








生物技术通报 ›› 2022, Vol. 38 ›› Issue (12): 214-222.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0290
王帅1,2( ), 袁坤2, 何其光2, 胡义钰2, 冯成天2, 王真辉2, 刘进平1, 刘辉2(
), 袁坤2, 何其光2, 胡义钰2, 冯成天2, 王真辉2, 刘进平1, 刘辉2( )
)
收稿日期:2022-03-30
出版日期:2022-12-26
发布日期:2022-12-29
作者简介:王帅,男,硕士研究生,研究方向:植物分子生物学;E-mail:基金资助:
WANG Shuai1,2( ), YUAN Kun2, HE Qi-guang2, HU Yi-yu2, FENG Cheng-tian2, WANG Zhen-hui2, LIU Jin-ping1, LIU Hui2(
), YUAN Kun2, HE Qi-guang2, HU Yi-yu2, FENG Cheng-tian2, WANG Zhen-hui2, LIU Jin-ping1, LIU Hui2( )
)
Received:2022-03-30
Published:2022-12-26
Online:2022-12-29
摘要:
硫氧还蛋白通过调控细胞内的氧化还原状态在植物生长发育及非生物胁迫应答等过程中发挥重要作用。研究硫氧还蛋白基因HbCXXS1在橡胶树各组织以及激素和非生物胁迫处理下的表达,为解析其生物学功能奠定基础。采用RT-PCR克隆HbCXXS1,对其进行生物信息学分析,并采用实时荧光定量PCR分析该基因在橡胶树不同组织及不同激素和非生物胁迫条件下的表达模式。结果表明,HbCXXS1开放阅读框为372 bp,编码123个氨基酸。HbCXXS1蛋白为亲水性蛋白,具有硫氧还蛋白保守结构域,活性中心为CXXS,属于h型硫氧还蛋白的第III组。HbCXXS1在橡胶树胶乳中的表达显著高于其他组织,且在死皮植株胶乳中的表达显著低于健康植株。脱落酸、茉莉酸甲酯、干旱和盐胁迫处理抑制了HbCXXS1的表达,乙烯利和低温处理上调了HbCXXS1的表达,而水杨酸和过氧化氢处理的不同时间点,HbCXXS1的表达既有上调也有下调。HbCXXS1可能在橡胶树产排胶和非生物胁迫应答中发挥重要调控作用。
王帅, 袁坤, 何其光, 胡义钰, 冯成天, 王真辉, 刘进平, 刘辉. 橡胶树硫氧还蛋白基因HbCXXS1的克隆及表达分析[J]. 生物技术通报, 2022, 38(12): 214-222.
WANG Shuai, YUAN Kun, HE Qi-guang, HU Yi-yu, FENG Cheng-tian, WANG Zhen-hui, LIU Jin-ping, LIU Hui. Cloning and Expression Analysis of HbCXXS1,a Thioredoxin Gene in Hevea brasiliensis[J]. Biotechnology Bulletin, 2022, 38(12): 214-222.

图2 HbCXXS1蛋白保守结构域(A)、跨膜结构域(B)和亲/疏水性(C)分析
Fig. 2 Conserved domain(A),transmembrane domain(B)and hydrophilicity/hydrophobicity(C)analysis of HbCXXS1 protein
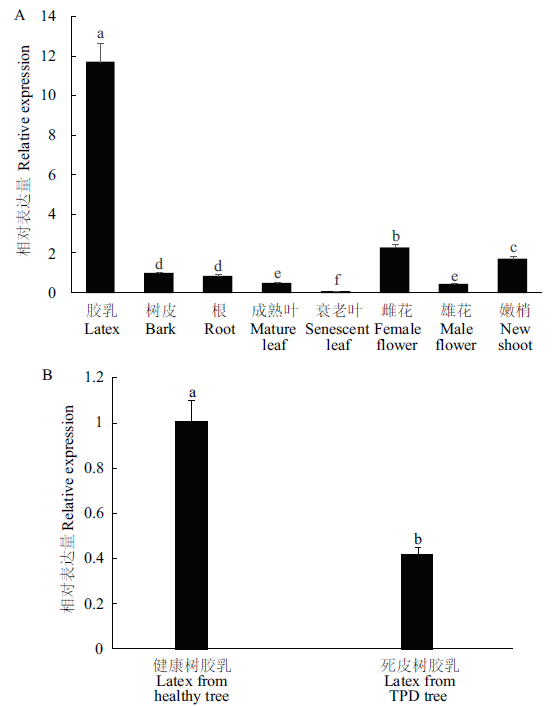
图4 HbCXXS1在橡胶树各组织(A)以及健康与死皮植株胶乳(B)中的表达 不同小写字母表示差异显著(P<0.05)。下同
Fig. 4 Expressions of HbCXXS1 in various tissues(A)and latex from healthy and TPD rubber tree(B) Different lowercase letters indicate a significant difference(P<0.05). The same below
| [1] |
Serrato AJ, Rojas-González JA, Torres-Romero D, et al. Thioredoxins m are major players in the multifaceted light-adaptive response in Arabidopsis thaliana[J]. Plant J, 2021, 108(1):120-133.
doi: 10.1111/tpj.15429 URL |
| [2] |
Bohrer AS, Massot V, Innocenti G, et al. New insights into the reduction systems of plastidial thioredoxins point out the unique properties of thioredoxin z from Arabidopsis[J]. J Exp Bot, 2012, 63(18):6315-6323.
doi: 10.1093/jxb/ers283 URL |
| [3] | 孙虎, 薛保国, 杨丽荣, 等. 植物硫氧还蛋白系统[J]. 基因组学与应用生物学, 2010, 29(4):748-753. |
| Sun H, Xue BG, Yang LR, et al. Plant thioredoxin system[J]. Genom Appl Biol, 2010, 29(4):748-753. | |
| [4] |
Collet JF, Messens J. Structure, function, and mechanism of thioredoxin proteins[J]. Antioxid Redox Signal, 2010, 13(8):1205-1216.
doi: 10.1089/ars.2010.3114 URL |
| [5] |
Gelhaye E, Rouhier N, Navrot N, et al. The plant thioredoxin system[J]. Cell Mol Life Sci, 2005, 62(1):24-35.
pmid: 15619004 |
| [6] |
Meyer Y, Belin C, Delorme-Hinoux V, et al. Thioredoxin and glutaredoxin systems in plants:molecular mechanisms, crosstalks, and functional significance[J]. Antioxid Redox Signal, 2012, 17(8):1124-1160.
doi: 10.1089/ars.2011.4327 URL |
| [7] |
Geigenberger P, Thormählen I, Daloso DM, et al. The unprecedented versatility of the plant thioredoxin system[J]. Trends Plant Sci, 2017, 22(3):249-262.
doi: S1360-1385(16)30221-7 pmid: 28139457 |
| [8] |
Gelhaye E, Rouhier N, Jacquot JP. The thioredoxin h system of higher plants[J]. Plant Physiol Biochem, 2004, 42(4):265-271.
doi: 10.1016/j.plaphy.2004.03.002 URL |
| [9] |
Chibani K, Wingsle G, Jacquot JP, et al. Comparative genomic study of the thioredoxin family in photosynthetic organisms with emphasis on Populus trichocarpa[J]. Mol Plant, 2009, 2(2):308-322.
doi: 10.1093/mp/ssn076 pmid: 19825616 |
| [10] | Meyer Y, Vignols F, Reichheld JP. Classification of plant thioredoxins by sequence similarity and intron position[J]. Methods Enzymol, 2002, 347:394-402. |
| [11] |
Serrato AJ, Guilleminot J, Meyer Y, et al. AtCXXS:atypical members of the Arabidopsis thaliana thioredoxin h family with a remarkably high disulfide isomerase activity[J]. Physiol Plant, 2008, 133(3):611-622.
doi: 10.1111/j.1399-3054.2008.01093.x URL |
| [12] |
Gelhaye E, Rouhier N, Jacquot JP. Evidence for a subgroup of thioredoxin h that requires GSH/Grx for its reduction[J]. FEBS Lett, 2003, 555(3):443-448.
pmid: 14675753 |
| [13] |
Renard M, Alkhalfioui F, Schmitt-Keichinger C, et al. Identification and characterization of thioredoxin h isoforms differentially expressed in germinating seeds of the model legume Medicago truncatula[J]. Plant Physiol, 2011, 155(3):1113-1126.
doi: 10.1104/pp.110.170712 pmid: 21239621 |
| [14] |
Ancín M, Fernández-San Millán A, Larraya L, et al. Overexpression of thioredoxin m in tobacco chloroplasts inhibits the protein kinase STN7 and alters photosynthetic performance[J]. J Exp Bot, 2018, 70(3):1005-1016.
doi: 10.1093/jxb/ery415 URL |
| [15] |
Richter AS, Pérez-Ruiz JM, Cejudo FJ, et al. Redox-control of chlorophyll biosynthesis mainly depends on thioredoxins[J]. FEBS Lett, 2018, 592(18):3111-3115.
doi: 10.1002/1873-3468.13216 pmid: 30076598 |
| [16] |
Zhang H, Zhang TT, Liu H, et al. Thioredoxin-mediated ROS homeostasis explains natural variation in plant regeneration[J]. Plant Physiol, 2018, 176(3):2231-2250.
doi: 10.1104/pp.17.00633 pmid: 28724620 |
| [17] |
Ying YH, Yue WH, Wang SD, et al. Two h-type thioredoxins interact with the E2 ubiquitin conjugase PHO2 to fine-tune phosphate homeostasis in rice[J]. Plant Physiol, 2017, 173(1):812-824.
doi: 10.1104/pp.16.01639 URL |
| [18] |
Bashandy T, Guilleminot J, Vernoux T, et al. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling[J]. Plant Cell, 2010, 22(2):376-391.
doi: 10.1105/tpc.109.071225 URL |
| [19] |
Luan JY, Dong JX, Song X, et al. Overexpression of Tamarix hispida ThTrx5 confers salt tolerance to Arabidopsis by activating stress response signals[J]. Int J Mol Sci, 2020, 21(3):1165.
doi: 10.3390/ijms21031165 URL |
| [20] |
Zhang CJ, Zhao BC, Ge WN, et al. An apoplastic h-type thioredoxin is involved in the stress response through regulation of the apoplastic reactive oxygen species in rice[J]. Plant Physiol, 2011, 157(4):1884-1899.
doi: 10.1104/pp.111.182808 URL |
| [21] |
Mata-Pérez C, Spoel SH. Thioredoxin-mediated redox signalling in plant immunity[J]. Plant Sci, 2019, 279:27-33.
doi: S0168-9452(17)31251-7 pmid: 30709489 |
| [22] |
Laloi C, Mestres-Ortega D, Marco Y, et al. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor[J]. Plant Physiol, 2004, 134(3):1006-1016.
pmid: 14976236 |
| [23] |
Torres-Rodríguez MD, Cruz-Zamora Y, Juárez-Díaz JA, et al. NaTrxh is an essential protein for pollen rejection in Nicotiana by increasing S-RNase activity[J]. Plant J, 2020, 103(4):1304-1317.
doi: 10.1111/tpj.14802 URL |
| [24] | 郭晋隆, 郑蕊, 凌辉, 等. 甘蔗硫氧还蛋白基因ScTRXh1的克隆及表达特性分析[J]. 基因组学与应用生物学, 2012, 31(6):574-581. |
| Guo JL, Zheng R, Ling H, et al. Cloning and expression analysis of a thioredoxin gene ScTRXh1 from sugarcane(Saccharum complex)[J]. Genom Appl Biol, 2012, 31(6):574-581. | |
| [25] | 李霞, 潘春柳, 苏桂军, 等. 花生硫氧还蛋白AhTRX h基因的克隆及表达分析[J]. 热带作物学报, 2021, 42(5):1252-1260. |
| Li X, Pan CL, Su GJ, et al. Cloning and expression analysis of thioredoxin gene AhTRX h in peanut(Arachis hypogaea)[J]. Chin J Trop Crops, 2021, 42(5):1252-1260. | |
| [26] | 李梦园, 樊亚栋, 张新宁, 等. 小麦TaTrxh9基因的序列特征及其对渗透胁迫的响应[J]. 西北植物学报, 2019, 39(5):896-903. |
| Li MY, Fan YD, Zhang XN, et al. Sequence characteristics of TaTrxh9 gene and its response to osmotic stresses in wheat[J]. Acta Bot Boreali Occidentalia Sin, 2019, 39(5):896-903. | |
| [27] |
Li HL, Lu HZ, Guo D, et al. Molecular characterization of a thioredoxin h gene(HbTRX1)from Hevea brasiliensis showing differential expression in latex between self-rooting juvenile clones and donor clones[J]. Mol Biol Rep, 2011, 38(3):1989-1994.
doi: 10.1007/s11033-010-0321-x URL |
| [28] | Montoro P, Wu S, Favreau B, et al. Transcriptome analysis in Hevea brasiliensis latex revealed changes in hormone signalling pathways during ethephon stimulation and consequent Tapping Panel Dryness[J]. Sci Reports, 2018, 8:8483. |
| [29] |
李双江, 冯成天, 胡义钰, 等. 橡胶树HbDHAR2基因克隆及表达分析[J]. 华北农学报, 2021, 36(3):25-32.
doi: 10.7668/hbnxb.20191875 |
| Li SJ, Feng CT, Hu YY, et al. Cloning and expression analysis of HbDHAR2 gene from Hevea brasiliensis[J]. Acta Agric Boreali Sin, 2021, 36(3):25-32. | |
| [30] |
Nuruzzaman M, Gupta M, Zhang CJ, et al. Sequence and expression analysis of the thioredoxin protein gene family in rice[J]. Mol Genet Genomics, 2008, 280(2):139-151.
doi: 10.1007/s00438-008-0351-4 pmid: 18491141 |
| [31] |
Balsera M, Buchanan BB. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress[J]. Free Radic Biol Med, 2019, 140:28-35.
doi: 10.1016/j.freeradbiomed.2019.03.003 URL |
| [32] |
Zhang Y, Leclercq J, Montoro P. Reactive oxygen species in Hevea brasiliensis latex and relevance to Tapping Panel Dryness[J]. Tree Physiol, 2017, 37(2):261-269.
doi: 10.1093/treephys/tpw106 pmid: 27903918 |
| [1] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [2] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [3] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [4] | 赵雪婷, 高利燕, 王俊刚, 沈庆庆, 张树珍, 李富生. 甘蔗AP2/ERF转录因子基因ShERF3的克隆、表达及其编码蛋白的定位[J]. 生物技术通报, 2023, 39(6): 208-216. |
| [5] | 李苑虹, 郭昱昊, 曹燕, 祝振洲, 王飞飞. 外源植物激素调控微藻生长及目标产物积累研究进展[J]. 生物技术通报, 2023, 39(6): 61-72. |
| [6] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [7] | 姜晴春, 杜洁, 王嘉诚, 余知和, 王允, 柳忠玉. 虎杖转录因子PcMYB2的表达特性和功能分析[J]. 生物技术通报, 2023, 39(5): 217-223. |
| [8] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [9] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [10] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [11] | 王艺清, 王涛, 韦朝领, 戴浩民, 曹士先, 孙威江, 曾雯. 茶树SMAS基因家族的鉴定及互作分析[J]. 生物技术通报, 2023, 39(4): 246-258. |
| [12] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [13] | 杨春洪, 董璐, 陈林, 宋丽. 大豆VAS1基因家族的鉴定及参与侧根发育的研究[J]. 生物技术通报, 2023, 39(3): 133-142. |
| [14] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [15] | 王涛, 漆思雨, 韦朝领, 王艺清, 戴浩民, 周喆, 曹士先, 曾雯, 孙威江. CsPPR和CsCPN60-like在茶树白化叶片中的表达分析及互作蛋白验证[J]. 生物技术通报, 2023, 39(3): 218-231. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||