








生物技术通报 ›› 2022, Vol. 38 ›› Issue (9): 96-105.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0089
• 细菌耐药性专题(专题主编: 刘雅红 教授 孙坚 教授) • 上一篇 下一篇
收稿日期:2022-01-19
出版日期:2022-09-26
发布日期:2022-10-11
作者简介:刘晓黎,男,硕士研究生,研究方向:生物表面活性剂在抗菌及抗生物膜方面应用;E-mail: 基金资助:
LIU Xiao-li( ), TONG Zhen-yi, ZHAO Liang, YIN Li, LIU Chen-guang(
), TONG Zhen-yi, ZHAO Liang, YIN Li, LIU Chen-guang( )
)
Received:2022-01-19
Published:2022-09-26
Online:2022-10-11
摘要:
幽门螺杆菌感染与多种胃部相关疾病(如胃溃疡、胃癌等)密切相关,是一种严重威胁人类健康的全球性流行病。过去临床上主要通过三联或四联疗法治疗幽门螺杆菌感染,然而由于抗生素的长期使用,幽门螺杆菌不断产生耐药性,导致临床治愈率逐年下降。针对这一问题,近年来非抗生素类抗菌活性物质由于不易诱导耐药性而逐渐受研究者更多关注。本文综述了非抗生素类活性物质(包括植物天然产物、糖脂、多不饱和脂肪酸、抗菌肽、尿素代谢抑制剂和益生菌)在抗幽门螺杆菌感染领域的研究概况和进展,重点介绍了不同活性物质的抗菌机制(如破坏细胞膜、抗生物膜、抑制幽门螺杆菌脲酶、竞争性抑制细菌粘附和分泌细菌素等)和对宿主机体的影响(如抗炎、抑制凋亡、促进黏膜和溃疡的修复及调节消化道菌群等),同时还比较了它们在临床上应用途径的差异。最后,提出了该领域未来需要解决的关键问题,以期为这类物质开发为药物并用于临床提供理论依据与参考。
刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105.
LIU Xiao-li, TONG Zhen-yi, ZHAO Liang, YIN Li, LIU Chen-guang. Research Progress in Non-antibiotic Active Substances Against Helicobacter pylori[J]. Biotechnology Bulletin, 2022, 38(9): 96-105.
| 化合物类型 Compound type | 活性成分 Ingredients | 作用机制 Mechanism | 结构式 Chemical structure | 参考文献 Reference |
|---|---|---|---|---|
| 有机硫Organic sulfur | 大蒜素Allicin | 裂解细胞壁Lyze cell war |  | [ |
| 生物碱Alkaloid | 小檗碱Berberine | 抑制生物膜形成、抗炎Inhibit biofilm formation & anti-inflammatory | 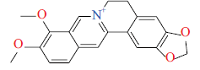 | [ |
| 原小檗碱家族Protoberberine group | 抑制脲酶活性、促进溃疡修复Inhibit urease activity & promote ulcer recovery | 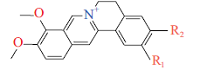 | [ | |
| 有机酸Organic acid | 鞣花酸Ellagic acid | 抑制能量代谢、促进黏膜修复Inhibit energy metabolism and promote mucosa recovery | 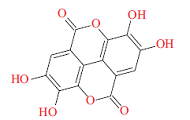 | [ |
| 萜类Terpenoid | 广藿香醇 Patchouli alcohol | 抑制脲酶活性、抑制细菌外排泵基因表达Inhibit urease activity & inhibit efflux pump gene express |  | [ |
| 广防风内酯Ovatodiolide | 抗炎、抑制细菌蛋白表达Anti-inflammation and inhibit protein express |  | [ | |
| 类黄酮Flavonoid | 二氢丹参酮Dihydrotanshinone | 抑制能量代谢、清除成熟生物膜 Inhibit energy metabolism & eradicate mature biofilm |  | [ |
| 橙皮苷Hesperetin | 下调细菌基因表达(脲酶、鞭毛及毒力因子)Down-regulate gene express(urease,flagella and virulence factors) | 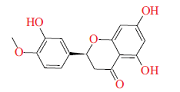 | [ | |
| 杨梅素Myricetin | 抑制细菌转型及生物膜形成 Inhibit cell transformation & biofilm formation |  | [ |
表1 抗幽门螺杆菌植物天然产物及其抑菌机制
Table 1 Anti-H. pylori products from nature plants and their antibacterial mechanism
| 化合物类型 Compound type | 活性成分 Ingredients | 作用机制 Mechanism | 结构式 Chemical structure | 参考文献 Reference |
|---|---|---|---|---|
| 有机硫Organic sulfur | 大蒜素Allicin | 裂解细胞壁Lyze cell war |  | [ |
| 生物碱Alkaloid | 小檗碱Berberine | 抑制生物膜形成、抗炎Inhibit biofilm formation & anti-inflammatory |  | [ |
| 原小檗碱家族Protoberberine group | 抑制脲酶活性、促进溃疡修复Inhibit urease activity & promote ulcer recovery |  | [ | |
| 有机酸Organic acid | 鞣花酸Ellagic acid | 抑制能量代谢、促进黏膜修复Inhibit energy metabolism and promote mucosa recovery |  | [ |
| 萜类Terpenoid | 广藿香醇 Patchouli alcohol | 抑制脲酶活性、抑制细菌外排泵基因表达Inhibit urease activity & inhibit efflux pump gene express |  | [ |
| 广防风内酯Ovatodiolide | 抗炎、抑制细菌蛋白表达Anti-inflammation and inhibit protein express |  | [ | |
| 类黄酮Flavonoid | 二氢丹参酮Dihydrotanshinone | 抑制能量代谢、清除成熟生物膜 Inhibit energy metabolism & eradicate mature biofilm |  | [ |
| 橙皮苷Hesperetin | 下调细菌基因表达(脲酶、鞭毛及毒力因子)Down-regulate gene express(urease,flagella and virulence factors) |  | [ | |
| 杨梅素Myricetin | 抑制细菌转型及生物膜形成 Inhibit cell transformation & biofilm formation |  | [ |
| 底物类型 Compound type | 活性物质 Active content | 合成模式 Synthesis method | 参考文献 Reference |
|---|---|---|---|
| 类黄酮 Flavonoid | 紫草醌 Shikonin | 半合成 Semi-synthesis | [ |
| 绿原酸 Chlorogenic acid | 半合成 Semi-synthesis | [ | |
| 萜类 Terpenoid | 香芹酮 Carvone | 半合成 Semi-synthesis | [ |
| 尿素类似物 Urea analogue | 硫脲 Thiourea | 全合成 Total-synthesis | [ |
| 咔唑-三嗪 Carbazole-triazine | 咔唑 Carbazole | 全合成 Total-synthesis | [ |
表2 新开发的脲酶抑制剂
Table 2 Novel discovered urease inhibitors
| 底物类型 Compound type | 活性物质 Active content | 合成模式 Synthesis method | 参考文献 Reference |
|---|---|---|---|
| 类黄酮 Flavonoid | 紫草醌 Shikonin | 半合成 Semi-synthesis | [ |
| 绿原酸 Chlorogenic acid | 半合成 Semi-synthesis | [ | |
| 萜类 Terpenoid | 香芹酮 Carvone | 半合成 Semi-synthesis | [ |
| 尿素类似物 Urea analogue | 硫脲 Thiourea | 全合成 Total-synthesis | [ |
| 咔唑-三嗪 Carbazole-triazine | 咔唑 Carbazole | 全合成 Total-synthesis | [ |
| 益生菌菌株 Probiotic strain | 抗H. pylori机制 Anti-H. pylori mechanism | 分子机制 Molecular mechanism | 参考文献Reference |
|---|---|---|---|
| 嗜热链球菌Streptococcus thermophilus CRL1190 | 降低炎症反应、抑制黏附Reduce inflammation & inhibit bacteria adhesion | 分泌胞外多糖1190 Secrete extra-polysaccharides 1190 | [ |
| 植物乳杆菌Lactiplantibacillus plantarum ATCC 14917T& R1012 | 抑制毒力因子分泌及脲酶活性Inhibit toxic factor secretion & urease activity | 分泌有机酸和拮抗因子Secrete organic acids & antagonistic factor | [ |
| 鼠李糖乳杆菌Lactobacillus rhamnosus GMNL-74 & 嗜酸乳杆菌Lactobacillus acidophilus GMNL-185 | 抑制黏附及毒力因子分泌Inhibit adhesion & toxic factor secretion | 降低血液中胆固醇水平Reduce cholesterol level in serum | [ |
| 干酪乳杆菌Lactobacillus casei DGDG & 嗜酸乳杆菌Lactobacillus acidophilus LA14 | 抑制脲酶活性、调节肠道菌群Inhibit urease activity & regulate intestinal flora | 分泌乳酸等有机酸Secrete lactic acid and other organic acids | [ |
| 鼠李糖乳杆菌Lactobacillus rhamnosus JB3 | 抑制毒力因子分泌及鞭毛运动Inhibit toxic factor secretion & flagellar movement | 降低宿主受体表达、分泌拮抗因子Reduce host receptor expression & secrete antagonistic factor | [ |
表3 不同益生菌对幽门螺杆菌的抑制作用及其机制
Table 3 Inhibitory activity and mechanisms of probiotics against H. pylori
| 益生菌菌株 Probiotic strain | 抗H. pylori机制 Anti-H. pylori mechanism | 分子机制 Molecular mechanism | 参考文献Reference |
|---|---|---|---|
| 嗜热链球菌Streptococcus thermophilus CRL1190 | 降低炎症反应、抑制黏附Reduce inflammation & inhibit bacteria adhesion | 分泌胞外多糖1190 Secrete extra-polysaccharides 1190 | [ |
| 植物乳杆菌Lactiplantibacillus plantarum ATCC 14917T& R1012 | 抑制毒力因子分泌及脲酶活性Inhibit toxic factor secretion & urease activity | 分泌有机酸和拮抗因子Secrete organic acids & antagonistic factor | [ |
| 鼠李糖乳杆菌Lactobacillus rhamnosus GMNL-74 & 嗜酸乳杆菌Lactobacillus acidophilus GMNL-185 | 抑制黏附及毒力因子分泌Inhibit adhesion & toxic factor secretion | 降低血液中胆固醇水平Reduce cholesterol level in serum | [ |
| 干酪乳杆菌Lactobacillus casei DGDG & 嗜酸乳杆菌Lactobacillus acidophilus LA14 | 抑制脲酶活性、调节肠道菌群Inhibit urease activity & regulate intestinal flora | 分泌乳酸等有机酸Secrete lactic acid and other organic acids | [ |
| 鼠李糖乳杆菌Lactobacillus rhamnosus JB3 | 抑制毒力因子分泌及鞭毛运动Inhibit toxic factor secretion & flagellar movement | 降低宿主受体表达、分泌拮抗因子Reduce host receptor expression & secrete antagonistic factor | [ |
| [1] |
Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection:systematic review and meta-analysis[J]. Gastroenterology, 2017, 153(2):420-429.
doi: 10.1053/j.gastro.2017.04.022 URL |
| [2] |
Kao CY, Sheu BS, Wu JJ. Helicobacter pylori infection:an overview of bacterial virulence factors and pathogenesis[J]. Biomed J, 2016, 39(1):14-23.
doi: 10.1016/j.bj.2015.06.002 URL |
| [3] |
Robinson K, Atherton JC. The spectrum of Helicobacter-mediated diseases[J]. Annu Rev Pathol, 2021, 16:123-144.
doi: 10.1146/annurev-pathol-032520-024949 pmid: 33197219 |
| [4] |
Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis[J]. Gut, 2015, 64(9):1353-1367.
doi: 10.1136/gutjnl-2015-309252 URL |
| [5] | Malfertheiner P, Mégraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection-The Maastricht 2-2000 Consensus Report[J]. Aliment Pharmacol Ther, 2002, 16(2):167-180. |
| [6] |
Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection[J]. Helicobacter, 2018, 23(2):e12475.
doi: 10.1111/hel.12475 URL |
| [7] |
Preston A, Mandrell RE, Gibson BW, et al. The lipooligosaccharides of pathogenic gram-negative bacteria[J]. Crit Rev Microbiol, 1996, 22(3):139-180.
pmid: 8894399 |
| [8] |
Hu Y, Zhang M, Lu B, et al. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem[J]. Helicobacter, 2016, 21(5):349-363.
doi: 10.1111/hel.12299 URL |
| [9] | 陈小楠, 申元娜, 李彭宇, 等. 细菌生物膜的特征及抗细菌生物膜策略[J]. 药学学报, 2018, 53(12):2040-2049. |
| Chen XN, Shen YN, Li PY, et al. Bacterial biofilms:characteristics and combat strategies[J]. Acta Pharm Sin, 2018, 53(12):2040-2049. | |
| [10] |
Gupta P, Sarkar S, Das B, et al. Biofilm, pathogenesis and prevention-a journey to break the wall:a review[J]. Arch Microbiol, 2016, 198(1):1-15.
doi: 10.1007/s00203-015-1148-6 URL |
| [11] |
Liu Y, Busscher HJ, Zhao BR, et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms[J]. ACS Nano, 2016, 10(4):4779-4789.
doi: 10.1021/acsnano.6b01370 pmid: 26998731 |
| [12] |
Salehi B, Sharopov F, Martorell M, et al. Phytochemicals in Helicobacter pylori infections:what are we doing now?[J]. Int J Mol Sci, 2018, 19(8):2361.
doi: 10.3390/ijms19082361 URL |
| [13] | Siddiqui MA, Sadiq O, Iqbal U, et al. Incidence of Clostridium difficile in patient treated with Helicobacter pylori eradication therapy[J]. Gastroenterology, 2017, 152(5):S951. |
| [14] | Baker DA. Plants against Helicobacter pylori to combat resistance:an ethnopharmacological review[J]. Biotechnol Rep(Amst), 2020, 26:e00470. |
| [15] |
Cañizares P, Gracia I, Gómez LA, et al. Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori[J]. Biotechnol Prog, 2004, 20(1):397-401.
doi: 10.1021/bp034143b URL |
| [16] |
Liu WH, Hsu CC, Yin MC. In vitro anti-Helicobacter pylori activity of diallyl sulphides and protocatechuic acid[J]. Phytother Res, 2008, 22(1):53-57.
doi: 10.1002/ptr.2259 URL |
| [17] |
Wu XX, Li X, Dang ZQ, et al. Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor[J]. J Cell Biochem, 2018, 119(7):5373-5381.
doi: 10.1002/jcb.26681 URL |
| [18] |
Shen YN, Zou YQ, Chen XN, et al. Antibacterial self-assembled nanodrugs composed of berberine derivatives and rhamnolipids against Helicobacter pylori[J]. J Control Release, 2020, 328:575-586.
doi: 10.1016/j.jconrel.2020.09.025 URL |
| [19] |
Zhou JT, Li CL, Tan LH, et al. Inhibition of Helicobacter pylori and its associated urease by palmatine:investigation on the potential mechanism[J]. PLoS One, 2017, 12(1):e0168944.
doi: 10.1371/journal.pone.0168944 URL |
| [20] |
Wang L, Wang X, Zhu XM, et al. Gastroprotective effect of alkaloids from cortex phellodendri on gastric ulcers in rats through neurohumoral regulation[J]. Planta Med, 2017, 83(3/4):277-284.
doi: 10.1055/s-0042-114044 URL |
| [21] | Luo PP, Huang YQ, Hang XD, et al. Dihydrotanshinone I is effective against drug-resistant Helicobacter pylori in vitro and in vivo[J]. Antimicrob Agents Chemother, 2021, 65(3):e01921-e01920. |
| [22] |
Kim HW, Woo HJ, Yang JY, et al. Hesperetin inhibits expression of virulence factors and growth of Helicobacter pylori[J]. Int J Mol Sci, 2021, 22(18):10035.
doi: 10.3390/ijms221810035 URL |
| [23] |
Krzyżek P, Migdał P, Paluch E, et al. Myricetin as an antivirulence compound interfering with a morphological transformation into coccoid forms and potentiating activity of antibiotics against Helicobacter pylori[J]. Int J Mol Sci, 2021, 22(5):2695.
doi: 10.3390/ijms22052695 URL |
| [24] |
Si XB, Zhang XM, Wang S, et al. Allicin as add-on therapy for Helicobacter pylori infection:a systematic review and meta-analysis[J]. World J Gastroenterol, 2019, 25(39):6025-6040.
doi: 10.3748/wjg.v25.i39.6025 URL |
| [25] |
Hu Q, Peng Z, Li LL, et al. The efficacy of berberine-containing quadruple therapy on Helicobacter pylori eradication in China:a systematic review and meta-analysis of randomized clinical trials[J]. Front Pharmacol, 2020, 10:1694.
doi: 10.3389/fphar.2019.01694 URL |
| [26] |
Tan LH, Li CL, Chen HB, et al. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean:Susceptibility and mechanism[J]. Eur J Pharm Sci, 2017, 110:77-86.
doi: 10.1016/j.ejps.2017.02.004 URL |
| [27] |
De R, Sarkar A, Ghosh P, et al. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice[J]. J Antimicrob Chemother, 2018, 73(6):1595-1603.
doi: 10.1093/jac/dky079 URL |
| [28] | Lian DW, Xu YF, Ren WK, et al. Mechanism of anti-Helicobacter pylori urease activity of patchouli alcohol[J]. Zhongguo Zhong Yao Za Zhi, 2017, 42(3):562-566. |
| [29] |
Zhong YZ, Tang LY, Deng QH, et al. Unraveling the novel effect of patchouli alcohol against the antibiotic resistance of Helicobacter pylori[J]. Front Microbiol, 2021, 12:674560.
doi: 10.3389/fmicb.2021.674560 URL |
| [30] |
Lien HM, Wu HY, Hung CL, et al. Antibacterial activity of ovatodiolide isolated from Anisomeles indica against Helicobacter pylori[J]. Sci Rep, 2019, 9(1):4205.
doi: 10.1038/s41598-019-40735-y URL |
| [31] |
Shu Q, Lou HH, Wei TY, et al. Contributions of glycolipid biosurfactants and glycolipid-modified materials to antimicrobial strategy:a review[J]. Pharmaceutics, 2021, 13(2):227.
doi: 10.3390/pharmaceutics13020227 URL |
| [32] |
Shen YN, Li PY, Chen XN, et al. Activity of sodium lauryl sulfate, rhamnolipids, and N-acetylcysteine against biofilms of five common pathogens[J]. Microb Drug Resist, 2020, 26(3):290-299.
doi: 10.1089/mdr.2018.0385 URL |
| [33] |
Chen XN, Li PY, Shen YN, et al. Rhamnolipid-involved antibiotics combinations improve the eradication of Helicobacter pylori biofilm in vitro:a comparison with conventional triple therapy[J]. Microb Pathog, 2019, 131:112-119.
doi: 10.1016/j.micpath.2019.04.001 URL |
| [34] |
Li PY, Chen XN, Shen YN, et al. Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of Helicobacter pylori biofilm[J]. J Control Release, 2019, 300:52-63.
doi: 10.1016/j.jconrel.2019.02.039 URL |
| [35] |
Wille JJ, Kydonieus A. Palmitoleic acid isomer(C16:1delta6)in human skin sebum is effective against gram-positive bacteria[J]. Skin Pharmacol Appl Skin Physiol, 2003, 16(3):176-187.
pmid: 12677098 |
| [36] |
Khulusi S, Ahmed HA, Patel P, et al. The effects of unsaturated fatty acids on Helicobacter pylori in vitro[J]. J Med Microbiol, 1995, 42(4):276-282.
pmid: 7707336 |
| [37] |
Petschow BW, Batema RP, Ford LL. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids[J]. Antimicrob Agents Chemother, 1996, 40(2):302-306.
pmid: 8834870 |
| [38] |
Bergsson G, Steingrímsson O, Thormar H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori[J]. Int J Antimicrob Agents, 2002, 20(4):258-262.
doi: 10.1016/S0924-8579(02)00205-4 URL |
| [39] |
Correia M, Michel V, Matos AA, et al. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization[J]. PLoS One, 2012, 7(4):e35072.
doi: 10.1371/journal.pone.0035072 URL |
| [40] |
Obonyo M, Zhang L, Thamphiwatana S, et al. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori[J]. Mol Pharm, 2012, 9(9):2677-2685.
doi: 10.1021/mp300243w URL |
| [41] |
Thamphiwatana S, Gao WW, Obonyo M, et al. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation[J]. PNAS, 2014, 111(49):17600-17605.
doi: 10.1073/pnas.1418230111 pmid: 25422427 |
| [42] |
Cong Y, Geng JY, Wang HY, et al. Ureido-modified carboxymethyl chitosan-graft-stearic acid polymeric nano-micelles as a targeted delivering carrier of clarithromycin for Helicobacter pylori:preparation and in vitro evaluation[J]. Int J Biol Macromol, 2019, 129:686-692.
doi: 10.1016/j.ijbiomac.2019.01.227 URL |
| [43] | Huang YQ, Hang XD, Jiang XQ, et al. In vitro and in vivo activities of zinc linolenate, a selective antibacterial agent against Helicobacter pylori[J]. Antimicrob Agents Chemother, 2019, 63(6):e00004-e00019. |
| [44] |
Zhang L, Wu WKK, Gallo RL, et al. Critical role of antimicrobial peptide cathelicidin for controlling Helicobacter pylori survival and infection[J]. J Immunol, 2016, 196(4):1799-1809.
doi: 10.4049/jimmunol.1500021 pmid: 26800870 |
| [45] |
Pero R, Coretti L, Nigro E, et al. β-defensins in the fight against Helicobacter pylori[J]. Molecules, 2017, 22(3):424-440.
doi: 10.3390/molecules22030424 URL |
| [46] |
Neshani A, Zare H, Akbari Eidgahi MR, et al. Review of antimicrobial peptides with anti-Helicobacter pylori activity[J]. Helicobacter, 2019, 24(1):e12555.
doi: 10.1111/hel.12555 URL |
| [47] |
Kang HK, Kim C, Seo CH, et al. The therapeutic applications of antimicrobial peptides(AMPs):a patent review[J]. J Microbiol, 2017, 55(1):1-12.
doi: 10.1007/s12275-017-6452-1 URL |
| [48] | 彭建, 赵行行, 吴兆颖, 等. 抗菌肽Cec4的结构改造及抗菌活性研究[J]. 生物技术, 2019, 29(4):330-335. |
| Peng J, Zhao XX, Wu ZY, et al. Structural modification and antibacterial related activity study of antimicrobial peptide Cec4[J]. Biotechnology, 2019, 29(4):330-335. | |
| [49] |
Olleik H, Baydoun E, Perrier J, et al. Temporin-SHa and its analogs as potential candidates for the treatment of Helicobacter pylori[J]. Biomolecules, 2019, 9(10):598-620.
doi: 10.3390/biom9100598 URL |
| [50] |
Ramsay KST, Wafo P, Ali Z, et al. Chemical constituents of Stereospermum acuminatissimum and their urease and α-chymotrypsin inhibitions[J]. Fitoterapia, 2012, 83(1):204-208.
doi: 10.1016/j.fitote.2011.10.014 URL |
| [51] |
Nagata K, Takagi E, Satoh H, et al. Growth inhibition of Ureaplasma urealyticum by the proton pump inhibitor lansoprazole:direct attribution to inhibition by lansoprazole of urease activity and urea-induced ATP synthesis in U. urealyticum[J]. Antimicrob Agents Chemother, 1995, 39(10):2187-2192.
doi: 10.1128/AAC.39.10.2187 pmid: 8619564 |
| [52] |
Sivapriya K, Suguna P, Banerjee A, et al. Facile one-pot synthesis of thio and selenourea derivatives:a new class of potent urease inhibitors[J]. Bioorg Med Chem Lett, 2007, 17(22):6387-6391.
doi: 10.1016/j.bmcl.2007.07.085 URL |
| [53] |
Ni WW, Liu Q, Ren SZ, et al. The synthesis and evaluation of phenoxyacylhydroxamic acids as potential agents for Helicobacter pylori infections[J]. Bioorg Med Chem, 2018, 26(14):4145-4152.
doi: 10.1016/j.bmc.2018.07.003 URL |
| [54] |
Domínguez MJ, Sanmartín C, Font M, et al. Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors[J]. J Agric Food Chem, 2008, 56(10):3721-3731.
doi: 10.1021/jf072901y URL |
| [55] |
Yang YS, Su MM, Zhang XP, et al. Developing potential Helicobacter pylori urease inhibitors from novel oxoindoline derivatives:Synthesis, biological evaluation and in silico study[J]. Bioorg Med Chem Lett, 2018, 28(19):3182-3186.
doi: 10.1016/j.bmcl.2018.08.025 URL |
| [56] |
Li WY, Ni WW, Ye YX, et al. N-monoarylacetothioureas as potent urease inhibitors:synthesis, SAR, and biological evaluation[J]. J Enzyme Inhib Med Chem, 2020, 35(1):404-413.
doi: 10.1080/14756366.2019.1706503 URL |
| [57] |
Kataria R, Khatkar A. In-silico design, synthesis, ADMET studies and biological evaluation of novel derivatives of chlorogenic acid against urease protein and H. pylori bacterium[J]. BMC Chem, 2019, 13(1):41-57.
doi: 10.1186/s13065-019-0556-0 pmid: 31384789 |
| [58] |
Kozioł A, Macegoniuk K, Grela E, et al. Synthesis of terpenoid oxo derivatives with antiureolytic activity[J]. Mol Biol Rep, 2019, 46(1):51-58.
doi: 10.1007/s11033-018-4442-y pmid: 30350237 |
| [59] |
Ibrar A, Kazmi M, Khan A, et al. Robust therapeutic potential of carbazole-triazine hybrids as a new class of urease inhibitors:a distinctive combination of nitrogen-containing heterocycles[J]. Bioorg Chem, 2020, 95:103479.
doi: 10.1016/j.bioorg.2019.103479 URL |
| [60] |
Reid G. Probiotics:definition, scope and mechanisms of action[J]. Best Pract Res Clin Gastroenterol, 2016, 30(1):17-25.
doi: 10.1016/j.bpg.2015.12.001 URL |
| [61] |
Gong Y, Li Y, Sun Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori:a meta-analysis of randomized controlled trials[J]. Int J Clin Exp Med, 2015, 8(4):6530-6543.
pmid: 26131283 |
| [62] |
Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy[J]. Aliment Pharmacol Ther, 2001, 15(2):163-169.
doi: 10.1046/j.1365-2036.2001.00923.x URL |
| [63] |
Oh B, Kim JW, Kim BS. Changes in the functional potential of the gut microbiome following probiotic supplementation during Helicobacter pylori treatment[J]. Helicobacter, 2016, 21(6):493-503.
doi: 10.1111/hel.12306 URL |
| [64] |
Feng JR, Wang F, Qiu X, et al. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children:a systematic review and network meta-analysis[J]. Eur J Clin Pharmacol, 2017, 73(10):1199-1208.
doi: 10.1007/s00228-017-2291-6 URL |
| [65] |
Gotteland M, Brunser O, Cruchet S. Systematic review:are probiotics useful in controlling gastric colonization by Helicobacter pylori?[J]. Aliment Pharmacol Ther, 2006, 23(8):1077-1086.
doi: 10.1111/j.1365-2036.2006.02868.x URL |
| [66] |
Lee JS, Paek NS, Kwon OS, et al. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling(SOCS)expression and signaling in Helicobacter pylori infection:a novel mechanism[J]. J Gastroenterol Hepatol, 2010, 25(1):194-202.
doi: 10.1111/j.1440-1746.2009.06127.x URL |
| [67] |
Mukai TK, Asasaka T, Sato E, et al. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri[J]. FEMS Immunol Med Microbiol, 2002, 32(2):105-110.
doi: 10.1111/j.1574-695X.2002.tb00541.x URL |
| [68] |
Kim TS, Hur JW, Yu MA, et al. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria[J]. J Food Prot, 2003, 66(1):3-12.
doi: 10.4315/0362-028X-66.1.3 URL |
| [69] |
Şirvan BN, Usta MK, Kizilkan NU, et al. Are synbiotics added to the standard therapy to eradicate Helicobacter pylori in children beneficial? A randomized controlled study[J]. Euroasian J Hepatogastroenterol, 2017, 7(1):17-22.
doi: 10.5005/jp-journals-10018-1205 pmid: 29201766 |
| [70] |
Marcial G, Villena J, Faller G, et al. Exopolysaccharide-producing Streptococcus thermophilus CRL1190 reduces the inflammatory response caused by Helicobacter pylori[J]. Benef Microbes, 2017, 8(3):451-461.
doi: 10.3920/BM2016.0186 pmid: 28504579 |
| [71] |
Hu JF, Tian XQ, Wei T, et al. Anti- Helicobacter pylori activity of a Lactobacillus sp. PW-7 exopolysaccharide[J]. Foods, 2021, 10(10):2453.
doi: 10.3390/foods10102453 URL |
| [72] |
Whiteside SA, Mohiuddin MM, Shlimon S, et al. In vitro framework to assess the anti-Helicobacter pylori potential of lactic acid bacteria secretions as alternatives to antibiotics[J]. Int J Mol Sci, 2021, 22(11):5650.
doi: 10.3390/ijms22115650 URL |
| [73] |
Chen YH, Tsai WH, Wu HY, et al. Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation[J]. J Clin Med, 2019, 8(1):90.
doi: 10.3390/jcm8010090 URL |
| [74] | Saracino IM, Pavoni M, Saccomanno L, et al. Antimicrobial efficacy of five probiotic strains against Helicobacter pylori[J]. Antibiotics(Basel), 2020, 9(5):244. |
| [75] | Do AD, Chang CC, Su CH, et al. Lactobacillus rhamnosus JB3 inhibits Helicobacter pylori infection through multiple molecular actions[J]. Helicobacter, 2021, 26(3):e12806. |
| [1] | 周璐祺, 崔婷茹, 郝楠, 赵雨薇, 赵斌, 刘颖超. 化学蛋白质组学在天然产物分子靶标鉴定中的应用[J]. 生物技术通报, 2023, 39(9): 12-26. |
| [2] | 陈勇, 李亚鑫, 王亚瑄, 梁露洁, 冯思源, 田国宝. MCR-1介导多黏菌素耐药性的分子机制研究进展[J]. 生物技术通报, 2023, 39(6): 102-108. |
| [3] | 周闪闪, 黄远龙, 黄建忠, 李善仁. 溶杆菌中活性天然产物的研究进展[J]. 生物技术通报, 2023, 39(10): 41-49. |
| [4] | 文畅, 刘晨, 卢诗韵, 许忠兵, 艾超凡, 廖汉鹏, 周顺桂. 一株新的多重耐药福氏志贺菌噬菌体生物学特性及基因组分析[J]. 生物技术通报, 2022, 38(9): 127-135. |
| [5] | 胡功政, 崔小蝶, 翟亚军, 贺丹丹. 细菌黏菌素耐药性及其逆转机制研究进展[J]. 生物技术通报, 2022, 38(9): 28-34. |
| [6] | 刘艺云, 邓利敏, 岳慧颖, 岳超, 刘健华. 质粒接合转移及其抑制剂的研究进展[J]. 生物技术通报, 2022, 38(9): 35-46. |
| [7] | 刘成程, 胡小芳, 冯友军. 细菌耐药:生化机制与应对策略[J]. 生物技术通报, 2022, 38(9): 4-16. |
| [8] | 陈福暖, 黄瑜, 蔡佳, 王忠良, 简纪常, 王蓓. ABC转运蛋白结构及其在细菌致病性中的研究进展[J]. 生物技术通报, 2022, 38(6): 43-52. |
| [9] | 赵海晴, 李耘, 梁严内, 刘哲, 任亚林, 李金娟. 联合用药对嗜水气单胞菌耐药性影响研究进展[J]. 生物技术通报, 2022, 38(6): 53-65. |
| [10] | 朱浩, 张严伟, 刘润, 梁艳, 杨奕, 徐天乐, 杨章平. 抗生素佐剂与抗生素联用的抑菌作用研究进展[J]. 生物技术通报, 2022, 38(6): 66-73. |
| [11] | 张国宁, 冯婧娴, 杨颖博, 陈万生, 肖莹. 环糊精葡萄糖基转移酶在天然产物糖基化修饰中的应用[J]. 生物技术通报, 2022, 38(3): 246-255. |
| [12] | 成温玉, 张博昕, 赵鸿远, 陈艳, 谢娟平. 天然产物抗猪流行性腹泻病毒研究进展[J]. 生物技术通报, 2022, 38(12): 127-136. |
| [13] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [14] | 赵鸿远, 王朝, 成温玉, 马宁宁, 李曼, 魏小丽. 抗非洲猪瘟病毒制剂的研究进展[J]. 生物技术通报, 2021, 37(5): 174-181. |
| [15] | 龚晓惠, 杨敏, 李舒婷, 林晟豪, 许文涛. 银纳米簇抗菌机理、活性及其应用的研究进展[J]. 生物技术通报, 2021, 37(5): 212-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||