1G Intervening 1G(1G) | CGGTGGTGGCGGTGGTG | 17 |
| 间隔插入3G Intervening 3G(3G) | CGGTGGTGGGGCGGTGGTG | 19 |
| 间隔插入4G Intervening 4G(4G) | CGGTGGTGGGGGCGGTGGTG | 20 |
| 间隔插入5G Intervening 5G(5G) | CGGTGGTGGGGGGCGGTGGTG | 21 |
表1 实验所用序列
Table 1 Sequences used in experiment
| 名称Name | 序列Sequence(5'-3') | 碱基数Number of bases/nt |
|---|---|---|
| 四环素适配体 Tetracycline aptamer(TCA) | CGGTGGTG | 8 |
| 双价裁剪适配体 Bivalent shortened aptamer(TCSA) | CGGTGGTGCGGTGGTG | 16 |
| 间隔插入2T Intervening 2T(2T) | CGGTGGTGTTCGGTGGTG | 18 |
| 间隔插入2A Intervening 2A(2A) | CGGTGGTGAACGGTGGTG | 18 |
| 间隔插入2C Intervening 2C(2C) | 5CGGTGGTGCCCGGTGGTG | 18 |
| 间隔插入2G Intervening 2G(2G) | 5CGGTGGTGGGCGGTGGTG | 18 |
| 间隔插入1G Intervening 1G(1G) | CGGTGGTGGCGGTGGTG | 17 |
| 间隔插入3G Intervening 3G(3G) | CGGTGGTGGGGCGGTGGTG | 19 |
| 间隔插入4G Intervening 4G(4G) | CGGTGGTGGGGGCGGTGGTG | 20 |
| 间隔插入5G Intervening 5G(5G) | CGGTGGTGGGGGGCGGTGGTG | 21 |
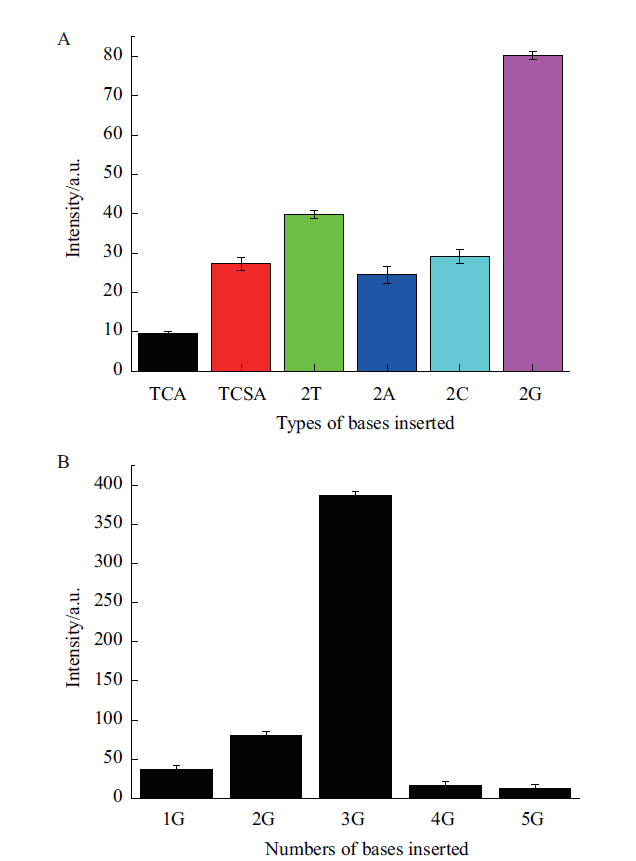
图2 双价适配体间隔插入序列激发ThT能力评估 A:双价适配体间隔插入不同类型碱基的荧光强度;B:双价适配体间隔插入不同数量G碱基的荧光强度
Fig. 2 Ability of bivalent aptamer spacer insert sequences to trigger ThT fluorescence A:Fluorescence intensity of different types of bases inserted at bivalent aptamer spacer. B:Fluorescence intensity of different numbers of G bases inserted at bivalent aptamer spacer
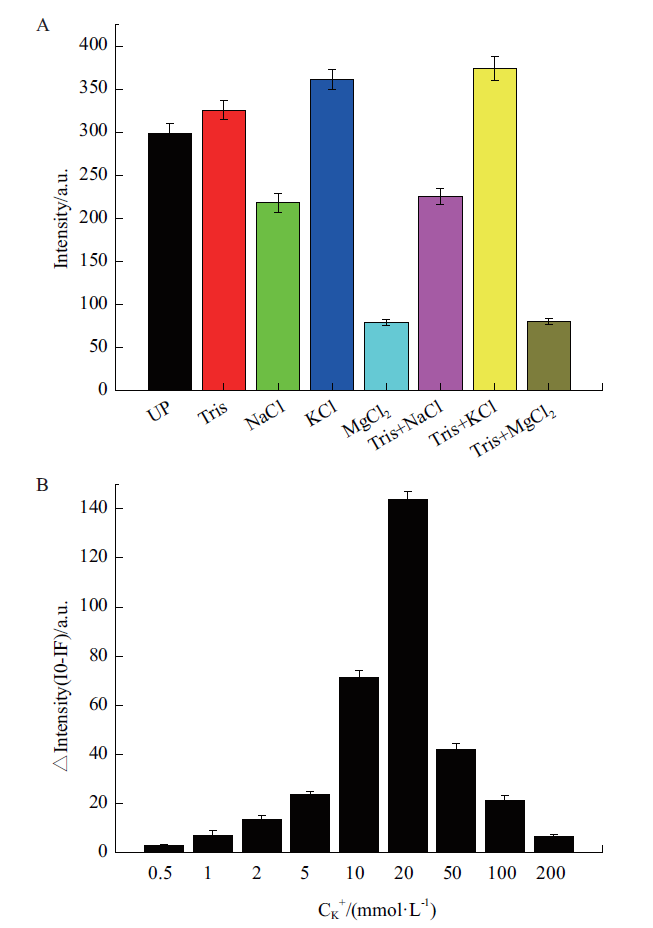
图7 反应体系及离子浓度优化 A:不同缓冲液体系的荧光强度;Tris:20 mmol/L;NaCl:20 mmol/L;KCl:20 mmol/L;MgCl2:20 mmol/L;B:加入靶标前后不同K+浓度条件下荧光强度的变化
Fig. 7 Optimization of reaction system and ion concen-tration A:Fluorescence intensity of different buffer systems;Tris:20 mmol/L;NaCl:20 mmol/L;KCl:20 mmol/L;MgCl2:20 mmol/L. B:Changes of fluorescence intensity under different K+ concentration conditions before and after adding the target
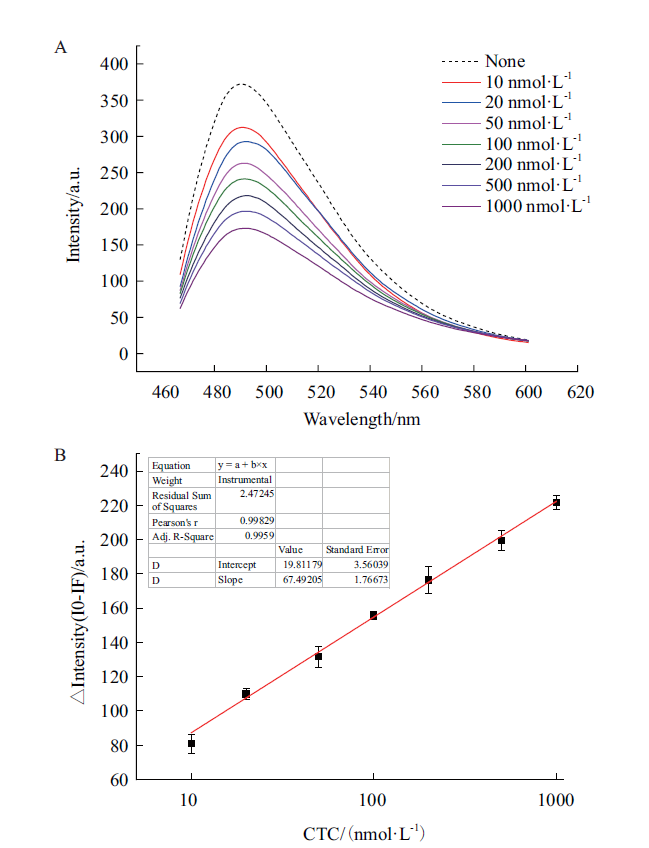
图8 TC传感器的建立 A:不同TC浓度下传感器荧光光谱;B:标准曲线
Fig. 8 Establishment of TC sensor A:Fluorescence spectrum of sensor under different TC concentration. B:Standard curve
| 添加浓度 Added concentration/(nmol·L-1) | △荧光强度 △ Fluorescence intensity/(I0-IF) | 回收浓度 Recycling concentration/(nmol·L-1) | 平均浓度 Average concentration/(nmol·L-1)+相对标准偏差 RSD | 回收率 Recovery rate/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| 20 | 109.7 | 110.8 | 108.4 | 21.5 | 22.3 | 20.5 | 21.4±0.7 | 107.1666667 | |
| 100 | 155.9 | 156.0 | 155.2 | 103.9 | 104.1 | 101.4 | 103.1±1.2 | 103.1333334 | |
| 500 | 204.1 | 202.7 | 203.6 | 537.7 | 512.6 | 510.8 | 520.4±12.3 | 104.0733334 | |
表2 牛奶样品加标回收
Table 2 Standard addition and recovery of milk samples
| 添加浓度 Added concentration/(nmol·L-1) | △荧光强度 △ Fluorescence intensity/(I0-IF) | 回收浓度 Recycling concentration/(nmol·L-1) | 平均浓度 Average concentration/(nmol·L-1)+相对标准偏差 RSD | 回收率 Recovery rate/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| 20 | 109.7 | 110.8 | 108.4 | 21.5 | 22.3 | 20.5 | 21.4±0.7 | 107.1666667 | |
| 100 | 155.9 | 156.0 | 155.2 | 103.9 | 104.1 | 101.4 | 103.1±1.2 | 103.1333334 | |
| 500 | 204.1 | 202.7 | 203.6 | 537.7 | 512.6 | 510.8 | 520.4±12.3 | 104.0733334 | |
| [1] |
Chatten LG, Krause SI. Colorimetric assay of tetracycline antibiotics[J]. J Pharm Sci, 1971, 60(1):107-110.
doi: 10.1002/jps.2600600121 URL |
| [2] |
Oka H, Ito Y, Matsumoto H. Chromatographic analysis of tetracycline antibiotics in foods[J]. J Chromatogr A, 2000, 882(1/2):109-133.
doi: 10.1016/S0021-9673(99)01316-3 URL |
| [3] |
Sun CY, Su RF, Bie JX, et al. Label-free fluorescent sensor based on aptamer and thiazole orange for the detection of tetracycline[J]. Dye Pigment, 2018, 149:867-875.
doi: 10.1016/j.dyepig.2017.11.031 URL |
| [4] | Ibarra IS, Rodriguez JA, Miranda JM, et al. Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis[J]. J Chromatogr A, 2011, 1218(16):2196-2202. |
| [5] | Zaitseva NV, Shur PZ, Atiskova NG, et al. Human health hazards associated with tetracycline drugs residues in food[J]. International Journal of Advanced Research, 2014, 2:488-495. |
| [6] | 于晓雯, 索全义. 畜禽粪便中四环素类抗生素的残留及危害[J]. 北方农业学报, 2018, 46(3):83-88. |
| Yu XW, Suo QY. Residues and hazards of tetracycline antibiotics in livestock and poultry manure[J]. J North Agric, 2018, 46(3):83-88. | |
| [7] |
Pastor-Navarro N, Morais S, Maquieira A, et al. Synjournal of haptens and development of a sensitive immunoassay for tetracycline residues. Application to honey samples[J]. Anal Chim Acta, 2007, 594(2):211-218.
pmid: 17586117 |
| [8] |
Samanidou VF, Nisyriou SA, Papadoyannis IN. Development and validation of an HPLC method for the determination of penicillin antibiotics residues in bovine muscle according to the European Union Decision 2002/657/EC[J]. J Sep Sci, 2007, 30(18):3193-3201.
pmid: 17960837 |
| [9] |
Kargin ID, Sokolova LS, Pirogov AV, et al. HPLC determination of tetracycline antibiotics in milk with post-column derivatization and fluorescence detection[J]. Inorg Mater, 2016, 52(14):1365-1369.
doi: 10.1134/S0020168516140065 URL |
| [10] |
Jiang T, Peng Z, Xie MP, et al. Rapid analysis of tetracycline in honey by microwave plasma torch mass spectrometry with ablation samples[J]. Anal Methods, 2020, 12(4):535-543.
doi: 10.1039/C9AY01887E URL |
| [11] |
Jing T, Gao XD, Wang P, et al. Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography-tandem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction[J]. Anal Bioanal Chem, 2009, 393(8):2009-2018.
doi: 10.1007/s00216-009-2641-z pmid: 19214484 |
| [12] |
Zhang Y, Lu S, Liu W, et al. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk[J]. J Agric Food Chem, 2007, 55(2):211-218.
doi: 10.1021/jf062627s URL |
| [13] |
Tang Y, Huang X, Wang X, et al. G-quadruplex DNAzyme as peroxidase mimetic in a colorimetric biosensor for ultrasensitive and selective detection of trace tetracyclines in foods[J]. Food Chem, 2022, 366:130560.
doi: 10.1016/j.foodchem.2021.130560 URL |
| [14] |
Fu Q, Long C, Qin L, et al. Fluorescent and colorimetric dual-mode detection of tetracycline in wastewater based on heteroatoms-doped reduced state carbon dots[J]. Environ Pollut, 2021, 283:117109.
doi: 10.1016/j.envpol.2021.117109 URL |
| [15] |
Huy BT, Nghia NN, Lee YI. Highly sensitive colorimetric paper-based analytical device for the determination of tetracycline using green fluorescent carbon nitride nanoparticles[J]. Microchem J, 2020, 158:105151.
doi: 10.1016/j.microc.2020.105151 URL |
| [16] |
Shen L, Chen J, Li N, et al. Rapid colorimetric sensing of tetracycline antibiotics with in situ growth of gold nanoparticles[J]. Anal Chim Acta, 2014, 839:83-90.
doi: 10.1016/j.aca.2014.05.021 pmid: 25066722 |
| [17] |
Song JL, Huang MH, Lin XH, et al. Novel Fe-based metal-organic framework(MOF)modified carbon nanofiber as a highly selective and sensitive electrochemical sensor for tetracycline detection[J]. Chem Eng J, 2022, 427:130913.
doi: 10.1016/j.cej.2021.130913 URL |
| [18] |
Verma S, Ravichandiran V, Ranjan N. Beyond amyloid proteins:Thioflavin T in nucleic acid recognition[J]. Biochimie, 2021, 190:111-123.
doi: 10.1016/j.biochi.2021.06.003 URL |
| [19] |
Renaud de la Faverie A, Guédin A, Bedrat A, et al. Thioflavin T as a fluorescence light-up probe for G4 formation[J]. Nucleic Acids Res, 2014, 42(8):e65.
doi: 10.1093/nar/gku111 URL |
| [20] |
Amdursky N, Erez Y, Huppert D. Molecular rotors:what lies behind the high sensitivity of the thioflavin-T fluorescent marker[J]. Acc Chem Res, 2012, 45(9):1548-1557.
doi: 10.1021/ar300053p URL |
| [21] |
Mohanty J, Barooah N, Dhamodharan V, et al. Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA[J]. J Am Chem Soc, 2013, 135(1):367-376.
doi: 10.1021/ja309588h URL |
| [22] | Kim YS, Gu MB. Advances in aptamer screening and small molecule aptasensors[J]. Adv Biochem Eng Biotechnol, 2014, 140:29-67. |
| [23] |
Song KM, Lee S, Ban C. Aptamers and their biological applications[J]. Sensors:Basel, 2012, 12(1):612-631.
doi: 10.3390/s120100612 URL |
| [24] |
Wang T, Chen C, Larcher LM, et al. Three decades of nucleic acid aptamer technologies:Lessons learned, progress and opportunities on aptamer development[J]. Biotechnol Adv, 2019, 37(1):28-50.
doi: S0734-9750(18)30177-0 pmid: 30408510 |
| [25] | Kwon YS, Ahmad Raston NH, Gu MB. An ultra-sensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity[J]. Chem Commun Camb Engl, 2014, 50(1):40-42. |
| [26] |
Ramezani M, Mohammad Danesh N, Lavaee P, et al. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline[J]. Biosens Bioelectron, 2015, 70:181-187.
doi: 10.1016/j.bios.2015.03.040 pmid: 25814407 |
| [27] |
Zhang Z, Tao C, Yin J, et al. Enhancing the response rate of strand displacement-based electrochemical aptamer sensors using bivalent binding aptamer-cDNA probes[J]. Biosens Bioelectron, 2018, 103:39-44.
doi: 10.1016/j.bios.2017.12.027 URL |
| [28] | 沈永聪, 李守军, 杨林. 牛奶中抗生素残留检测技术进展[J]. 畜牧兽医科技信息, 2006(5):87-89. |
| Shen YC, Li SJ, Yang L. Progress in detection technology of antibiotic residues in milk[J]. Chin J Animal Husb Vet Med, 2006(5):87-89. | |
| [29] | 朱红. G-quadruplex结构和稳定性的分子模拟研究[D]. 合肥:中国科学技术大学, 2015. |
| Zhu H. Structural Properties and Stability of G-quadruplex Studied by Molecular Simulations[D]. Hefei:University of Science and Technoloay of China, 2015. | |
| [30] |
Choudhury SD, Mohanty J, Pal H, et al. Cooperative metal ion binding to a cucurbit[7]uril-thioflavin T complex:demonstration of a stimulus-responsive fluorescent supramolecular capsule[J]. J Am Chem Soc, 2010, 132(4):1395-1401.
doi: 10.1021/ja908795y URL |
| [31] |
Stsiapura VI, Maskevich AA, Kuzmitsky VA, et al. Thioflavin T as a molecular rotor:fluorescent properties of thioflavin T in solvents with different viscosity[J]. J Phys Chem B, 2008, 112(49):15893-15902.
doi: 10.1021/jp805822c URL |
| [32] |
Xue WF, Hellewell AL, Gosal WS, et al. Fibril fragmentation enhances amyloid cytotoxicity[J]. J Biol Chem, 2009, 284(49):34272-34282.
doi: 10.1074/jbc.M109.049809 URL |
| [33] |
Biancardi A, Biver T, Burgalassi A, et al. Mechanistic aspects of thioflavin-T self-aggregation and DNA binding:evidence for dimer attack on DNA grooves[J]. Phys Chem Chem Phys, 2014, 16(37):20061-20072.
doi: 10.1039/c4cp02838d pmid: 25130260 |
| [1] | 刘宁宁, 王鑫昕, 兰欣悦, 褚华硕, 陈旭, 常世敏, 李腾飞, 许文涛. G-三链体可视化核酸传感器用于四环素的检测[J]. 生物技术通报, 2022, 38(10): 106-114. |
| [2] | 丰敏, 李舒婷, 张洋子, 粟元, 朱龙佼, 曹际娟, 刘海燕, 许文涛. 基于荧光自淬灭引物的沙门氏菌新型荧光定量PCR方法的开发[J]. 生物技术通报, 2021, 37(11): 285-292. |
| [3] | 吴学玲, 周翔宇, 吴晓燕, 罗奎, 顾怡超, 周晗, 廖婉晴, 曾伟民. 四环素降解菌共培养体系构建及废水修复的群落分析[J]. 生物技术通报, 2020, 36(10): 116-126. |
| [4] | 肖冰, 罗云波, 黄昆仑, 张园, 许文涛. 功能核酸荧光标记型定量统一化检测技术的研究进展[J]. 生物技术通报, 2019, 35(7): 213-221. |
| [5] | 肖冰, 刘榜, 罗云波, 黄昆仑, 张园, 李夏莹, 张秀杰, 许文涛, 周翔. 功能核酸荧光免标记型定量统一化检测技术的研究进展[J]. 生物技术通报, 2019, 35(3): 194-202. |
| [6] | 张永丽, 袁伟, 杨清香. 纳米TiO2和四环素暴露对耐药大肠杆菌的影响[J]. 生物技术通报, 2019, 35(11): 124-131. |
| [7] | 高志强, 汪琳, 蒲静, 尹羿, 张伟, 赵相鹏, 姚震宇. 双重实时荧光PCR定量检测动物产品中牛源性成分[J]. 生物技术通报, 2018, 34(9): 190-194. |
| [8] | 吴学玲,吴晓燕,李交昆,申丽,余润兰,曾伟民. 一株四环素高效降解菌的分离及降解特性[J]. 生物技术通报, 2018, 34(5): 172-178. |
| [9] | 于晓俊, 曹绍玉, 董玉梅, 毕保良, 张应华, 许俊强. 基于四环素调控系统的叶绿体启动子在大肠杆菌中活性检测[J]. 生物技术通报, 2017, 33(2): 149-154. |
| [10] | 张欣阳,蔡婷静,许旭萍. 一株高效四环素降解菌的分离鉴定及其降解性能研究[J]. 生物技术通报, 2015, 31(1): 173-180. |
| [11] | 白荷露, 刘蕊, 朱乃硕. 乙肝病毒X 基因可调控表达细胞模型的构建[J]. 生物技术通报, 2013, 0(2): 140-146. |
| [12] | 黄新;张琰;侯立华;张祺;陈洪俊;朱水芳;. 转基因水稻“科丰6号”实时荧光PCR定性定量检测方法研究[J]. , 2010, 0(02): 90-93. |
| [13] | 张艳;李秋芬;王晓熙;. 应用竞争PCR方法检测亚硝化细菌的研究[J]. , 2009, 0(S1): 394-397. |
| [14] | 张小男;李玉霞;凌焱;梁龙;陈珊;陈惠鹏;. 基于四环素调控系统的病毒载体在基因治疗中的应用[J]. , 2009, 0(10): 49-54. |
| [15] | 王建波;任杰;张映;. 实时荧光定量PCR在猪肺炎支原体检测中的应用[J]. , 2009, 0(07): 60-62. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||