








生物技术通报 ›› 2023, Vol. 39 ›› Issue (9): 168-175.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0150
刘玉玲1,2( ), 王梦瑶2, 孙琦1, 马利花1, 朱新霞1(
), 王梦瑶2, 孙琦1, 马利花1, 朱新霞1( )
)
收稿日期:2023-02-22
出版日期:2023-09-26
发布日期:2023-10-24
通讯作者:
朱新霞,女,博士,副教授,研究方向:植物生物技术;E-mail: xinxiazh@shzu.edu.cn作者简介:刘玉玲,女,硕士,研究方向:环境生物技术;E-mail: 839779394@qq.com
基金资助:
LIU Yu-ling1,2( ), WANG Meng-yao2, SUN Qi1, MA Li-hua1, ZHU Xin-xia1(
), WANG Meng-yao2, SUN Qi1, MA Li-hua1, ZHU Xin-xia1( )
)
Received:2023-02-22
Published:2023-09-26
Online:2023-10-24
摘要:
探究逆境诱导启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响,为SikCDPK1基因在植物遭遇低温、干旱时更好发挥作用奠定基础。采用基因重组技术构建RD29A启动子驱动SikCDPK1基因的植物表达载体,通过农杆菌介导法遗传转化烟草,分别观察、测定和比较分析低温、干旱处理后,RD29A∷SikCDPK1转基因烟草、35S∷SikCDPK1转基因烟草和非转基因烟草的表型、POD活性、SOD活性、叶绿素含量、MDA含量和相对电导率的差异。结果显示,干旱和低温胁迫后,RD29A∷SikCDPK1转基因烟草的生长状况优于35S∷SikCDPK1转基因烟草,更优于非转基因烟草;同时,RD29A∷SikCDPK1转基因烟草的POD活性、SOD活性、叶绿素含量显著高于35S∷SikCDPK1转基因烟草,极显著高于非转基因烟草;但MDA含量与相对电导率显著低于35S∷SikCDPK1转基因烟草,极显著低于非转基因烟草。表明启动子RD29A可通过减缓叶绿素降解速率、提高抗氧化系统酶活性、减小膜通透性,使转基因烟草表现出更强的干旱、低温耐受性。
刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175.
LIU Yu-ling, WANG Meng-yao, SUN Qi, MA Li-hua, ZHU Xin-xia. Effect of RD29A Promoter on the Stress Resistance of Transgenic Tobacco with SikCDPK1 Gene from Saussurea involucrata[J]. Biotechnology Bulletin, 2023, 39(9): 168-175.
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| RD29A-F | GAATTCCGACTCAAAACAAACTTACGAA | 启动子克隆Promoter cloing |
| RD29A-R | CCCGGGAATCAAACCCTTTATTCCTGA | 启动子克隆Promoter cloing |
| SikCDPK1-F | GGATCCATGGGGAATACTTGTGTTGGAC | 基因鉴定 Gene identification |
| SikCDPK1-R | GTCGACCCGTCGATACCGGAAAAAAC | 基因鉴定 Gene identification |
表1 引物序列信息
Table 1 Primers information
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| RD29A-F | GAATTCCGACTCAAAACAAACTTACGAA | 启动子克隆Promoter cloing |
| RD29A-R | CCCGGGAATCAAACCCTTTATTCCTGA | 启动子克隆Promoter cloing |
| SikCDPK1-F | GGATCCATGGGGAATACTTGTGTTGGAC | 基因鉴定 Gene identification |
| SikCDPK1-R | GTCGACCCGTCGATACCGGAAAAAAC | 基因鉴定 Gene identification |

图1 RD29A∷SikCDPK1重组质粒双酶切鉴定 A: 重组质粒双酶切(M:Marker III;1:质粒对照; 2:酶切产物);B: 重组质粒双酶切(M:Trans 5 K DNA marker;1-2:酶切产物;3:质粒对照)
Fig. 1 Double enzyme digestion of RD29A∷SikCDPK1 recombinant plasmid A: Recombinant plasmids was digested with double enzymes(M: Marker III.1: Plasmid control. 2: Digestion products). B: Recombinant plasmids were digested with double enzymes(M: Trans 5 K DNA marker. 1-2: Digestion products.3: Plasmid control)
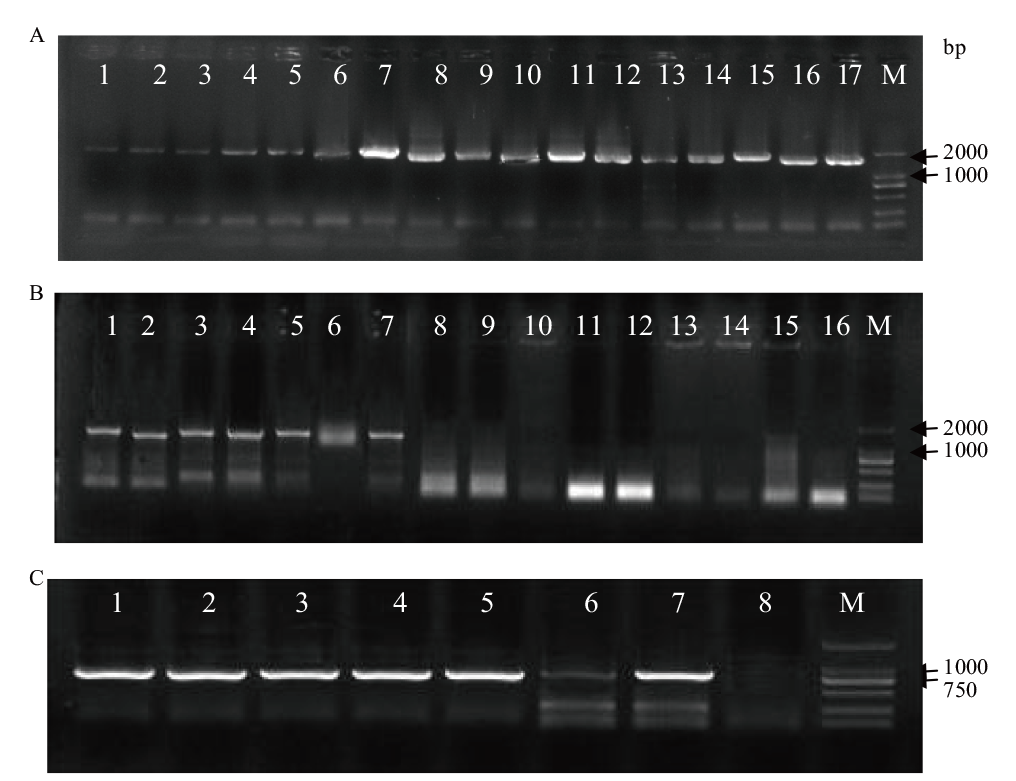
图2 转基因烟草PCR鉴定 A:35S∷SikCDPK1转基因烟草PCR鉴定(M:DL2000 DNA marker;1-17:转基因植株);B:RD29A∷SikCDPK1转基因烟草SikCDPK1基因PCR检测(M:DL2000 DNA marker;1-7:SikCDPK1基因PCR检测;8-16:非转基因烟草PCR对照);C:RD29A∷SikCDPK1转基因烟草RD29A启动子PCR检测(M:Marker III;1-7:RD29A启动子PCR检测;8:非转基因烟草PCR对照)
Fig. 2 Identification of transgenic tobacco by PCR A: PCR identification of 35S∷SikCDPK1 transgenic tobacco(M: DL2000 DNA marker. 1-17: Transgenic plant). B: PCR detection of RD29A∷SikCDPK1 transgenic tobacco SikCDPK1 gene(M: DL2000 DNA marker. 1-7: Detection of SikCDPK1 gene PCR. 8-16: WT PCR control). C: PCR detection of RD29A∷SikCDPK1 transgenic tobacco RD29A promoter(M: Marker III. 1-7: RD29A promoter PCR detection. 8: WT PCR control)

图3 非转基因烟草与转基因烟草逆境胁迫前后表型变化 A,C:正常生长的烟草;B:干旱30 d的烟草;D:低温处理48 h 后的烟草; Line 1,Line 2:不同株系
Fig. 3 Phenotypic characteristics of WT tobacco and transgenic tobacco under stress A, C: Normally growing tobacco. B: Withheld watering 30 d. D: Low temperature treatment of tobacco after 48 h. Line 1, Line 2: Two different tobacco lines
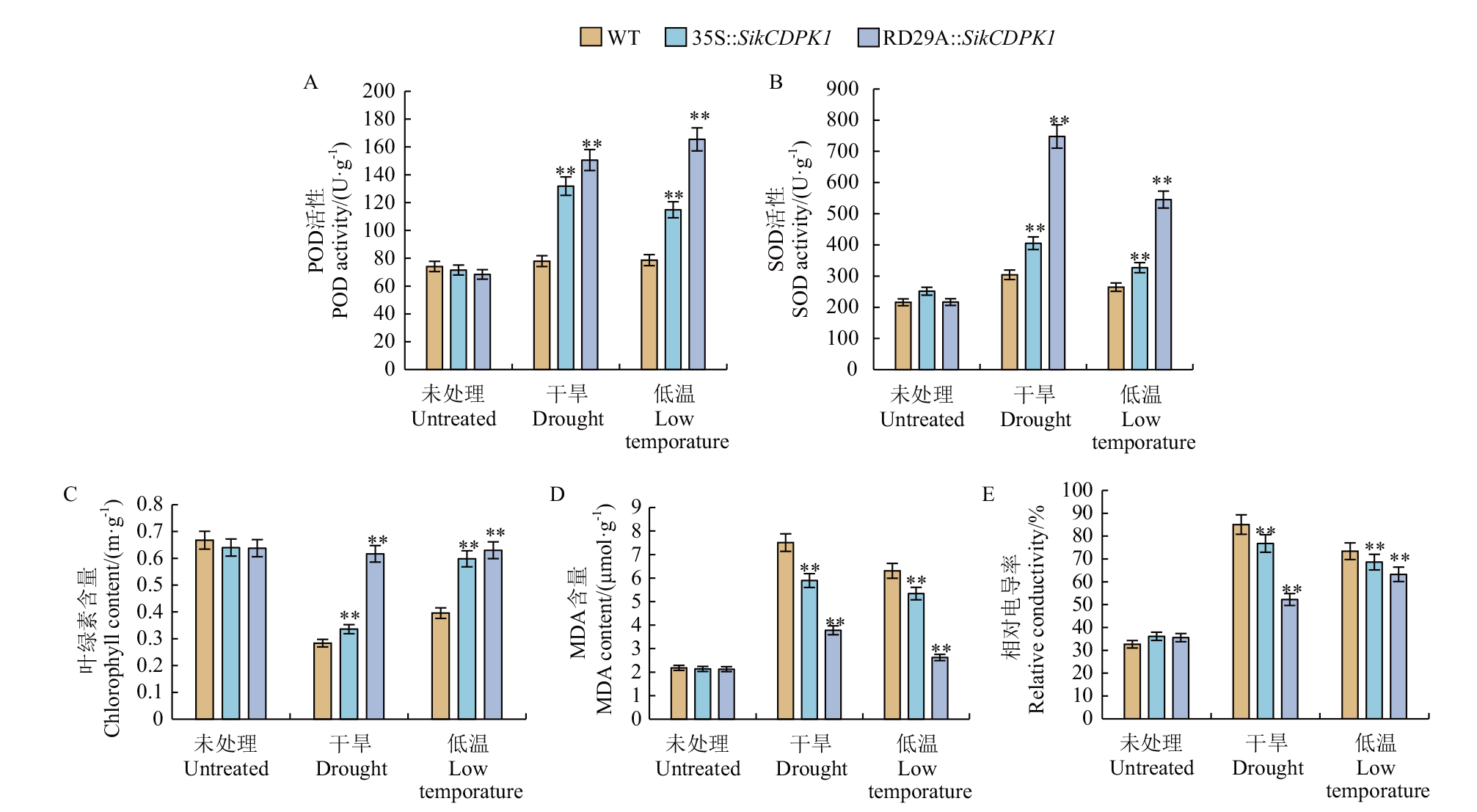
图4 非转基因烟草与转基因烟草胁迫处理前后生理指标测定 *与**分别表示相关性达显著(P<0.05)和极显著(P <0.01)水平
Fig. 4 Physiological indexes of non-transgenic tobacco and transgenic tobacco determined before and after stress * and * * showed significant correlations at 0.05 and 0.01 levels, respectively
| [1] |
Li YY, Zhang HX, Liang SB, et al. Identification of CDPK gene family in Solanum habrochaites and its function analysis under stress[J]. Int J Mol Sci, 2022, 23(8): 4227.
doi: 10.3390/ijms23084227 URL |
| [2] |
Bi ZZ, Wang YH, Li PC, et al. Evolution and expression analysis of CDPK genes under drought stress in two varieties of potato[J]. Biotechnol Lett, 2021, 43(2): 511-521.
doi: 10.1007/s10529-020-03037-2 |
| [3] | 李建鑫. 番茄钙依赖蛋白激酶CPK28在植物响应高温胁迫中的功能及机制研究[D]. 杭州: 浙江大学, 2021. |
| Li JX. The function and mechanism of calcium-dependent protein kinases CPK28 in tomato response to heat stress[D]. Hangzhou: Zhejiang University, 2021. | |
| [4] | 朱丽萍, 于壮, 邹翠霞, 等. 植物逆境相关启动子及功能[J]. 遗传, 2010, 32(3): 229-234. |
| Zhu LP, Yu Z, Zou CX, et al. Plant stress-inducible promoters and their function[J]. Hereditas, 2010, 32(3): 229-234. | |
| [5] |
Orbović V, Ali Ravanfar S, Acanda Y, et al. Stress-inducible Arabidopsis thaliana RD29A promoter constitutively drives Citrus sinensis APETALA1 and LEAFY expression and precocious flowering in transgenic Citrus spp[J]. Transgenic Res, 2021, 30(5): 687-699.
doi: 10.1007/s11248-021-00260-z |
| [6] |
Bihmidine S, Lin JS, Stone JM, et al. Activity of the Arabidopsis RD29A and RD29B promoter elements in soybean under water stress[J]. Planta, 2013, 237(1): 55-64.
doi: 10.1007/s00425-012-1740-9 pmid: 22983672 |
| [7] | 聂利珍, 于肖夏, 李国婧, 等. Rd29A启动子驱动AtCDPK1基因转化马铃薯的研究[J]. 中国生物工程杂志, 2015, 35(11): 13-22. |
| Nie LZ, Yu XX, Li GJ, et al. Study on transgenic potato contained AtCDPK1 gene drived by Rd29A promoter[J]. China Biotechnol, 2015, 35(11): 13-22. | |
| [8] |
Rai GK, Rai NP, Rathaur S, et al. Expression of RD29A∷AtDRE-B1A/CBF3 in tomato alleviates drought-induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants[J]. Plant Physiol Biochem, 2013, 69: 90-100.
doi: 10.1016/j.plaphy.2013.05.002 URL |
| [9] |
Estrada-Melo AC, Chao, Reid MS, et al. Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia[J]. Hortic Res, 2015, 2: 15013.
doi: 10.1038/hortres.2015.13 |
| [10] |
Das A, Basu PS, Kumar M, et al. Transgenic chickpea(Cicer arietinum L.) harbouring AtDREB1a are physiologically better adapted to water deficit[J]. BMC Plant Biol, 2021, 21(1): 39.
doi: 10.1186/s12870-020-02815-4 |
| [11] |
Kang HH, Zhang M, Zhou SM, et al. Overexpression of wheat ubiquitin gene, Ta-Ub2, improves abiotic stress tolerance of Brachypodium distachyon[J]. Plant Sci, 2016, 248: 102-115.
doi: 10.1016/j.plantsci.2016.04.015 URL |
| [12] |
Gu XB, Chen YH, Gao ZH, et al. Transcription factors and anthocyanin genes related to low-temperature tolerance in RD29A∷RdreB1BI transgenic strawberry[J]. Plant Physiol Biochem, 2015, 89: 31-43.
doi: 10.1016/j.plaphy.2015.02.004 URL |
| [13] | 田晓涵, 庞学兵, 祝建波, 等. 过表达天山雪莲SikCDPK1基因提高转基因烟草耐低温能力的机制初探[J]. 中国烟草学报, 2016, 22(6): 98-103. |
| Tian XH, Pang XB, Zhu JB, et al. Effect of over-expression of Saussurea involucrata SikCDPK1 gene on improving cold tolerance in transgenic tobacco[J]. Acta Tabacaria Sin, 2016, 22(6): 98-103. | |
| [14] | 王金胜, 吕淑霞, 张宁. 基础生物化学[M]. 2版. 北京: 中国农业出版社, 2021. |
| Wang JS, Lv SX, Zhang N. Fundamental biochemistry[M]. 2nd ed. Beijing: China Agriculture Press, 2021. | |
| [15] |
Zhang HM, Zhu JH, Gong ZZ, et al. Abiotic stress responses in plants[J]. Nat Rev Genet, 2022, 23(2): 104-119.
doi: 10.1038/s41576-021-00413-0 |
| [16] |
Asano T, Hayashi N, Kikuchi S, et al. CDPK-mediated abiotic stress signaling[J]. Plant Signal Behav, 2012, 7(7): 817-821.
doi: 10.4161/psb.20351 pmid: 22751324 |
| [17] |
McAinsh MR, Pittman JK. Shaping the calcium signature[J]. New Phytol, 2009, 181(2): 275-294.
doi: 10.1111/j.1469-8137.2008.02682.x pmid: 19121028 |
| [18] |
Shi SJ, Li SG, Asim M, et al. The Arabidopsis calcium-dependent protein kinases(CDPKs)and their roles in plant growth regulation and abiotic stress responses[J]. Int J Mol Sci, 2018, 19(7): 1900.
doi: 10.3390/ijms19071900 URL |
| [19] |
Boudsocq M, Willmann MR, McCormack M, et al. Differential innate immune signalling via Ca2+ sensor protein kinases[J]. Nature, 2010, 464(7287): 418-422.
doi: 10.1038/nature08794 |
| [20] |
Zou JJ, Wei FJ, Wang C, et al. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress[J]. Plant Physiol, 2010, 154(3): 1232-1243.
doi: 10.1104/pp.110.157545 URL |
| [21] |
Ampo S, Baldrich P, Messeguer J, et al. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation[J]. Plant Physiol, 2014, 165(2): 688-704.
pmid: 24784760 |
| [22] |
Kiselev KV, Aleynova OA, Ogneva ZV, et al. 35S promoter-driven transgenes are variably expressed in different organs of Arabidopsis thaliana and in response to abiotic stress[J]. Mol Biol Rep, 2021, 48(3): 2235-2241.
doi: 10.1007/s11033-021-06235-x |
| [23] |
Zhou SM, Sun XD, Yin SH, et al. The role of the F-box gene TaFBA1 from wheat(Triticum aestivum L.) in drought tolerance[J]. Plant Physiol Biochem, 2014, 84: 213-223.
doi: 10.1016/j.plaphy.2014.09.017 URL |
| [24] | Wei T, Deng KJ, Zhang QX, et al. Modulating AtDREB1C expression improves drought tolerance in Salvia miltiorrhiza[J]. Front Plant Sci, 2017, 8: 52. |
| [25] |
Pino MT, Skinner JS, Park EJ, et al. Use of a stress inducible promoter to drive ectopic AtCBF expression improves potato freezing tolerance while minimizing negative effects on tuber yield[J]. Plant Biotechnol J, 2007, 5(5): 591-604.
doi: 10.1111/pbi.2007.5.issue-5 URL |
| [26] |
Li F, Han YY, Feng YN, et al. Expression of wheat expansin driven by the RD29 promoter in tobacco confers water-stress tolerance without impacting growth and development[J]. J Biotechnol, 2013, 163(3): 281-291.
doi: 10.1016/j.jbiotec.2012.11.008 pmid: 23183383 |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 刘雯锦, 马瑞, 刘升燕, 杨江伟, 张宁, 司怀军. 马铃薯StCIPK11的克隆及响应干旱胁迫分析[J]. 生物技术通报, 2023, 39(9): 147-155. |
| [3] | 王贵芳, 姚元涛, 许海峰, 相昆, 梁家慧, 张淑辉, 王文茹, 张明娟, 张美勇, 陈新. 核桃JrSnRK1α1.1调控种子油脂合成与积累[J]. 生物技术通报, 2023, 39(9): 183-191. |
| [4] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [5] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [6] | 丁凯鑫, 王立春, 田国奎, 王海艳, 李凤云, 潘阳, 庞泽, 单莹. 烯效唑缓解植物干旱损伤的研究进展[J]. 生物技术通报, 2023, 39(6): 1-11. |
| [7] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [8] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [9] | 马芳芳, 刘冠闻, 庞冰, 蒋春美, 师俊玲. 强化细胞外排提高工程菌类黄酮产量的策略[J]. 生物技术通报, 2023, 39(5): 63-76. |
| [10] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [11] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [12] | 蒋路园, 丰美静, 杜雨晴, 邸葆, 陈段芬, 邱德有, 杨艳芳. 红豆杉低温半致死温度和低温胁迫下紫杉烷含量[J]. 生物技术通报, 2023, 39(3): 232-242. |
| [13] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [14] | 吕宇婧, 吴丹丹, 孔春艳, 杨宇, 龚明. 小桐子XTH基因家族和与之互作的miRNAs的全基因组鉴定及其在低温适应中的作用[J]. 生物技术通报, 2023, 39(2): 147-160. |
| [15] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||