








生物技术通报 ›› 2022, Vol. 38 ›› Issue (11): 70-79.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0193
收稿日期:2022-02-17
出版日期:2022-11-26
发布日期:2022-12-01
作者简介:陈宇捷,男,研究方向:水污染治理;E-mail:基金资助:
CHEN Yu-jie( ), ZHENG Hua-bao, ZHOU Xin-yan(
), ZHENG Hua-bao, ZHOU Xin-yan( )
)
Received:2022-02-17
Published:2022-11-26
Online:2022-12-01
摘要:
研究藻华水体中浮游植物群落对除藻剂的响应,可为在实际应用中选择合适的除藻剂提供理论依据。近年来,高通量测序技术已逐步用于表征藻类群落结构,然而它无法区分群落中的“死亡”和“存活”个体。本研究利用经叠氮溴化丙锭(PMA)前处理和未经PMA前处理的高通量测序技术,探究Ca(ClO)2、CuSO4和一种市售生物除藻剂对某一存在藻华的景观水体中藻类群落组成、多样性和标志物种的影响。结果显示,3种除藻剂均能有效降低水样的藻密度;初始水样中绿藻门的单针藻属(Monoraphidium)相对丰度最大(74.34%),是造成藻华的优势藻;与非PMA处理组相比,经PMA处理后的测序结果更能体现不同除藻剂处理后活藻群落组成和多样性差异:在除藻剂作用下,绿藻门相对丰度下降至1.50%-24.61%,蓝藻门优势种Leptolyngbya boryana相对丰度上升至27.85%-41.52%;50 mg/L生物除藻剂能显著提升群落的Chao1指数、observed species指数、Pielou's evenness指数和Shannon指数,增加了群落的多样性、均匀性和稳定性;主坐标分析表明投加量增大不会显著改变Ca(ClO)2和生物除藻剂处理组的活藻群落结构;物种差异分析显示,Chroococcidiopsis sp.这一典型的逆境环境优势藻种可在1.0 mg/LCuSO4和50 mg/L生物除藻剂处理组中富集。基于PMA前处理的高通量测序技术,为研究不同类型除藻剂处理后藻华水体中活藻群落结构的变化提供了一种有效的技术手段。
陈宇捷, 郑华宝, 周昕彦. 改良高通量测序技术揭示除藻剂对藻类群落的影响[J]. 生物技术通报, 2022, 38(11): 70-79.
CHEN Yu-jie, ZHENG Hua-bao, ZHOU Xin-yan. Modified High-throughput Sequencing Reveals the Effects of Different Algicides towards Algal Community[J]. Biotechnology Bulletin, 2022, 38(11): 70-79.
| 氨氮Ammonia nitrogen/(mg·L-1) | 总氮Total nitrogen/(mg·L-1) | 总磷Total phosphorus/(mg·L-1) | 化学需氧量Chemical oxidation demand/(mg·L-1) | 浊度 Turbidity/(mg·L-1) | 藻细胞密度 Algal cell density/(106 cell·mL-1) | 叶绿素a Chlorophyll a/(μg·L-1) |
|---|---|---|---|---|---|---|
| 0.60 | 0.79 | 0.05 | 35 | 23.7±1.1 | 1.41 | 184 |
表1 景观池塘水基本水质参数
Table 1 Basic water quality parameters of the landscape pond
| 氨氮Ammonia nitrogen/(mg·L-1) | 总氮Total nitrogen/(mg·L-1) | 总磷Total phosphorus/(mg·L-1) | 化学需氧量Chemical oxidation demand/(mg·L-1) | 浊度 Turbidity/(mg·L-1) | 藻细胞密度 Algal cell density/(106 cell·mL-1) | 叶绿素a Chlorophyll a/(μg·L-1) |
|---|---|---|---|---|---|---|
| 0.60 | 0.79 | 0.05 | 35 | 23.7±1.1 | 1.41 | 184 |
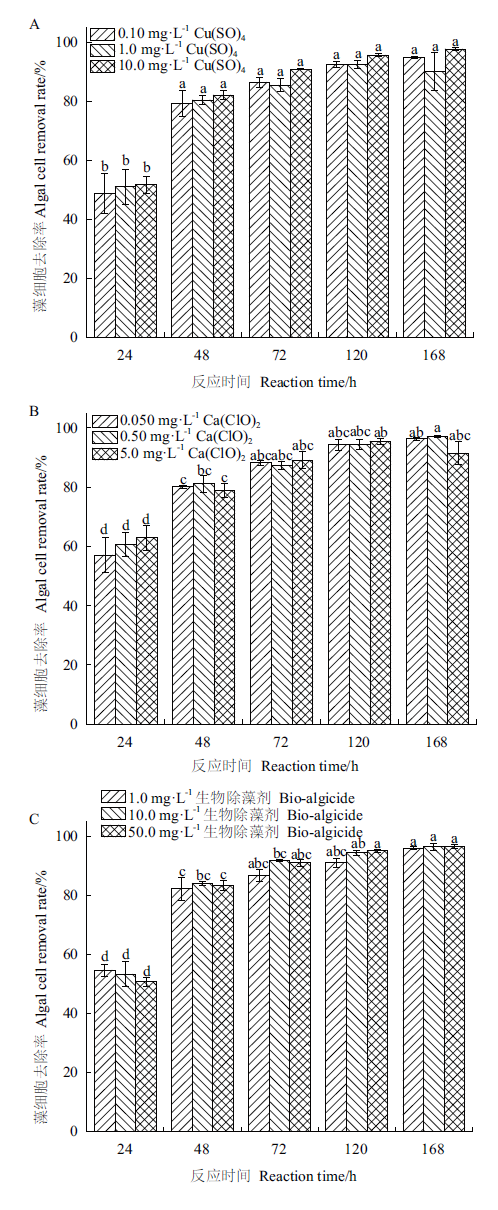
图1 3种除藻剂对藻细胞的去除效果 图中数据经Tukey检验,柱子上不同字母表示在0.05水平差异显著
Fig.1 Removal efficiencies of algal cells by 3 algicides Data of the bar marked by different letters indicate significant difference at 0.05 levels by Tukey’s test

图2 3种除藻剂对藻类群落组成(门水平)的影响 Initial代表初始时刻藻类群落;CK代表对照组;Cl、Cu和Bio分别代表Ca(ClO)2、CuSO4和生物除藻剂;数字代表除藻剂的投加量(单位:mg/L);NPMA代表未经PMA处理的样本;PMA代表经PMA处理的样本,下同
Fig. 2 Influences of 3 algicides on algal community compositions(phylum level) “Initial” refers to the initial algal community;“CK” refers to control check;“Cl”,“Cu” and “Bio” refers to Ca(ClO)2,CuSO4 and bio-algicide,respectively;number indicates the dosage of algicide(mg/L);NPMA refers to the samples without PMA pre-treatment;PMA refers to the samples with PMA pre-treatment,the same below
| 物种名称 Species name | Initial /% | CKNPMA /% | Cl-0.5NPMA /% | Cl-5.0NPMA /% | Cu-0.1NPMA /% | Cu-1.0NPMA /% | Bio-10NPMA /% | Bio-50NPMA /% | CKPMA /% | Cl-0.5PMA /% | Cl-5.0PMA /% | Cu-0.1PMA /% | Cu-1.0PMA /% | Bio-10PMA /% | Bio-50PMA /% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptolyngbya boryana* | 0.27 | 0.44 | 0.33 | 0.49 | 0.22 | 0.23 | 0.50 | 0.87 | 20.60 | 28.01 | 28.67 | 41.52 | 18.18 | 27.85 | 28.01 |
| Chamaesiphon minutus* | <0.01 | 0.02 | <0.01 | 0.03 | <0.01 | 0.01 | <0.01 | 0.02 | 0.29 | 0.62 | 0.56 | 0.66 | 0.79 | 0.65 | 1.51 |
| Nannochloris normandinae** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.047 | 0.33 | 0.73 | 0.43 | 0.27 | 0.12 | 0.41 |
| Xylochloris irregularis** | 0.02 | 0.02 | 0.02 | 0.04 | <0.01 | 0.01 | <0.01 | <0.01 | 0.07 | 0.07 | 0.61 | 0.31 | 0.20 | 0.24 | 0.27 |
| Pectinodesmus pectinatus** | <0.01 | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.14 | 0.25 | 0.11 | <0.01 | 0.46 | 0.18 | 0.44 |
| Lobosphaera incisa** | 0.57 | 0.02 | 0.02 | 0.18 | <0.01 | 0.08 | <0.01 | 0.18 | 0.03 | 0.03 | 0.16 | 0.18 | <0.01 | 0.03 | 0.18 |
| Dinophysis acuta*** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.56 | <0.01 | <0.01 |
| Pleurocapsa sp. 04302-00001* | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.57 | <0.01 | <0.01 | <0.01 |
| Tetradesmus obliquus** | 0.04 | 0.05 | 0.02 | 0.04 | 0.02 | 0.01 | 0.04 | 0.06 | 0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.10 |
| Neochloris aquatica** | <0.01 | 0.02 | 0.03 | 0.05 | <0.01 | 0.02 | 0.02 | 0.03 | 0.05 | <0.01 | 0.03 | <0.01 | <0.01 | 0.02 | <0.01 |
表2 3种除藻剂处理下藻类在种水平的群落组成(相对丰度前10的物种)
Table 2 Algal community compositions in species level under 3 algicides treatment(species with top 10 relative abundance)
| 物种名称 Species name | Initial /% | CKNPMA /% | Cl-0.5NPMA /% | Cl-5.0NPMA /% | Cu-0.1NPMA /% | Cu-1.0NPMA /% | Bio-10NPMA /% | Bio-50NPMA /% | CKPMA /% | Cl-0.5PMA /% | Cl-5.0PMA /% | Cu-0.1PMA /% | Cu-1.0PMA /% | Bio-10PMA /% | Bio-50PMA /% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptolyngbya boryana* | 0.27 | 0.44 | 0.33 | 0.49 | 0.22 | 0.23 | 0.50 | 0.87 | 20.60 | 28.01 | 28.67 | 41.52 | 18.18 | 27.85 | 28.01 |
| Chamaesiphon minutus* | <0.01 | 0.02 | <0.01 | 0.03 | <0.01 | 0.01 | <0.01 | 0.02 | 0.29 | 0.62 | 0.56 | 0.66 | 0.79 | 0.65 | 1.51 |
| Nannochloris normandinae** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.047 | 0.33 | 0.73 | 0.43 | 0.27 | 0.12 | 0.41 |
| Xylochloris irregularis** | 0.02 | 0.02 | 0.02 | 0.04 | <0.01 | 0.01 | <0.01 | <0.01 | 0.07 | 0.07 | 0.61 | 0.31 | 0.20 | 0.24 | 0.27 |
| Pectinodesmus pectinatus** | <0.01 | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.14 | 0.25 | 0.11 | <0.01 | 0.46 | 0.18 | 0.44 |
| Lobosphaera incisa** | 0.57 | 0.02 | 0.02 | 0.18 | <0.01 | 0.08 | <0.01 | 0.18 | 0.03 | 0.03 | 0.16 | 0.18 | <0.01 | 0.03 | 0.18 |
| Dinophysis acuta*** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.56 | <0.01 | <0.01 |
| Pleurocapsa sp. 04302-00001* | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.57 | <0.01 | <0.01 | <0.01 |
| Tetradesmus obliquus** | 0.04 | 0.05 | 0.02 | 0.04 | 0.02 | 0.01 | 0.04 | 0.06 | 0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.10 |
| Neochloris aquatica** | <0.01 | 0.02 | 0.03 | 0.05 | <0.01 | 0.02 | 0.02 | 0.03 | 0.05 | <0.01 | 0.03 | <0.01 | <0.01 | 0.02 | <0.01 |
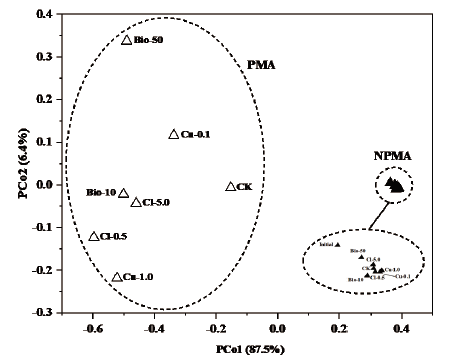
图5 基于OTU水平下藻类群落的PCoA分析图 实心三角形代表NPMA样本,空心三角形代表PMA样本
Fig. 5 PCoA analysis of algal communities based on OTU level Filled triangles refer to NPMA samples,and hollow triangles refer to PMA samples
| [1] | 李安定, 张彦, 周北海, 等. 城市景观河道中藻华暴发对水体中DOM特征的影响[J]. 光谱学与光谱分析, 2018, 38(1):188-193. |
| Li AD, Zhang Y, Zhou BH, et al. Influence of algae blooms on DOM characteristic in water bodies in urban landscape river[J]. Spectrosc Spectr Anal, 2018, 38(1):188-193. | |
| [2] | 陈修康. 水环境治理中藻华的危害及其防控技术[J]. 绿色环保建材, 2021(10):27-28. |
| Chen XK. Harm of algal bloom and its pretection and control technology in water environment treatment[J]. Green Environ Prot Build Mater, 2021(10):27-28. | |
| [3] | 刘佩蕊, 洪喻, 谢兴. 藻华防控方法及灭活与捕获新技术研究进展[J]. 环境科学与技术, 2021, 44(2):171-185. |
| Liu PR, Hong Y, Xie X. Research progress on the prevention and control methods of algal bloom and the new technologies for algal capture and inactivation[J]. Environ Sci Technol, 2021, 44(2):171-185. | |
| [4] |
Cardoso SJ, Roland F, Loverde-Oliveira SM, et al. Phytoplankton abundance, biomass and diversity within and between Pantanal wetland habitats[J]. Limnologica, 2012, 42(3):235-241.
doi: 10.1016/j.limno.2012.01.002 URL |
| [5] | 方雨博, 王趁义, 汤唯唯, 等. 除藻技术的优缺点比较、应用现状与新技术进展[J]. 工业水处理, 2020, 40(9):1-6. |
| Fang YB, Wang CY, Tang WW, et al. Comparison of advantages and disadvantages of algae removal technology, application status and new technology progress[J]. Ind Water Treat, 2020, 40(9):1-6. | |
| [6] | 黄振兴, 李晨晨, 金旭琴, 等. 除藻剂新洁尔灭与鲱鱼精子DNA的相互作用[J]. 环境化学, 2016, 35(8):1636-1641. |
| Huang ZX, Li CC, Jin XQ, et al. Interaction between the algaecide bromogeramine and herring sperm DNA[J]. Environ Chem, 2016, 35(8):1636-1641. | |
| [7] |
Manoylov KM. Taxonomic identification of algae(morphological and molecular):species concepts, methodologies, and their implications for ecological bioassessment[J]. J Phycol, 2014, 50(3):409-424.
doi: 10.1111/jpy.12183 pmid: 26988316 |
| [8] |
Penna A, Casabianca S, Guerra AF, et al. Analysis of phytoplankton assemblage structure in the Mediterranean Sea based on high-throughput sequencing of partial 18S rRNA sequences[J]. Mar Genomics, 2017, 36:49-55.
doi: 10.1016/j.margen.2017.06.001 URL |
| [9] | 贺玉晓, 郑永坤, 李卫国, 等. 丹江口水库早春真核浮游植物群落结构特征及其与环境因子的关系[J]. 环境科学学报, 2021, 41(6):2192-2200. |
| He YX, Zheng YK, Li WG, et al. Characteristics of eukaryotic phytoplankton community structure in early spring and its relationship with environmental factors in Danjiangkou Reservoir[J]. Acta Sci Circumstantiae, 2021, 41(6):2192-2200. | |
| [10] | 乔玲, 常志强, 李健, 等. 基于形态学和高通量测序的海水池塘生态养殖系统中浮游植物多样性比较[J]. 渔业科学进展, 2022, 43(2):32-43. |
| Qiao L, Chang ZQ, Li J, et al. Comparison of phytoplankton community diversity in the ecological aquaculture system of a marine pond using morphological analysis and high-throughput sequencing[J]. Prog Fish Sci, 2022, 43(2):32-43. | |
| [11] |
Wang Y, Yan Y, Thompson KN, et al. Whole microbial community viability is not quantitatively reflected by propidium monoazide sequencing approach[J]. Microbiome, 2021, 9(1):17.
doi: 10.1186/s40168-020-00961-3 pmid: 33478576 |
| [12] | Nocker A, Richter-Heitmann T, Montijn R, et al. Discrimination between live and dead cells in bacterial communities from environmental water samples analyzed by 454 pyrosequencing[J]. Int Microbiol, 2010, 13(2):59-65. |
| [13] | Joo S, Park P, Park S. Applicability of propidium monoazide(PMA)for discrimination between living and dead phytoplankton cells[J]. PLoS One, 2019, 14(6):e0218924. |
| [14] | 张继周. LNG接收站工程冷排水及余氯排放对海洋生物的影响研究[J]. 科技创新与应用, 2013(17):64. |
| Zhang JZ. Study on the impacts of cold water and chlorine residual discharge from LNG terminal project to marine creatures[J]. Technol Innov Appl, 2013(17):64. | |
| [15] |
Chen ZW, Song SF, Wen YZ, et al. Toxicity of Cu(II)to the green alga Chlorella vulgaris:a perspective of photosynthesis and oxidant stress[J]. Environ Sci Pollut Res Int, 2016, 23(18):17910-17918.
doi: 10.1007/s11356-016-6997-2 URL |
| [16] |
Yin K, Wang QN, Lv M, et al. Microorganism remediation strategies towards heavy metals[J]. Chem Eng J, 2019, 360:1553-1563.
doi: 10.1016/j.cej.2018.10.226 |
| [17] | 卢兰兰, 李根保, 沈银武, 等. 溶藻细菌DC-L5的分离、鉴定及其溶藻特性[J]. 水生生物学报, 2009, 33(5):860-865. |
|
Lu LL, Li GB, Shen YW, et al. Isolation, identification and characterization of a strain of blue-green algae-lysing bacterium from lake Dianchi[J]. Acta Hydrobiol Sin, 2009, 33(5):860-865.
doi: 10.3724/SP.J.1035.2009.50860 URL |
|
| [18] | 黄廷林, 朱倩, 邱晓鹏, 等. 扬水曝气技术对周村水库藻类的控制[J]. 环境工程学报, 2017, 11(4):2255-2260. |
| Huang TL, Zhu Q, Qiu XP, et al. Algae control by water-lifting aerator in Zhoucun reservoir[J]. Chin J Environ Eng, 2017, 11(4):2255-2260. | |
| [19] |
Zhu F, Yang M, Luo ZX, et al. Bioaccumulation and biotransformation of arsenic in Leptolyngbya boryana[J]. Environ Sci Pollut Res Int, 2020, 27(24):29993-30000.
doi: 10.1007/s11356-020-09294-y URL |
| [20] |
Assunção J, Amaro HM, Lopes G, et al. Exploration of marine genus Chroococcidiopsis sp. :a valuable source for antioxidant industry?[J]. J Appl Phycol, 2021, 33(4):2169-2187.
doi: 10.1007/s10811-021-02435-x URL |
| [21] | 穆海热姆·艾则孜, 阿曼古力·海瓦尔, 帕孜来提·拜合提, 等. 抗重金属土壤微藻F1对Cu2+胁迫的耐受生理机制研究[J]. 西北植物学报, 2017, 37(12):2452-2459. |
| Muharram E, Amangul H, Pazilat B, et al. Tolerance mechanism of soil microalgae F1 against Cu2+ stress isolated from metal mining area in Fuyun County[J]. Acta Bot Boreali Occidentalia Sin, 2017, 37(12):2452-2459. | |
| [22] | Casero MC, Ascaso C, Quesada A, et al. Response of endolithic Chroococcidiopsis strains from the polyextreme Atacama desert to light radiation[J]. Front Microbiol, 2021, 11:614875. |
| [23] | 魏静, 林莉, 潘雄, 等. 不同环境胁迫因子对藻类分子生物学特性的影响研究进展[J]. 长江科学院院报, 2020, 37(4):14-24. |
| Wei J, Lin L, Pan X, et al. Research progress about the effects of different environmental stress factors on algae in molecular biology[J]. J Yangtze River Sci Res Inst, 2020, 37(4):14-24. | |
| [24] | 董立新, 周绪申. 浮游植物多样性指数在内陆水体污染类型评价中的应用简述[J]. 海河水利, 2017(5):57-60. |
| Dong LX, Zhou XS. A breif introduction to the application of phytoplankton diversity indeces in the evaluation of inland water pollution types[J]. Haihe Water Resour, 2017(5):57-60. |
| [1] | 余洋, 刘天海, 刘理旭, 唐杰, 彭卫红, 陈阳, 谭昊. 羊肚菌菌种生产车间气溶胶微生物群落研究[J]. 生物技术通报, 2023, 39(5): 267-275. |
| [2] | 李善家, 雷雨昕, 孙梦格, 刘海锋, 王兴敏. 种子内生细菌多样性与植物互馈作用研究进展[J]. 生物技术通报, 2023, 39(4): 166-175. |
| [3] | 徐小文, 李金仓, 海都, 查玉平, 宋菲, 王义勋. 核桃炭疽菌携带病毒种类鉴定及多样性分析[J]. 生物技术通报, 2023, 39(3): 278-289. |
| [4] | 孙海航, 官会林, 王旭, 王童, 李泓霖, 彭文洁, 刘柏桢, 樊芳玲. 生物炭对三七连作土壤性质及真菌群落的影响[J]. 生物技术通报, 2023, 39(2): 221-231. |
| [5] | 王子夜, 王志刚, 阎爱华. 不同树龄桑根际土壤原生生物群落组成多样性[J]. 生物技术通报, 2022, 38(8): 206-215. |
| [6] | 陈天赐, 武少兰, 杨国辉, 江丹霞, 江玉姬, 陈炳智. 无柄灵芝醇提物对小鼠睡眠及肠道菌群的影响[J]. 生物技术通报, 2022, 38(8): 225-232. |
| [7] | 高小宁, 刘睿, 吴自林, 吴嘉云. 宿根矮化病抗感甘蔗品种茎部内生真菌和细菌群落特征分析[J]. 生物技术通报, 2022, 38(6): 166-173. |
| [8] | 徐扬, 张冠初, 丁红, 秦斐斐, 张智猛, 戴良香. 土壤类型对花生根际土壤细菌群落多样性和产量的影响[J]. 生物技术通报, 2022, 38(6): 221-234. |
| [9] | 钟辉, 刘亚军, 王滨花, 和梦洁, 吴兰. 分析方法对细菌群落16S rRNA基因扩增测序分析结果的影响[J]. 生物技术通报, 2022, 38(6): 81-92. |
| [10] | 周晓楠, 徐金青, 雷雨晴, 王海庆. 基于GBS-seq的青藏扁蓿豆SNP标记开发[J]. 生物技术通报, 2022, 38(4): 303-310. |
| [11] | 谢果珍, 唐圆, 宁晓妹, 邱集慧, 谭周进. 铁皮石斛多糖对高脂饮食小鼠肠黏膜结构及菌群的影响[J]. 生物技术通报, 2022, 38(2): 150-157. |
| [12] | 赵林艳, 官会林, 向萍, 李泽诚, 柏雨龙, 宋洪川, 孙世中, 徐武美. 白及根腐病植株根际土壤微生物群落组成特征分析[J]. 生物技术通报, 2022, 38(2): 67-74. |
| [13] | 刘爽, 姚佳妮, 沈聪, 代金霞. 荒漠植物柠条根际土壤nifH基因荧光定量及固氮菌多样性分析[J]. 生物技术通报, 2022, 38(12): 252-262. |
| [14] | 李婷婷, 邓旭辉, 李若尘, 刘红军, 沈宗专, 李荣, 沈其荣. 番茄青枯病发生对土壤真菌群落多样性的影响[J]. 生物技术通报, 2022, 38(10): 195-203. |
| [15] | 颜珲璘, 芦光新, 邓晔, 顾松松, 颜程良, 马坤, 赵阳安, 张海娟, 王英成, 周学丽, 窦声云. 高寒地区根瘤菌拌种对禾/豆混播土壤微生物群落的影响[J]. 生物技术通报, 2022, 38(10): 204-215. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||