








生物技术通报 ›› 2022, Vol. 38 ›› Issue (9): 158-166.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1444
陈光1,2,3( ), 李佳4, 杜瑞英1,2,3, 王旭1,2,3(
), 李佳4, 杜瑞英1,2,3, 王旭1,2,3( )
)
收稿日期:2021-11-18
出版日期:2022-09-26
发布日期:2022-10-11
作者简介:陈光,男,博士,副研究员,研究方向:植物营养与环境胁迫;E-mail: 基金资助:
CHEN Guang1,2,3( ), LI Jia4, DU Rui-ying1,2,3, WANG Xu1,2,3(
), LI Jia4, DU Rui-ying1,2,3, WANG Xu1,2,3( )
)
Received:2021-11-18
Published:2022-09-26
Online:2022-10-11
摘要:
通过盐胁迫筛选EMS诱变的粳稻突变体库,挖掘盐逆境响应关键基因,鉴定出一个盐敏感突变体。在100 mmol/LNaCl处理下,突变体严重萎蔫,叶片褪绿黄化,地上部Na+的积累显著高于野生型。盐胁迫后,突变体叶片的光合速率和叶绿素含量均显著低于野生型,并且源叶中的蔗糖含量增加,而根中的蔗糖只有野生型的83%。突变体源叶中参与糖转运的几个关键基因表达下调,进一步表明糖分向库器官根系的分配受抑制。突变体库组织中较低的蔗糖含量不能满足生长发育对碳营养的需求,导致水稻耐盐性降低。通过图位克隆确定候选基因,将其命名为SS2,互补实验可以恢复突变体的盐敏感表型,盐胁迫下生长的互补转基因株系与野生型植株无显著差异。综上所述,SS2通过调控体内糖分转运,对水稻的生长和盐胁迫响应起重要作用。
陈光, 李佳, 杜瑞英, 王旭. 水稻盐敏感突变体ss2的鉴定与基因功能分析[J]. 生物技术通报, 2022, 38(9): 158-166.
CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. Identification and Gene Functional Analysis of Salinity-hypersensitive Mutant ss2 in Rice[J]. Biotechnology Bulletin, 2022, 38(9): 158-166.
| 基因名称 Gene name | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsSUT4 | CGCCGGCGGTGGCGGCCTCA | CGTGAGGAGCGAGAGCTGA |
| OsSWEET11 | GACGTTCTTGCAGGTGTACA | TAGCGGACGATGTAGGCGGC |
| OsSWEET14 | TTCCCAACGTGCTGGGCTTCT | GCACCTCGCGGGTCTTGACG |
| OsMT | GCTGCCAGGCAGGAAGCT | GGTTCCAGTTTCACCACGACA |
表1 荧光定量PCR所用引物
Table 1 Primer sequences used for RT-qPCR assays
| 基因名称 Gene name | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsSUT4 | CGCCGGCGGTGGCGGCCTCA | CGTGAGGAGCGAGAGCTGA |
| OsSWEET11 | GACGTTCTTGCAGGTGTACA | TAGCGGACGATGTAGGCGGC |
| OsSWEET14 | TTCCCAACGTGCTGGGCTTCT | GCACCTCGCGGGTCTTGACG |
| OsMT | GCTGCCAGGCAGGAAGCT | GGTTCCAGTTTCACCACGACA |
| 标记 Marker | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| SS11 | GCAACTGGTGGAGTCTATTT | CATGCTAACATGAGGTGATC |
| SS12 | ACGCCTCCCAAGTCGAAAGG | GGTGGGCCTCGATTGTAAGTAG |
| SS10 | TTGGCTCTTCTCCTTAGTAT | CATTTGTATCTTGTGAACGT |
| SS3 | CCAATGTTTGCTCCAGAT | TTCAATGACCCACGTCCC |
| SS4 | TGGTTTTCCTTGTTGCTG | GCTTGCGGCTCTGCTTAC |
| SS20 | TACAGGTATGCTGCTTTTCC | CTGGTCCTTTTCATTCTAAC |
| SS21 | AAAACATGCTCCAACAGCCT | CCAAATGTAGCCAGTGAGGA |
| SS22 | GGAGGAGTTCATTTGAGGCG | CTGGGTGGGCTAGGAAGTAA |
| SS23 | ATACCTCCTTGTATTCGCACT | CGATCGATTGCCACATTATA |
| SS24 | AGCGTGAATCTAATAGCACT | CGTTCAACAAGACCCAATAC |
| SS25 | TTGTAACCGTCGATTTCGTTC | CCGCTCCGTCACTCTACTACC |
| SS16 | CCTCCGACCTCAGCACCTGC | GTTGGCGTCCGCTGCTCCTG |
| SS17 | AGGTAGGCGTGGCGATCAAC | CTTCTCCGGTCACCATCCAC |
| SS27 | TTGGCGATTAATGATCCGGGAAC | CGTTCGTGCCGGTGATGTCG |
| SS29 | ACAACAGTTCTTCACCAGAG | GTAGTATAAATTGTAATAGCTCAA |
| SS32 | GTTAAATGAATCATCAGGAT | AGTAGTCTTGAATTCGCTGT |
表2 基因定位所用的标记引物
Table 2 Marked primer used for gene mapping
| 标记 Marker | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| SS11 | GCAACTGGTGGAGTCTATTT | CATGCTAACATGAGGTGATC |
| SS12 | ACGCCTCCCAAGTCGAAAGG | GGTGGGCCTCGATTGTAAGTAG |
| SS10 | TTGGCTCTTCTCCTTAGTAT | CATTTGTATCTTGTGAACGT |
| SS3 | CCAATGTTTGCTCCAGAT | TTCAATGACCCACGTCCC |
| SS4 | TGGTTTTCCTTGTTGCTG | GCTTGCGGCTCTGCTTAC |
| SS20 | TACAGGTATGCTGCTTTTCC | CTGGTCCTTTTCATTCTAAC |
| SS21 | AAAACATGCTCCAACAGCCT | CCAAATGTAGCCAGTGAGGA |
| SS22 | GGAGGAGTTCATTTGAGGCG | CTGGGTGGGCTAGGAAGTAA |
| SS23 | ATACCTCCTTGTATTCGCACT | CGATCGATTGCCACATTATA |
| SS24 | AGCGTGAATCTAATAGCACT | CGTTCAACAAGACCCAATAC |
| SS25 | TTGTAACCGTCGATTTCGTTC | CCGCTCCGTCACTCTACTACC |
| SS16 | CCTCCGACCTCAGCACCTGC | GTTGGCGTCCGCTGCTCCTG |
| SS17 | AGGTAGGCGTGGCGATCAAC | CTTCTCCGGTCACCATCCAC |
| SS27 | TTGGCGATTAATGATCCGGGAAC | CGTTCGTGCCGGTGATGTCG |
| SS29 | ACAACAGTTCTTCACCAGAG | GTAGTATAAATTGTAATAGCTCAA |
| SS32 | GTTAAATGAATCATCAGGAT | AGTAGTCTTGAATTCGCTGT |

图1 正常和盐胁迫条件下SS2突变对幼苗期水稻生长的影响 A:幼苗期ss2突变体及WT在正常和盐胁迫条件下的生长情况,黄色标尺=1 cm,白色标尺=5 cm;B-E:在正常和盐胁迫条件下ss2突变体及WT的株高(B),地上部鲜重(C),Na+浓度(D)和钠钾浓度比(E)。数值显示的是平均值±SE(n=5),不同的字母表示在P<0.05水平下具有显著差异,下同。DW:干重
Fig.1 Effects of SS2 mutation on the growth performance of seedlings under normal and salinity stress A:Growth performance of WT and ss2 mutants seedling under normal or NaCl treatment. Yellow bar = 1 cm. White bar = 5 cm. B-E:Shoot height(B),shoot fresh weight(C),Na+ concentration([Na])(D)and[Na]/[K]ratio in the shoots(E)of the ss2 mutants under normal and salinity stress conditions. The values are means ± SE of 5 replicates. Significant differences at P < 0.05 are indicated with different letters,the same below. DW:Dry weight
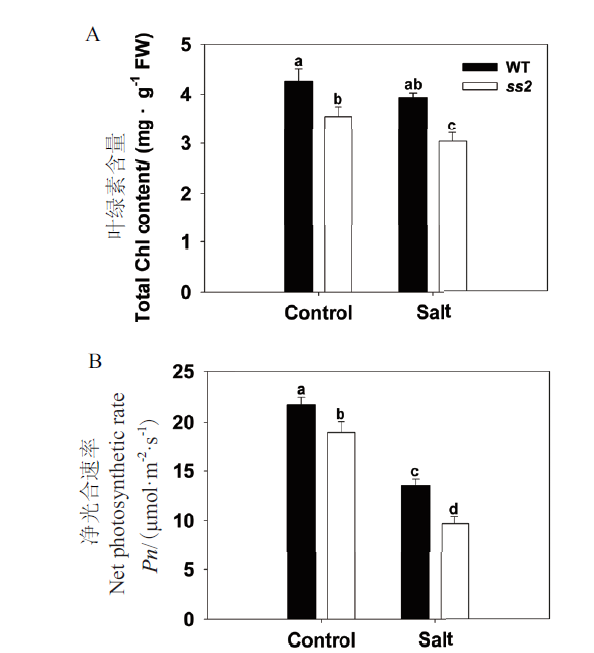
图2 分蘖期WT和ss2对盐胁迫的生理响应 A:总叶绿素含量;B:叶片净光合速率。FW:鲜重,下同
Fig. 2 Physiological responses of WT and ss2 to salinity stress during tillering stage A:Total chlorophyll content. B:Net photosynthetic rate of the leaf. FW:Fresh weight,the same below
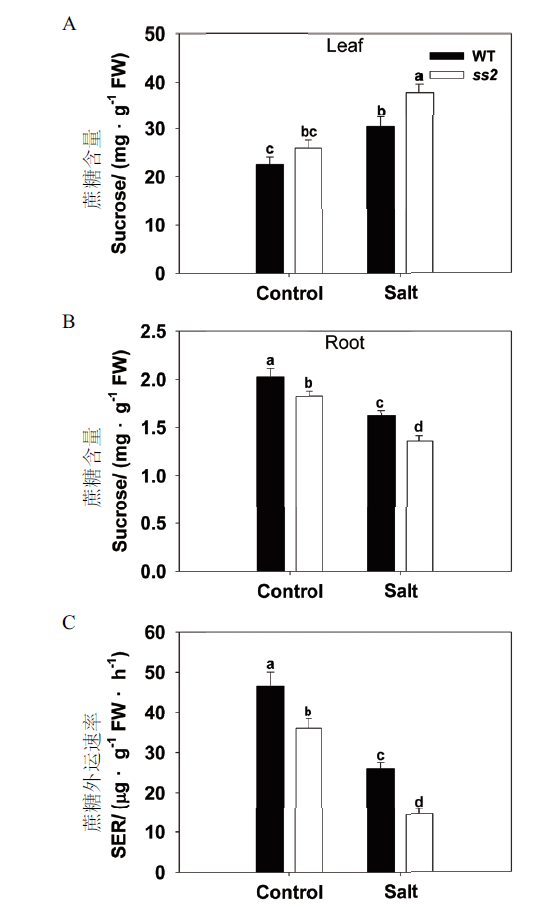
图3 盐胁迫下WT和ss2的蔗糖转运差异分析 A:源叶中蔗糖含量;B:根中蔗糖含量;C:蔗糖外运速率
Fig. 3 Comparison of sucrose transportation between WT and ss2 in response to salinity stress A:Sucrose contents of the leaf. B:Sucrose content of the root. C:Rate of sucrose export from the leaf(SER)
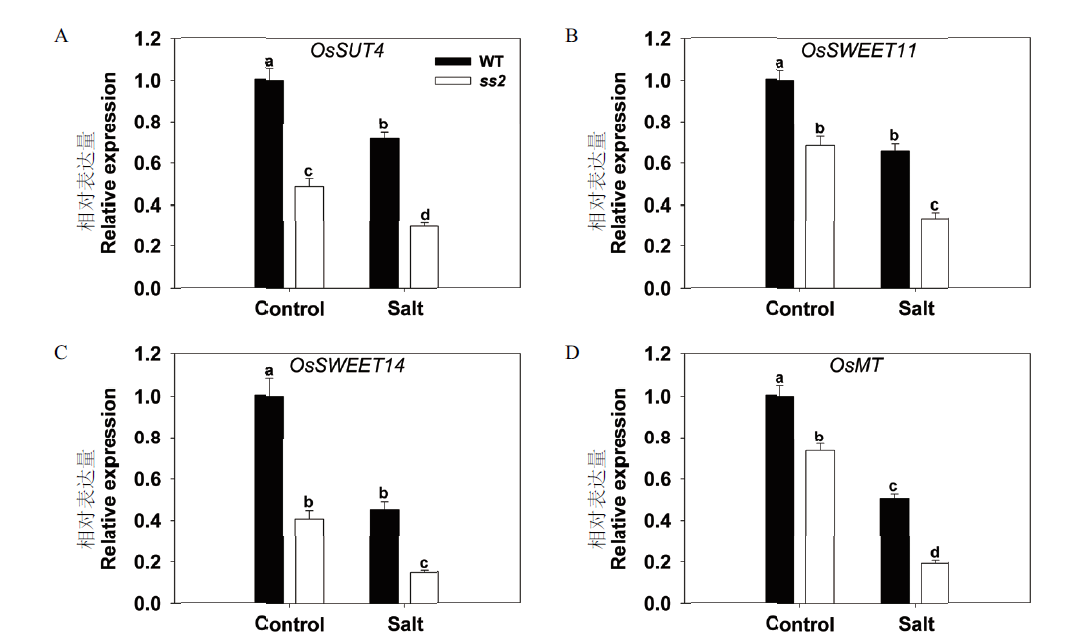
图4 SS2突变对WT和ss2源叶中糖转运相关基因表达的影响 定量检测的基因有OsSUT4(A),OsSWEET11(B),OsSWEET14(C)和OsMT(D)。UBQ5基因作为内参,待测基因在正常生长的WT中的表达量设为1
Fig.4 Effects of SS2 mutation on the expressions of genes related to sugar transport in WT and ss2 leaves The genes assayed were OsSUT4(A),OsSWEET11(B),OsSWEET14(C)and OsMT(D). UBQ5 was chosen as the reference sequence. The expression of to-be-detected genes in non-stressed WT plants was set to 1
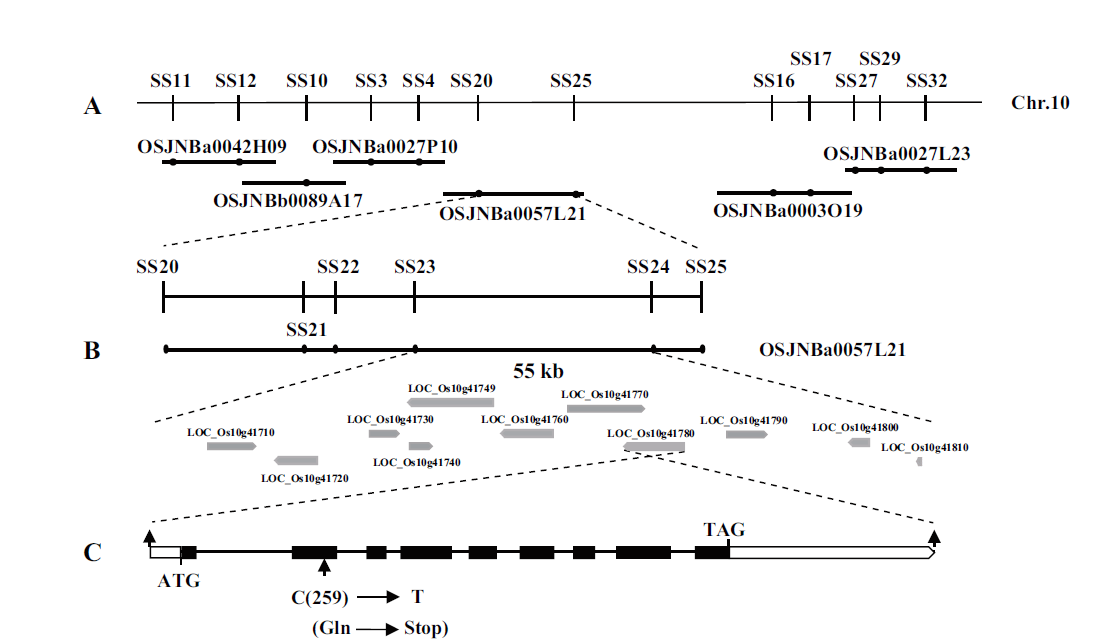
图5 SS2的图位克隆 A:SS2初步定位在第5号染色体上,并用基于BAC克隆OSJNBa0057L21序列开发的标记进行精细定位;B:SS2被精细定位到标记SS23和SS24之间55 kb的物理区域,11个开放阅读框位于该区间;C:SS2候选基因LOC_Os10g41780的基因结构,箭头指示ss2的突变位点
Fig.5 Map-based cloning of SS2 A:SS2 was primarily mapped on chromosome 5 and fine mapping with the markers developed based on the sequence of BAC clone OSJNBa0057L21. B:The SS2 locus was narrowed to a 55 kb genomic DNA region between markers SS23 and SS24. Eleven open reading frames were located in the region. C:Gene structure of the SS2 candidate LOC_Os10g41780. Arrows show mutation sites of ss2

图6 盐胁迫下WT和互补转基因株系的生长差异分析 A:幼苗期转基因互补株系及WT在正常和盐胁迫条件下的生长情况,黄色标尺=1 cm,白色标尺=5 cm。B-E:在正常和盐胁迫条件下转基因互补株系及WT的地上部生物量(B),根系生物量(C),H2O2(D)和MDA含量(E)
Fig.6 Difference analysis in the growth performance of complementary transgenic plant compared with WT in response to salinity stress A:Growth performance of WT and complementary transgenic plants under normal or 100 mmol/L NaCl treatment. Yellow bar = 1 cm. White bar = 5 cm. B-E:Shoot biomass(B),root biomass(C),H2O2(D)and MDA(E)content in the shoots of the seedlings under normal and salinity stress conditions
| [1] |
Campbell MT, Bandillo N, Al Shiblawi FRA, et al. Allelic variants of OsHKT1;1 underlie the divergence between indica and japonica subspecies of rice(Oryza sativa)for root sodium content[J]. PLoS Genet, 2017, 13(6):e1006823.
doi: 10.1371/journal.pgen.1006823 URL |
| [2] |
Khan I, Khan S, Zhang Y, et al. CRISPR-Cas technology based genome editing for modification of salinity stress tolerance responses in rice(Oryza sativa L.)[J]. Mol Biol Rep, 2021, 48(4):3605-3615.
doi: 10.1007/s11033-021-06375-0 URL |
| [3] |
Machado R, Serralheiro R. Soil salinity:effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization[J]. Horticulturae, 2017, 3(2):30.
doi: 10.3390/horticulturae3020030 URL |
| [4] |
Qin H, Li YX, Huang RF. Advances and challenges in the breeding of salt-tolerant rice[J]. Int J Mol Sci, 2020, 21(21):8385.
doi: 10.3390/ijms21218385 URL |
| [5] | 刘奕媺, 于洋, 方军. 盐碱胁迫及植物耐盐碱分子机制研究[J]. 土壤与作物, 2018, 7(2):201-211. |
| Liu YM, Yu Y, Fang J. Saline-alkali stress and molecular mechanism of saline-alkali tolerance in plants[J]. Soils Crops, 2018, 7(2):201-211. | |
| [6] |
Lim JD, Cho JI, Park YI, et al. Sucrose transport from source to sink seeds in rice[J]. Physiol Plant, 2006, 126(4):572-584.
doi: 10.1111/j.1399-3054.2006.00654.x URL |
| [7] |
Eom JS, Choi SB, Ward JM, et al. The mechanism of phloem loading in rice(Oryza sativa)[J]. Mol Cells, 2012, 33(5):431-438.
doi: 10.1007/s10059-012-0071-9 URL |
| [8] |
Kerepesi I, Galiba G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings[J]. Crop Sci, 2000, 40(2):482-487.
doi: 10.2135/cropsci2000.402482x URL |
| [9] |
Watanabe S, Kojima K, Ide Y, et al. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro[J]. Plant Cell Tissue Organ Cult, 2000, 63(3):199-206.
doi: 10.1023/A:1010619503680 URL |
| [10] |
Feng G, Zhang FS, Li XL, et al. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots[J]. Mycorrhiza, 2002, 12(4):185-190.
pmid: 12189473 |
| [11] |
Mathan J, Singh A, Ranjan A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice[J]. Physiol Plant, 2021, 171(4):620-637.
doi: 10.1111/ppl.13210 URL |
| [12] |
Chen G, Feng HM, Hu QD, et al. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development[J]. Plant Biotechnol J, 2015, 13(6):833-848.
doi: 10.1111/pbi.12320 URL |
| [13] |
Chen G, Liu CL, Gao ZY, et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a japonica rice cultivar[J]. Front Plant Sci, 2018, 8:2216.
doi: 10.3389/fpls.2017.02216 URL |
| [14] |
Chen G, Liu CL, Gao ZY, et al. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice[J]. Front Plant Sci, 2017, 8:1885.
doi: 10.3389/fpls.2017.01885 pmid: 29163608 |
| [15] |
Chen G, Hu J, Dong LL, et al. The tolerance of salinity in rice requires the presence of a functional copy of FLN2[J]. Biomolecules, 2019, 10(1):17.
doi: 10.3390/biom10010017 URL |
| [16] |
Chen G, Wu C, He L, et al. Knocking out the gene RLS1 induces hypersensitivity to oxidative stress and premature leaf senescence in rice[J]. Int J Mol Sci, 2018, 19(10):2853.
doi: 10.3390/ijms19102853 URL |
| [17] |
Chen G, Hu J, Lian J, et al. Functional characterization of OsHAK1 promoter in response to osmotic/drought stress by deletion analysis in transgenic rice[J]. Plant Growth Regul, 2019, 88(3):241-251.
doi: 10.1007/s10725-019-00504-3 URL |
| [18] |
Lee S, Kim JH, Yoo ES, et al. Differential regulation of chlorophyll a oxygenase genes in rice[J]. Plant Mol Biol, 2005, 57(6):805-818.
pmid: 15952067 |
| [19] |
Yang YL, Xu J, Huang LC, et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice[J]. J Exp Bot, 2016, 67(5):1297-1310.
doi: 10.1093/jxb/erv529 pmid: 26709310 |
| [20] |
Ferrandino A, Lovisolo C. Abiotic stress effects on grapevine(Vitis vinifera L.):focus on abscisic acid-mediated consequences on secondary metabolism and berry quality[J]. Environ Exp Bot, 2014, 103:138-147.
doi: 10.1016/j.envexpbot.2013.10.012 URL |
| [21] |
Aoki N, Hirose T, Scofield GN, et al. The sucrose transporter gene family in rice[J]. Plant Cell Physiol, 2003, 44(3):223-232.
pmid: 12668768 |
| [22] |
Gong X, Liu ML, Zhang LJ, et al. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway[J]. Physiol Plant, 2015, 153(1):119-136.
doi: 10.1111/ppl.12225 pmid: 24814155 |
| [23] |
Jia WQ, Zhang LJ, Wu D, et al. Sucrose transporter AtSUC9 mediated by a low sucrose level is involved in Arabidopsis abiotic stress resistance by regulating sucrose distribution and ABA accumulation[J]. Plant Cell Physiol, 2015, 56(8):1574-1587.
doi: 10.1093/pcp/pcv082 URL |
| [24] |
Ma QJ, Sun MH, Kang H, et al. A CIPK protein kinase targets sucrose transporter MdSUT2. 2 at Ser254 for phosphorylation to enhance salt tolerance[J]. Plant Cell Environ, 2019, 42(3):918-930.
doi: 10.1111/pce.13349 URL |
| [25] |
Ma QJ, Sun MH, Lu J, et al. An apple sucrose transporter MdSUT2.2is a phosphorylation target for protein kinase MdCIPK22 in response to drought[J]. Plant Biotechnol J, 2019, 17(3):625-637.
doi: 10.1111/pbi.13003 URL |
| [26] | Ibraheem O, Dealtry G, Roux S, et al. The effect of drought and salinity on the expressional levels of sucrose transporters in rice(Oryza sativa Nipponbare)cultivar plants[J]. Plant Omics, 2011, 4(2):68-74. |
| [27] |
Siahpoosh MR, Sanchez DH, Schlereth A, et al. Modification of OsSUT1 gene expression modulates the salt response of rice Oryza sativa cv. Taipei 309[J]. Plant Sci, 2012, 182:101-111.
doi: 10.1016/j.plantsci.2011.01.001 pmid: 22118621 |
| [28] |
Zhou AM, Ma HP, Feng S, et al. A novel sugar transporter from Dianthus spiculifolius, DsSWEET12, affects sugar metabolism and confers osmotic and oxidative stress tolerance in Arabidopsis[J]. Int J Mol Sci, 2018, 19(2):497.
doi: 10.3390/ijms19020497 URL |
| [29] |
Zhou AM, Ma HP, Feng S, et al. DsSWEET17, a tonoplast-localized sugar transporter from Dianthus spiculifolius, affects sugar metabolism and confers multiple stress tolerance in Arabidopsis[J]. Int J Mol Sci, 2018, 19(6):1564.
doi: 10.3390/ijms19061564 URL |
| [30] |
Platten JD, Egdane JA, Ismail AM. Salinity tolerance, Na+ exclusion and allele mining of HKT1;5 in Oryza sativa and O. glaberrima:many sources, many genes, one mechanism?[J]. BMC Plant Biol, 2013, 13:32.
doi: 10.1186/1471-2229-13-32 pmid: 23445750 |
| [31] |
Yeo AR, Yeo ME, Flowers SA, et al. Screening of rice(Oryza sativa L.)genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance[J]. Theor Appl Genet, 1990, 79(3):377-384.
doi: 10.1007/BF01186082 pmid: 24226357 |
| [32] |
Rahman MA, Thomson MJ, Shah-E-Alam M, et al. Exploring novel genetic sources of salinity tolerance in rice through molecular and physiological characterization[J]. Ann Bot, 2016, 117(6):1083-1097.
doi: 10.1093/aob/mcw030 URL |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [3] | 李雪琪, 张素杰, 于曼, 黄金光, 周焕斌. 基于CRISPR/CasX介导的水稻基因组编辑技术的建立[J]. 生物技术通报, 2023, 39(9): 40-48. |
| [4] | 吴元明, 林佳怡, 柳雨汐, 李丹婷, 张宗琼, 郑晓明, 逄洪波. 基于BSA-seq和RNA-seq挖掘水稻株高相关QTL[J]. 生物技术通报, 2023, 39(8): 173-184. |
| [5] | 姚莎莎, 王晶晶, 王俊杰, 梁卫红. 植物激素信号通路调控水稻粒型的分子机制[J]. 生物技术通报, 2023, 39(8): 80-90. |
| [6] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [7] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [8] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [9] | 任沛东, 彭健玲, 刘圣航, 姚姿婷, 朱桂宁, 陆光涛, 李瑞芳. 沙福芽孢杆菌GX-H6的分离鉴定及对水稻细菌性条斑病的防病效果[J]. 生物技术通报, 2023, 39(5): 243-253. |
| [10] | 李怡君, 吴晨晨, 李睿, 王喆, 何山文, 韦善君, 张晓霞. 水稻内生细菌新资源分离培养方案探究[J]. 生物技术通报, 2023, 39(4): 201-211. |
| [11] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [12] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [13] | 卢振万, 李雪琪, 黄金光, 周焕斌. 利用胞嘧啶碱基编辑技术创制耐草甘膦水稻[J]. 生物技术通报, 2023, 39(2): 63-69. |
| [14] | 杨茂, 林宇丰, 戴阳朔, 潘素君, 彭伟业, 严明雄, 李魏, 王冰, 戴良英. OsDIS1通过抗氧化途径负调控水稻耐旱性[J]. 生物技术通报, 2023, 39(2): 88-95. |
| [15] | 蒋铭轩, 李康, 罗亮, 刘建祥, 芦海平. 植物表达外源蛋白研究进展及展望[J]. 生物技术通报, 2023, 39(11): 110-122. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||