








生物技术通报 ›› 2023, Vol. 39 ›› Issue (9): 147-155.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0062
刘雯锦1( ), 马瑞2,3, 刘升燕3, 杨江伟1,2, 张宁1,2, 司怀军1,2(
), 马瑞2,3, 刘升燕3, 杨江伟1,2, 张宁1,2, 司怀军1,2( )
)
收稿日期:2023-01-31
出版日期:2023-09-26
发布日期:2023-10-24
通讯作者:
司怀军,男,博士,教授,博士生导师,研究方向:马铃薯生物技术育种与基因工程;E-mail: hjsi@gsau.edu.cn作者简介:刘雯锦,女,硕士,研究方向:植物学;E-mail: 1490826168@qq.com
基金资助:
LIU Wen-jin1( ), MA Rui2,3, LIU Sheng-yan3, YANG Jiang-wei1,2, ZHANG Ning1,2, SI Huai-jun1,2(
), MA Rui2,3, LIU Sheng-yan3, YANG Jiang-wei1,2, ZHANG Ning1,2, SI Huai-jun1,2( )
)
Received:2023-01-31
Published:2023-09-26
Online:2023-10-24
摘要:
明确马铃薯StCIPK11在响应干旱胁迫信号传导中的功能和作用机制,为深入研究StCIPK11响应马铃薯抗旱调控的分子机制提供理论依据。利用同源重组法和人工microRNA技术构建马铃薯StCIPK11过表达载体和干扰表达载体,通过根癌农杆菌介导法分别将其转入马铃薯栽培品种‘大西洋’中。RT-qPCR结果表明,过表达植株StCIPK11的表达量是非转基因植株(NT)的11.59和21.76倍,干扰表达植株StCIPK11干扰程度达到78%。经PEG模拟干旱胁迫,过表达植株叶片中丙二醛含量显著高于NT植株,脯氨酸含量、抗氧化酶(超氧化物歧化酶、过氧化物酶)活性均低于NT植株;StCIPK11干扰表达植株则表现出相反的趋势。StCIPK11参与了干旱胁迫应答过程,StCIPK11干扰表达可以降低马铃薯植株对水分胁迫的敏感性。
刘雯锦, 马瑞, 刘升燕, 杨江伟, 张宁, 司怀军. 马铃薯StCIPK11的克隆及响应干旱胁迫分析[J]. 生物技术通报, 2023, 39(9): 147-155.
LIU Wen-jin, MA Rui, LIU Sheng-yan, YANG Jiang-wei, ZHANG Ning, SI Huai-jun. Cloning of StCIPK11 Gene and Analysis of Its Response to Drought Stress in Solanum tuberosum[J]. Biotechnology Bulletin, 2023, 39(9): 147-155.
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| StCIPK11-F | CGGGGGACGAGCTCGGTACCATGGCCTTATTCTCTTCTTCGG | PCR扩增 PCR amplification |
| StCIPK11-R | CCATGTCGACTCTAGATTATGGATCCACCTCTGTTGAAATC | |
| StCIPK11-I | GATTCTATTTCCGCGCTAGCCTCTCTCTCTTTTGTATTCC | amiR-StCIPK11 PCR扩增 amiR-StCIPK11 for PCR amplification |
| StCIPK11-II | GAGAGGCTAGCGCGGAAATAGAATCAAAGAGAATCAATGA | |
| StCIPK11-III | GAGAAGCTAGCGCGGTAATAGATTCACAGGTCGTGATATG | |
| StCIPK11-IV | GAATCTATTACCGCGCTAGCTTCTCTACATATATATTCCT | |
| StCIPK11-A | CTGCAAGGCGATTAAGTTGGGTAAC | |
| StCIPK11-B | GCGGATAACAATTTCACACAGGAAACAG | |
| Q- StCIPK11-F | TACGACACCCACACATCGTC | 实时荧光定量PCR Quantitative real-time PCR |
| Q-StCIPK11-R | TTTGCGAACAATTCGCCTCC | |
| StEf1a-F | CAAGGATGACCCAGCCAAG | 内参基因Reference gene |
| StEf1a-R | TTCCTTACCTGAACGCCTGT | |
| HYG-F | GTGATTTCATATGCGCGATTGCTG | HYG基因PCR扩增PCR amplification for HYG gene |
| HYG-R | ACGAGTGCTGGGGCGTCGGTTTCC | |
| NPT II-F | GCTATGACTGGGCACAACAG | NPT II基因PCR扩增PCR amplification for NPT II gene |
| NPT II-R | ATACCGTAAAGCACGAGGAA |
表 1 本研究所用的引物序列
Table 1 Primer sequences used in the study
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| StCIPK11-F | CGGGGGACGAGCTCGGTACCATGGCCTTATTCTCTTCTTCGG | PCR扩增 PCR amplification |
| StCIPK11-R | CCATGTCGACTCTAGATTATGGATCCACCTCTGTTGAAATC | |
| StCIPK11-I | GATTCTATTTCCGCGCTAGCCTCTCTCTCTTTTGTATTCC | amiR-StCIPK11 PCR扩增 amiR-StCIPK11 for PCR amplification |
| StCIPK11-II | GAGAGGCTAGCGCGGAAATAGAATCAAAGAGAATCAATGA | |
| StCIPK11-III | GAGAAGCTAGCGCGGTAATAGATTCACAGGTCGTGATATG | |
| StCIPK11-IV | GAATCTATTACCGCGCTAGCTTCTCTACATATATATTCCT | |
| StCIPK11-A | CTGCAAGGCGATTAAGTTGGGTAAC | |
| StCIPK11-B | GCGGATAACAATTTCACACAGGAAACAG | |
| Q- StCIPK11-F | TACGACACCCACACATCGTC | 实时荧光定量PCR Quantitative real-time PCR |
| Q-StCIPK11-R | TTTGCGAACAATTCGCCTCC | |
| StEf1a-F | CAAGGATGACCCAGCCAAG | 内参基因Reference gene |
| StEf1a-R | TTCCTTACCTGAACGCCTGT | |
| HYG-F | GTGATTTCATATGCGCGATTGCTG | HYG基因PCR扩增PCR amplification for HYG gene |
| HYG-R | ACGAGTGCTGGGGCGTCGGTTTCC | |
| NPT II-F | GCTATGACTGGGCACAACAG | NPT II基因PCR扩增PCR amplification for NPT II gene |
| NPT II-R | ATACCGTAAAGCACGAGGAA |
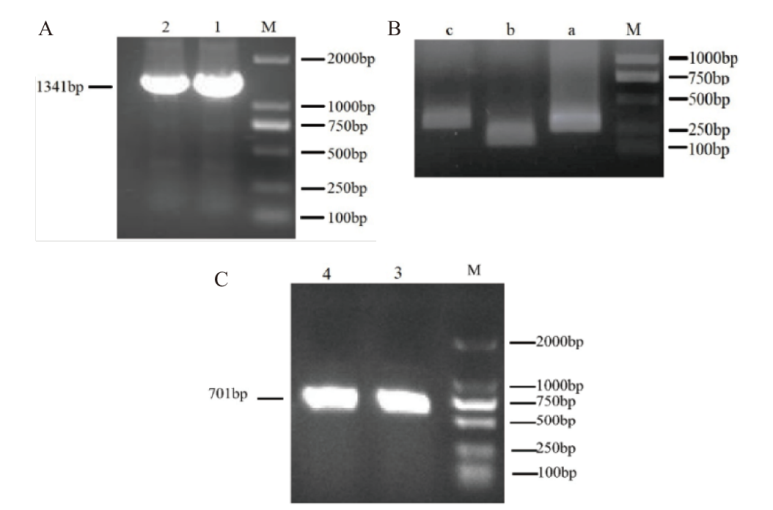
图1 StCIPK11的扩增产物条带及 amiR-StCIPK11前体片段扩增 A: StCIPK11扩增产物条带;B: a、b和 c小片段;C: d 片段;M: DNA marker DL2000;1-2:基因目的片段;3-4:d片段
Fig. 1 StCIPK11 amplified product bands and amiR-StCIPK11 precursor fragments amplification A: StCIPK11 amplification product bands. B: a, b and c are small fragments. C: d fragment. M: DNA marker DL2000. 1-2: Gene fragment. 3-4: d fragment

图2 StCIPK11蛋白序列及其他物种同源序列的多重比对 黑色:完全相同序列;粉色:序列相似度75%以上;蓝色:序列相似度50%以上;白色:序列相似度小于30%
Fig. 2 Multiple alignments of homologous sequences between StCIPK11 protein and other species Black: Completely identitical sequence. Pink: Sequence identity above 75%. Blue: Sequence identity above 50%. White: Sequence identity below 30%

图4 重组质粒双酶切验证 A: p1300-StCIPK11双酶切验证;B: pBI121-amiR-StCIPK11双酶切验证;M: DNA marker DL2000;1-2: 酶切片段
Fig. 4 Double-digestion verification of recombinant plasmid A: Double-digestion verification of p1300-StCIPK11. B: Double-digestion verification of pBI121-amiR-StCIPK11. M: DNA marker DL2000. 1-2: Digestion fragment
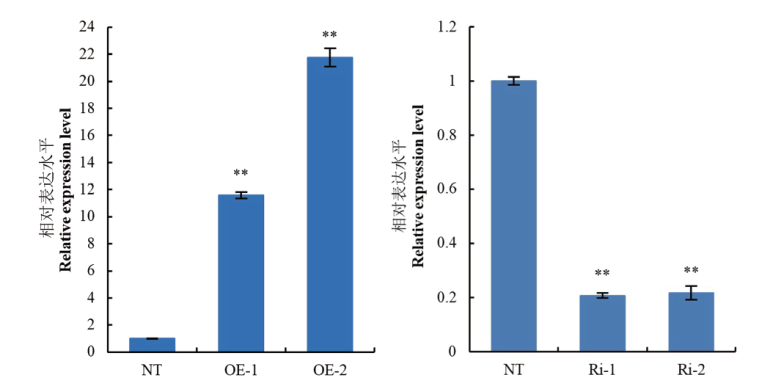
图5 转基因植株的RT-qPCR检测 OE-1、OE-2:过表达植株;Ri-1、Ri-2:干扰表达植株;NT:非转基因植株;试验数据为3个生物学重复的平均值,数据为LSD法进行显著性分析。*:P< 0.05显著差异;**:P< 0.01极显著差异。下同
Fig. 5 RT-qPCR detection of the transgenic plants OE-1, OE-2: The overexpressed plants. Ri-1, Ri-2: The interfering expression plants. NT: Non-transgenic plants. Data are mean ±SD(n=3). LSD post hoc test were used for significant analysis. *: P< 0.05; **: P< 0.01. The same below

图6 PEG6000处理下转基因植株和NT植株生理指标分析 A: MDA含量;B: Pro含量;C: SOD活性;D: POD活性
Fig. 6 Analysis of physiological indices of transgenic and NT potato plants under PEG6000 treatment A: MDA content; B: proline content; C: SOD activity; D: POD activity
| [1] | 陈玉珍, 唐广彬, 马宪新, 等. 马铃薯块茎发育的四大调控途径[J]. 作物杂志, 2022(4): 9-13. |
| Chen YZ, Tang GB, Ma XX, et al. Four major regulatory pathways of potato tuber development[J]. Crops, 2022(4): 9-13. | |
| [2] |
The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato[J]. Nature, 2011, 475(7355): 189-195.
doi: 10.1038/nature10158 |
| [3] | 贾立国, 陈玉珍, 樊明寿, 等. 干旱对马铃薯光合特性及块茎形成的影响[J]. 干旱区资源与环境, 2018, 32(2): 188-193. |
| Jia LG, Chen YZ, Fan MS, et al. Influence of drought stress on potato photosynthetic characteristics and tuberization and its possible mechanism[J]. J Arid Land Resour Environ, 2018, 32(2): 188-193. | |
| [4] | 吴明阳. 转AtCIPK23基因马铃薯耐旱性的初步研究[D]. 雅安: 四川农业大学, 2012. |
| Wu MY. The preliminary study on the drought tolerance of transgenic ArCIPK23 potato[D]. Ya'an: Sichuan Agricultural University, 2012. | |
| [5] | 孙慧生. 马铃薯育种学[M]. 北京: 中国农业出版社, 2003: 242-254. |
| Sun HS. Potato breeding[M]. Beijing: China Agriculture Press, 2003: 242-254. | |
| [6] | Лetyxob CН, 陶金萍, 张相英. 马铃薯抗旱性状的遗传[J]. 杂粮作物, 2000, 20(6): 11-13. |
| Лetyxob CH, Tao JP, Zhang XY. Inheritance of drought-resistant traits in potatoes[J]. Rain Fed Crops, 2000, 20(6): 11-13. | |
| [7] |
Ranty B, Aldon D, Cotelle V, et al. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses[J]. Front Plant Sci, 2016, 7: 327.
doi: 10.3389/fpls.2016.00327 pmid: 27014336 |
| [8] |
Rudd JJ, Franklin-Tong VE. Unravelling response-specificity in Ca2+ signaling pathways in plant cells[J]. New Phytol, 2001, 151(1): 7-33.
doi: 10.1046/j.1469-8137.2001.00173.x URL |
| [9] |
Boudsocq M, Sheen J. CDPKs in immune and stress signaling[J]. Trends Plant Sci, 2013, 18(1): 30-40.
doi: 10.1016/j.tplants.2012.08.008 pmid: 22974587 |
| [10] |
Luan S, Lan WZ, Lee SC. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL-CIPK network[J]. Curr Opin Plant Biol, 2009, 12(3): 339-346.
doi: 10.1016/j.pbi.2009.05.003 pmid: 19501014 |
| [11] |
Tang RJ, Zhao FG, Garcia VJ, et al. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis[J]. Proc Natl Acad Sci USA, 2015, 112(10): 3134-3139.
doi: 10.1073/pnas.1420944112 URL |
| [12] | 刘砚璞, 肖雪, 赵建明, 等. 中国野生燕山葡萄VyCIPK9基因的克隆与功能分析[J]. 果树学报, 2022, 39(10): 1748-1758. |
| Liu YP, Xiao X, Zhao JM, et al. Cloning and functional analysis of VyCIPK9 gene in Chinese wild grape(Vitis yeshanensis ‘Yanshan’)[J]. J Fruit Sci, 2022, 39(10): 1748-1758. | |
| [13] |
Kim KN, Cheong YH, Grant JJ, et al. CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis[J]. Plant Cell, 2003, 15(2): 411-423.
doi: 10.1105/tpc.006858 URL |
| [14] |
Kudla J, Xu QA, Harter K, et al. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals[J]. Proc Natl Acad Sci USA, 1999, 96(8): 4718-4723.
doi: 10.1073/pnas.96.8.4718 pmid: 10200328 |
| [15] |
Albrecht V, Ritz O, Linder S, et al. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases[J]. EMBO J, 2001, 20(5): 1051-1063.
doi: 10.1093/emboj/20.5.1051 pmid: 11230129 |
| [16] |
Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance[J]. J Exp Bot, 2014, 65(5): 1241-1257.
doi: 10.1093/jxb/ert430 pmid: 24368505 |
| [17] |
Qi XH, Tang X, Liu WG, et al. A potato RING-finger protein gene StRFP2 is involved in drought tolerance[J]. Plant Physiol Biochem, 2020, 146: 438-446.
doi: 10.1016/j.plaphy.2019.11.042 URL |
| [18] |
Armengaud P, Thiery L, Buhot N, et al. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features[J]. Physiol Plant, 2004, 120(3): 442-450.
doi: 10.1111/j.0031-9317.2004.00251.x pmid: 15032841 |
| [19] |
Zhang HY, Mao XG, Jing RL, et al. Characterization of a common wheat(Triticum aestivum L.) TaSnRK2.7 gene involved in abiotic stress responses[J]. J Exp Bot, 2011, 62(3): 975-988.
doi: 10.1093/jxb/erq328 URL |
| [20] |
Tsou PL, Lee SY, Allen NS, et al. An ER-targeted calcium-binding peptide confers salt and drought tolerance mediated by CIPK6 in Arabidopsis[J]. Planta, 2012, 235(3): 539-552.
doi: 10.1007/s00425-011-1522-9 URL |
| [21] |
Xiang Y, Huang YM, Xiong LZ. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement[J]. Plant Physiol, 2007, 144(3): 1416-1428.
doi: 10.1104/pp.107.101295 pmid: 17535819 |
| [22] |
Tripathi V, Parasuraman B, Laxmi A, et al. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants[J]. Plant J, 2009, 58(5): 778-790.
doi: 10.1111/tpj.2009.58.issue-5 URL |
| [23] |
Abdula SE, Lee HJ, Ryu H, et al. Overexpression of BrCIPK1 gene enhances abiotic stress tolerance by increasing proline biosynthesis in rice[J]. Plant Mol Biol Rep, 2016, 34(2): 501-511.
doi: 10.1007/s11105-015-0939-x URL |
| [24] | 柴畅. 番茄CIPK8基因在低温、盐和干旱胁迫下功能研究[D]. 哈尔滨: 东北农业大学, 2021. |
| Chai C. Function of CIPK8 gene under low temperature, salt and drought stress in tomato[D]. Harbin:Northeast Agricultural University, 2021. | |
| [25] |
Ma X, Li Y, Gai WX, et al. The CaCIPK3 gene positively regulates drought tolerance in pepper[J]. Hortic Res, 2021, 8(1): 216.
doi: 10.1038/s41438-021-00651-7 |
| [26] |
Ma R, Liu WG, Li SG, et al. Genome-wide identification, characterization and expression analysis of the CIPK gene family in potato(Solanum tuberosum L.) and the role of StCIPK10 in response to drought and osmotic stress[J]. Int J Mol Sci, 2021, 22(24): 13535.
doi: 10.3390/ijms222413535 URL |
| [27] | Huai S. An efficient protocol for Agrobacterium-mediated transformation with microtuber and the introduction of an antisense class I patatin gene into potato[J]. Acta Agronomica Sinica, 2003(6): 801-805. |
| [28] |
Osmolovskaya N, Shumilina J, Kim A, et al. Methodology of drought stress research: experimental setup and physiological characterization[J]. Int J Mol Sci, 2018, 19(12): 4089.
doi: 10.3390/ijms19124089 URL |
| [29] |
Ravikumar G, Manimaran P, Voleti SR, et al. Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice[J]. Transgenic Res, 2014, 23(3): 421-439.
doi: 10.1007/s11248-013-9776-6 pmid: 24398893 |
| [30] | 陈刚, 李胜. 植物生理学实验[M]. 北京: 高等教育出版社, 2016. |
| Chen G, Li S. Plant physiology experiment[M]. Beijing: Higher Education Press, 2016. | |
| [31] |
Lu L, Chen XY, Wang PK, et al. CIPK11: a calcineurin B-like protein-interacting protein kinase from Nitraria tangutorum, confers tolerance to salt and drought in Arabidopsis[J]. BMC Plant Biol, 2021, 21(1): 123.
doi: 10.1186/s12870-021-02878-x pmid: 33648456 |
| [32] |
苏倩, 杜文宣, 马琳, 等. 紫花苜蓿MsCIPK2的克隆及功能分析[J]. 中国农业科学, 2022, 55(19): 3697-3709.
doi: 10.3864/j.issn.0578-1752.2022.19.002 |
| Su Q, Du WX, Ma L, et al. Cloning and functional analyses of MsCIPK2 in Medicago sativa[J]. Sci Agric Sin, 2022, 55(19): 3697-3709. | |
| [33] | 洪鼎立, 安畅, 徐如宏, 等. 小麦GzCIPK19基因的克隆及表达分析[J]. 分子植物育种, 2022, 20(12): 3837-3846. |
| Hong DL, An C, Xu RH, et al. Cloning and expression analysis of wheat GzCIPK19 gene[J]. Mol Plant Breed, 2022, 20(12): 3837-3846. | |
| [34] |
Ma YL, Cao J, Chen QQ, et al. The kinase CIPK11 functions as a negative regulator in drought stress response in Arabidopsis[J]. Int J Mol Sci, 2019, 20(10): 2422.
doi: 10.3390/ijms20102422 URL |
| [35] |
Gratz R, Manishankar P, Ivanov R, et al. CIPK11-dependent phosphorylation modulates FIT activity to promote Arabidopsis iron acquisition in response to calcium signaling[J]. Dev Cell, 2019, 48(5): 726-740.e10.
doi: 10.1016/j.devcel.2019.01.006 URL |
| [36] |
陈小晶, 王东梅, 关红辉, 等. 玉米CIPK基因家族的鉴定及ZmCIPK3的抗旱性功能研究[J]. 植物遗传资源学报, 2022, 23(4): 1064-1075.
doi: 10.13430/j.cnki.jpgr.20220107006 |
| Chen XJ, Wang DM, Guan HH, et al. Identification of CIPK gene family members and investigation of the drought tolerance of ZmCIPK3 in maize[J]. J Plant Genet Resour, 2022, 23(4): 1064-1075. | |
| [37] | 杨宏羽, 李瑞瑞, 何雷, 等. 马铃薯StCIPK基因家族鉴定及表达特征分析[J]. 植物生理学报, 2022, 58(10): 1919-1934. |
| Yang HY, Li RR, He L, et al. Identification and expression characterization of StCIPK gene family in potato(Solanum tuberosum)[J]. Plant Physiol J, 2022, 58(10): 1919-1934. |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 丁凯鑫, 王立春, 田国奎, 王海艳, 李凤云, 潘阳, 庞泽, 单莹. 烯效唑缓解植物干旱损伤的研究进展[J]. 生物技术通报, 2023, 39(6): 1-11. |
| [3] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [4] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [5] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [6] | 于波, 秦晓惠, 赵杨. 植物感应干旱信号的机制[J]. 生物技术通报, 2023, 39(11): 6-17. |
| [7] | 陈楚怡, 杨小梅, 陈胜艳, 陈斌, 岳莉然. ABA和干旱胁迫下菊花脑ZF-HD基因家族的表达分析[J]. 生物技术通报, 2023, 39(11): 270-282. |
| [8] | 冯策婷, 江律, 刘鑫颖, 罗乐, 潘会堂, 张启翔, 于超. 单叶蔷薇NAC基因家族鉴定及干旱胁迫响应分析[J]. 生物技术通报, 2023, 39(11): 283-296. |
| [9] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| [10] | 关志秀, 汪燕, 梁成刚, 韦春玉, 黄娟, 陈庆富. 苦荞FtCBL基因的鉴定及对干旱与高钙胁迫的响应[J]. 生物技术通报, 2022, 38(8): 101-109. |
| [11] | 陈佳敏, 刘永杰, 马锦绣, 李丹, 公杰, 赵昌平, 耿洪伟, 高世庆. 小麦组蛋白甲基化酶在杂交种中干旱胁迫表达模式分析[J]. 生物技术通报, 2022, 38(7): 51-61. |
| [12] | 李一涵, 于浪柳, 李春燕, 张蒙蒙, 张晓勤, 方云霞, 薛大伟. 大麦NRAMP全基因组鉴定及重金属胁迫下基因表达分析[J]. 生物技术通报, 2022, 38(6): 103-111. |
| [13] | 刘自然, 甄珍, 陈强, 李玥莹, 王泽, 逄洪波. 植物响应Cd胁迫研究进展[J]. 生物技术通报, 2022, 38(6): 13-26. |
| [14] | 于国红, 刘朋程, 李磊, 李明哲, 崔海英, 郝洪波, 郭安强. 不同基因型马铃薯对干旱胁迫的生理响应[J]. 生物技术通报, 2022, 38(5): 56-63. |
| [15] | 高蒙, 李富婷, 魏湛林, 张赛行, 白如仟, 尚轶, 马玲. 二倍体马铃薯中SCFSLF复合体的组分分析[J]. 生物技术通报, 2022, 38(4): 117-125. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||