








生物技术通报 ›› 2023, Vol. 39 ›› Issue (3): 35-42.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0816
崔俊美1( ), 魏家萍1, 董小云2, 王莹2, 郑国强2, 刘自刚1(
), 魏家萍1, 董小云2, 王莹2, 郑国强2, 刘自刚1( )
)
收稿日期:2022-07-01
出版日期:2023-03-26
发布日期:2023-04-10
通讯作者:
刘自刚,男,博士,研究员,研究方向:作物育种与逆境调控;E-mail: lzgworking@163.com作者简介:崔俊美,女,博士,助理研究员,研究方向:植物逆境调控;E-mail: cuijm@gsau.edu.cn
基金资助:
CUI Jun-mei1( ), WEI Jia-ping1, DONG Xiao-yun2, WANG Ying2, ZHENG Guo-qiang2, LIU Zi-gang1(
), WEI Jia-ping1, DONG Xiao-yun2, WANG Ying2, ZHENG Guo-qiang2, LIU Zi-gang1( )
)
Received:2022-07-01
Published:2023-03-26
Online:2023-04-10
摘要:
植物内源性多肽由前体蛋白剪切而成,由天然氨基酸以不同组成和排列方式构成。多肽分泌至细胞外后,被其受体识别,从而调控植物生长发育和逆境响应。PIP(PAMP-induced secreted peptide)/PIP-Like(PIPL)是包含SGPS和GxGH基序的植物内源性多肽,可被受体激酶RLK7(receptor-like kinase 7)识别,在植物抵抗病原菌、病毒和盐害等逆境及调控生长发育过程中扮演重要角色。本文重点阐述了PIP/PIPL家族多肽在植物抵抗逆境胁迫和调控生长发育过程中的信号转导通路,并讨论了该领域尚待解决的一些科学问题和可能的应用方向,以期为PIP/PIPL的深入研究提供参考。
崔俊美, 魏家萍, 董小云, 王莹, 郑国强, 刘自刚. PIP/PIPL:一类调控植物逆境响应和发育的植物内源性多肽[J]. 生物技术通报, 2023, 39(3): 35-42.
CUI Jun-mei, WEI Jia-ping, DONG Xiao-yun, WANG Ying, ZHENG Guo-qiang, LIU Zi-gang. PIP/PIPL: A Kind of Endogenous Plant Peptide Regulating Plant Stress Response and Development[J]. Biotechnology Bulletin, 2023, 39(3): 35-42.
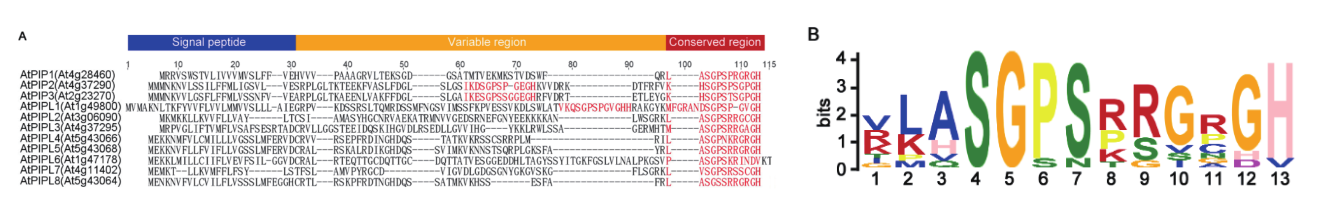
图1 AtPIP/PIPL家族蛋白质序列比对及保守基序分析 A:基于AtPIP/PIPL前体蛋白全长序列的蛋白质比对,保守基序用红色标注。 B:AtPIP/PIPL的保守序列特征
Fig. 1 Sequence alignment and conserved motif of AtPIP/PIPL family proteins A: Alignment based on full-length sequence of AtPIP/PIPL precursor proteins. The conserved motifs were marked in red. B: Conserved sequence features of AtPIP/PIPL

图2 RLK7蛋白结构及LRRs序列信息 A:RLK7蛋白结构信息;B:RLK7的LRRs基序信息
Fig. 2 Protein structure and LRRs sequence information of RLK7 A: Structural information of RLK7 protein. B: LRRs motif information of RLK7

图4 LRR XI亚家族成员及其他几个RLKs的激酶域序列比对分析 *:磷酸化或激酶活性位点
Fig. 4 Alignment analysis of kinase domain sequences of LRR XI subfamily members and other RLKs * indicates phosphorylation or kinase active sites
| [1] |
Matsubayashi Y, Sakagami Y. Peptide hormones in plants[J]. Annu Rev Plant Biol, 2006, 57: 649-674.
pmid: 16669777 |
| [2] |
Matsubayashi Y. Posttranslationally modified small-peptide signals in plants[J]. Annu Rev Plant Biol, 2014, 65: 385-413.
doi: 10.1146/annurev-arplant-050312-120122 pmid: 24779997 |
| [3] |
Lease KA, Walker JC. Bioinformatic identification of plant peptides[J]. Methods Mol Biol, 2010, 615: 375-383.
doi: 10.1007/978-1-60761-535-4_26 pmid: 20013221 |
| [4] |
Lease KA, Walker JC. The Arabidopsis unannotated secreted peptide database, a resource for plant peptidomics[J]. Plant Physiol, 2006, 142(3): 831-838.
doi: 10.1104/pp.106.086041 URL |
| [5] |
Takahashi F, Suzuki T, Osakabe Y, et al. A small peptide modulates stomatal control via abscisic acid in long-distance signalling[J]. Nature, 2018, 556(7700): 235-238.
doi: 10.1038/s41586-018-0009-2 |
| [6] |
Takahashi F, Hanada K, Kondo T, et al. Hormone-like peptides and small coding genes in plant stress signaling and development[J]. Curr Opin Plant Biol, 2019, 51: 88-95.
doi: S1369-5266(19)30028-7 pmid: 31265991 |
| [7] |
Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response[J]. PNAS, 2006, 103(26): 10098-10103.
doi: 10.1073/pnas.0603727103 pmid: 16785434 |
| [8] |
Stegmann M, Monaghan J, Smakowska-Luzan E, et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling[J]. Science, 2017, 355(6322): 287-289.
doi: 10.1126/science.aal2541 pmid: 28104890 |
| [9] |
Zhou HP, Xiao F, Zheng Y, et al. Pamp-induced secreted peptide 3 modulates salt tolerance through receptor-like kinase 7 in plants[J]. Plant Cell, 2022, 34(2): 927-944.
doi: 10.1093/plcell/koab292 URL |
| [10] |
Najafi J, Brembu T, Vie AK, et al. Pamp-induced secreted peptide 3(pip3)modulates immunity in Arabidopsis thaliana[J]. J Exp Bot, 2020, 71(3):850-864.
doi: 10.1093/jxb/erz482 pmid: 31665431 |
| [11] |
Hussain S, Wang W, Ahmed S, et al. PIP2, an auxin induced plant peptide hormone regulates root and hypocotyl elongation in Arabidopsis[J]. Front Plant Sci, 2021, 12: 646736.
doi: 10.3389/fpls.2021.646736 URL |
| [12] |
Hou SG, Wang X, Chen DH, et al. The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7[J]. PLoS Pathog, 2014, 10(9): e1004331.
doi: 10.1371/journal.ppat.1004331 URL |
| [13] |
Vie AK, Najafi J, Liu B, et al. The IDA/IDA-LIKE and PIP/PIP-LIKE gene families in Arabidopsis: phylogenetic relationship, expression patterns, and transcriptional effect of the PIPL3 peptide[J]. J Exp Bot, 2015, 66(17): 5351-5365.
doi: 10.1093/jxb/erv285 URL |
| [14] |
Combest MM, Moroz N, Tanaka K, et al. StPIP1, a PAMP-induced peptide in potato, elicits plant defenses and is associated with disease symptom severity in a compatible interaction with Potato virus Y[J]. J Exp Bot, 2021, 72(12): 4472-4488.
doi: 10.1093/jxb/erab078 pmid: 33681961 |
| [15] |
Ito Y, Nakanomyo I, Motose H, et al. Dodeca-CLE peptides as suppressors of plant stem cell differentiation[J]. Science, 2006, 313(5788): 842-845.
doi: 10.1126/science.1128436 pmid: 16902140 |
| [16] |
Meng L, Buchanan BB, Feldman LJ, et al. CLE-like(CLEL)peptides control the pattern of root growth and lateral root development in Arabidopsis[J]. Proc Natl Acad Sci USA, 2012, 109(5): 1760-1765.
doi: 10.1073/pnas.1119864109 URL |
| [17] |
Aalen RB, Wildhagen M, Stø IM, et al. IDA: a peptide ligand regulating cell separation processes in Arabidopsis[J]. J Exp Bot, 2013, 64(17): 5253-5261.
doi: 10.1093/jxb/ert338 URL |
| [18] |
Zipfel C. Plant pattern-recognition receptors[J]. Trends Immunol, 2014, 35(7): 345-351.
doi: 10.1016/j.it.2014.05.004 pmid: 24946686 |
| [19] |
Belkhadir Y, Yang L, Hetzel J, et al. The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors[J]. Trends Biochem Sci, 2014, 39(10): 447-456.
doi: 10.1016/j.tibs.2014.06.006 pmid: 25089011 |
| [20] |
Jones JDG, Dangl JL. The plant immune system[J]. Nature, 2006, 444(7117): 323-329.
doi: 10.1038/nature05286 |
| [21] |
Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis[J]. Mol Cell, 2000, 5(6): 1003-1011.
doi: 10.1016/s1097-2765(00)80265-8 pmid: 10911994 |
| [22] |
Böhm H, et al. A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis[J]. PLoS Pathog, 2014, 10(11): e1004491.
doi: 10.1371/journal.ppat.1004491 URL |
| [23] |
Sakamoto T, Deguchi M, Brustolini OJB, et al. The tomato RLK superfamily: phylogeny and functional predictions about the role of the LRRII-RLK subfamily in antiviral defense[J]. BMC Plant Biol, 2012, 12: 229.
doi: 10.1186/1471-2229-12-229 pmid: 23198823 |
| [24] |
Dunning FM, Sun WX, Jansen KL, et al. Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception[J]. Plant Cell, 2007, 19(10): 3297-3313.
doi: 10.1105/tpc.106.048801 pmid: 17933906 |
| [25] |
Tang J, Han ZF, Sun YD, et al. Structural basis for recognition of an endogenous peptide by the plant receptor kinase PEPR1[J]. Cell Res, 2015, 25(1): 110-120.
doi: 10.1038/cr.2014.161 pmid: 25475059 |
| [26] |
Zhang XX, Liu WJ, Nagae TT, et al. Structural basis for receptor recognition of pollen tube attraction peptides[J]. Nat Commun, 2017, 8(1): 1331.
doi: 10.1038/s41467-017-01323-8 pmid: 29109411 |
| [27] |
Hallgren J, Tsirigos KD, Pedersen MD, et al. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks[J]. bioRxiv, 2022, DOI:10.1101/2022.04.08.487609.
doi: 10.1101/2022.04.08.487609 |
| [28] |
Chen TS. Identification and characterization of the LRR repeats in plant LRR-RLKs[J]. BMC Mol Cell Biol, 2021, 22(1): 9.
doi: 10.1186/s12860-021-00344-y pmid: 33509084 |
| [29] |
Liebrand TWH, van den Burg HA, Joosten MHAJ. Two for all: receptor-associated kinases SOBIR1 and BAK1[J]. Trends Plant Sci, 2014, 19(2): 123-132.
doi: 10.1016/j.tplants.2013.10.003 pmid: 24238702 |
| [30] |
Roux M, Schwessinger B, Albrecht C, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens[J]. Plant Cell, 2011, 23(6): 2440-2455.
doi: 10.1105/tpc.111.084301 URL |
| [31] |
Liang XX, Zhou JM. Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling[J]. Annu Rev Plant Biol, 2018, 69: 267-299.
doi: 10.1146/annurev-arplant-042817-040540 pmid: 29719165 |
| [32] |
Zhang J, Li W, Xiang TT, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector[J]. Cell Host Microbe, 2010, 7(4): 290-301.
doi: 10.1016/j.chom.2010.03.007 pmid: 20413097 |
| [33] |
Lei JX, Finlayson SA, Salzman RA, et al. BOTRYTIS-INDUCED KINASE1 modulates Arabidopsis resistance to green peach aphids via PHYTOALEXIN DEFICIENT4[J]. Plant Physiol, 2014, 165(4): 1657-1670.
doi: 10.1104/pp.114.242206 URL |
| [34] |
Lu DP, Wu SJ, Gao XQ, et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity[J]. PNAS, 2010, 107(1): 496-501.
doi: 10.1073/pnas.0909705107 pmid: 20018686 |
| [35] |
Liu Z, Wu Y, Yang F, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity[J]. Proc Natl Acad Sci USA, 2013, 110(15): 6205-6210.
doi: 10.1073/pnas.1215543110 pmid: 23431184 |
| [36] |
Macho AP, Lozano-Durán R, Zipfel C. Importance of tyrosine phosphorylation in receptor kinase complexes[J]. Trends Plant Sci, 2015, 20(5): 269-272.
doi: S1360-1385(15)00050-3 pmid: 25795237 |
| [37] |
Macho AP, Schwessinger B, Ntoukakis V, et al. A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation[J]. Science, 2014, 343(6178): 1509-1512.
doi: 10.1126/science.1248849 pmid: 24625928 |
| [38] |
Perraki A, DeFalco TA, Derbyshire P, et al. Phosphocode-dependent functional dichotomy of a common co-receptor in plant signalling[J]. Nature, 2018, 561(7722): 248-252.
doi: 10.1038/s41586-018-0471-x |
| [39] |
Chinchilla D, Zipfel C, Robatzek S, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence[J]. Nature, 2007, 448(7152): 497-500.
doi: 10.1038/nature05999 |
| [40] |
Hartmann J, Fischer C, et al. Kinase activity and calmodulin binding are essential for growth signaling by the phytosulfokine receptor PSKR1[J]. Plant J, 2014, 78(2): 192-202.
doi: 10.1111/tpj.12460 URL |
| [41] |
Hou SG, Shen HX, Shao HW. PAMP-induced peptide 1 cooperates with salicylic acid to regulate stomatal immunity in Arabidopsis thaliana[J]. Plant Signal Behav, 2019, 14(11): 1666657.
doi: 10.1080/15592324.2019.1666657 URL |
| [42] |
Yamaguchi Y, Pearce G, Ryan CA. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells[J]. Proc Natl Acad Sci USA, 2006, 103(26): 10104-10109.
pmid: 16785433 |
| [43] |
Yamaguchi Y, Huffaker A, et al. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis[J]. Plant Cell, 2010, 22(2): 508-522.
doi: 10.1105/tpc.109.068874 URL |
| [44] |
Park CH, Bi Y, et al. Deconvoluting signals downstream of growth and immune receptor kinases by phosphocodes of the BSU1 family phosphatases[J]. Nat Plants, 2022, 8(6): 646-655.
doi: 10.1038/s41477-022-01167-1 pmid: 35697730 |
| [45] |
Toyokura K, Goh T, Shinohara H, et al. Lateral inhibition by a peptide hormone-receptor cascade during Arabidopsis lateral root founder cell formation[J]. Dev Cell, 2019, 48(1): 64-75.e5.
doi: S1534-5807(18)30987-0 pmid: 30581155 |
| [46] |
Okushima Y, Fukaki H, Onoda M, et al. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis[J]. Plant Cell, 2007, 19(1): 118-130.
doi: 10.1105/tpc.106.047761 pmid: 17259263 |
| [47] |
Jing YP, Zheng XJ, et al. Danger-associated peptides interact with PIN-dependent local auxin distribution to inhibit root growth in Arabidopsis[J]. Plant Cell, 2019, 31(8): 1767-1787.
doi: 10.1105/tpc.18.00757 URL |
| [1] | 胡海琳, 徐黎, 李晓旭, 王晨璨, 梅曼, 丁文静, 赵媛媛. 小肽激素调控植物生长发育及逆境生理研究进展[J]. 生物技术通报, 2023, 39(7): 13-25. |
| [2] | 李英, 岳祥华. DNA甲基化在解析毛竹自然变异中的应用[J]. 生物技术通报, 2023, 39(7): 48-55. |
| [3] | 崔学强, 黄昌艳, 邓杰玲, 李先民, 李秀玲, 张自斌. 基于SLAF-seq技术的石斛兰SNP标记开发及亲缘关系分析[J]. 生物技术通报, 2023, 39(6): 141-148. |
| [4] | 尹明华, 余锾媛, 肖心怡, 王玉婷. 江西铅山红芽芋叶绿体基因组特征及系统发育分析[J]. 生物技术通报, 2023, 39(6): 233-247. |
| [5] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [6] | 薛皦, 朱庆锋, 冯彦钊, 陈沛, 刘文华, 张爱霞, 刘勤坚, 张琪, 于洋. 植物基因上游开放阅读框的研究进展[J]. 生物技术通报, 2023, 39(4): 157-165. |
| [7] | 魏明, 王欣玉, 伍国强, 赵萌. NAD依赖型去乙酰化酶SRT在植物表观遗传调控中的作用[J]. 生物技术通报, 2023, 39(4): 59-70. |
| [8] | 桑田, 王鹏程. 植物SUMO化修饰研究进展[J]. 生物技术通报, 2023, 39(3): 1-12. |
| [9] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [10] | 杨春洪, 董璐, 陈林, 宋丽. 大豆VAS1基因家族的鉴定及参与侧根发育的研究[J]. 生物技术通报, 2023, 39(3): 133-142. |
| [11] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [12] | 赵艳侠, 张晶莹, 孙骏飞, 王绛辉, 孙家波, 吕晓惠. ‘重瓣红’玫瑰不同花发育阶段转录和代谢差异分析[J]. 生物技术通报, 2023, 39(3): 184-195. |
| [13] | 曲春娟, 朱悦, 江晨, 曲明静, 王向誉, 李晓. 铜绿丽金龟线粒体全基因组及其系统发育分析[J]. 生物技术通报, 2023, 39(2): 263-273. |
| [14] | 陈广霞, 李秀杰, 蒋锡龙, 单雷, 张志昌, 李勃. 植物小分子信号肽参与非生物逆境胁迫应答的研究进展[J]. 生物技术通报, 2023, 39(11): 61-73. |
| [15] | 马秋雨, 袁芳. 植物盐腺泌盐及发育研究进展[J]. 生物技术通报, 2023, 39(11): 74-85. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||