








生物技术通报 ›› 2023, Vol. 39 ›› Issue (12): 311-319.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0702
尚怡彤1,2( ), 闫欢欢1,2, 王丽红1,2, 田学琴1,2, 薛萍红1,2, 罗涛1, 胡志宏1,2(
), 闫欢欢1,2, 王丽红1,2, 田学琴1,2, 薛萍红1,2, 罗涛1, 胡志宏1,2( )
)
收稿日期:2023-07-21
出版日期:2023-12-26
发布日期:2024-01-11
通讯作者:
胡志宏,男,博士,副教授,研究方向:微生物分子生物学;E-mail: huzhihong426@163.com作者简介:尚怡彤,女,硕士研究生,研究方向:微生物分子生物学;E-mail: syt15735905986@163.com
基金资助:
SHANG Yi-tong1,2( ), YAN Huan-huan1,2, WANG Li-hong1,2, TIAN Xue-qin1,2, XUE Ping-hong1,2, LUO Tao1, HU Zhi-hong1,2(
), YAN Huan-huan1,2, WANG Li-hong1,2, TIAN Xue-qin1,2, XUE Ping-hong1,2, LUO Tao1, HU Zhi-hong1,2( )
)
Received:2023-07-21
Published:2023-12-26
Online:2024-01-11
摘要:
磷酸甲羟戊酸激酶(PMK)是甲羟戊酸(MVA)途径的关键酶。在真菌中,MVA途径是麦角甾醇生物合成的上游,因此PMK也被称为Erg8。为了研究磷酸甲羟戊酸激酶在米曲霉(Aspergillus oryzae)麦角甾醇合成通路中的作用,对米曲霉AoErg8基因功能进行研究。采用生物信息学方法鉴定米曲霉中的该基因,通过系统发育树和酵母异源互补分析其是否保守,利用实时荧光定量PCR(RT-qPCR)检测其表达模式,同时通过荧光蛋白标记对其亚细胞定位进行分析,最后测定AoErg8基因过表达对米曲霉生长和麦角甾醇含量的影响。结果表明,AoErg8进化保守,其表达量在不同生长时间和不同非生物胁迫下均发生了改变;AoErg8能恢复酿酒酵母erg8突变体的温度敏感表型;AoErg8定位于细胞质中;米曲霉中AoErg8过表达导致麦角甾醇含量降低,并且影响米曲霉生长和孢子形成。因此,米曲霉AoErg8的功能相对保守,其过表达可以降低麦角甾醇含量并影响菌落生长和孢子形成。该研究进一步揭示丝状真菌米曲霉麦角甾醇生物合成和调控机理,为米曲霉或其他真菌脂质代谢的基因工程奠定基础。
尚怡彤, 闫欢欢, 王丽红, 田学琴, 薛萍红, 罗涛, 胡志宏. 米曲霉磷酸甲羟戊酸激酶功能研究[J]. 生物技术通报, 2023, 39(12): 311-319.
SHANG Yi-tong, YAN Huan-huan, WANG Li-hong, TIAN Xue-qin, XUE Ping-hong, LUO Tao, HU Zhi-hong. Study on the Function of Phosphomevalonate Kinase in Aspergillus oryzae[J]. Biotechnology Bulletin, 2023, 39(12): 311-319.
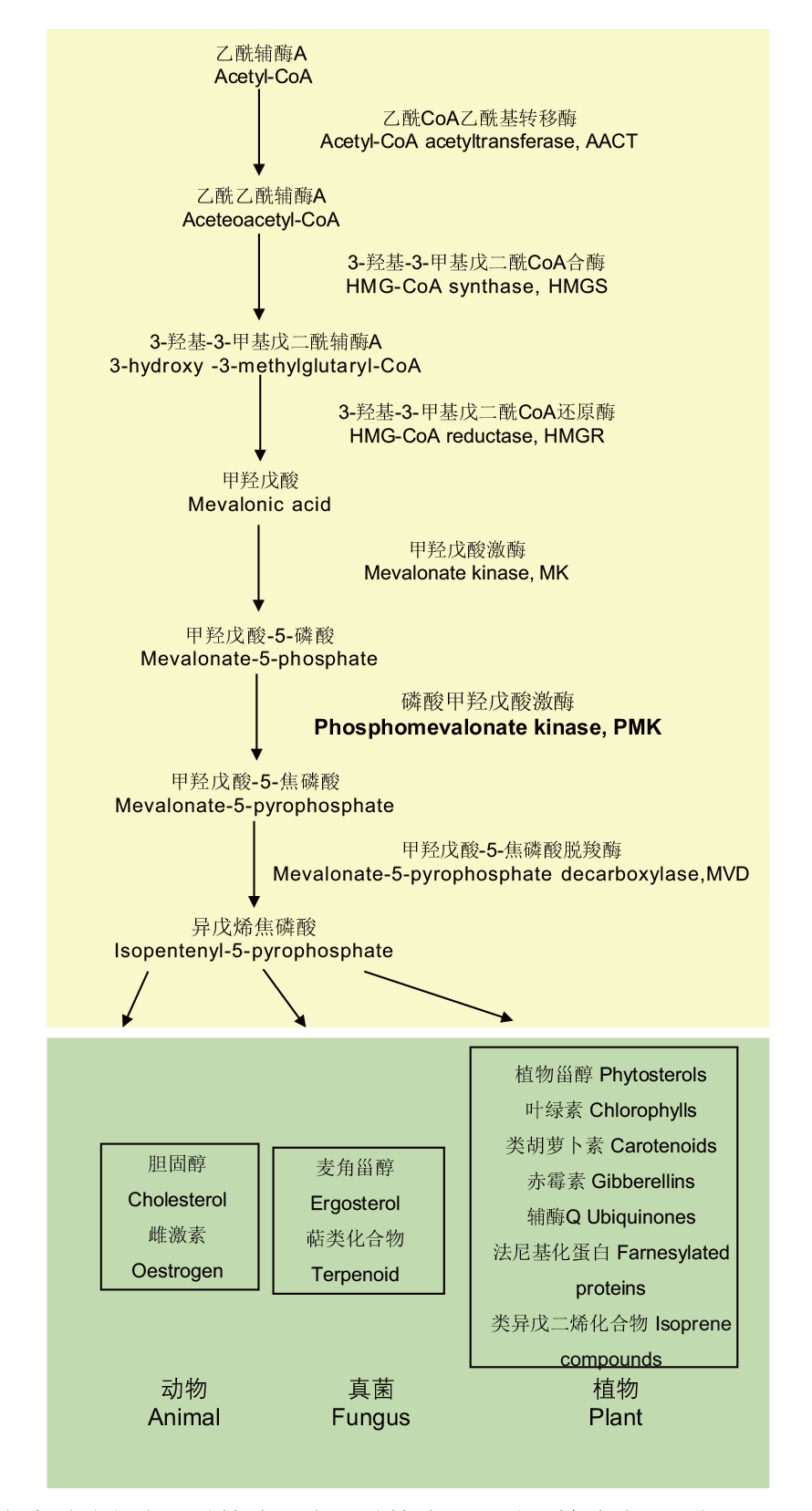
图1 MVA途径代谢通路 甲羟戊酸途径由乙酰辅酶A在乙酰辅酶A乙酰基转移酶、3-羟基-3-甲基戊二酰辅酶A合酶、3-羟基-3-甲基戊二酰辅酶A还原酶、甲羟戊酸激酶、磷酸甲羟戊酸激酶、甲羟戊酸-5-焦磷酸脱羧酶的催化下生成异戊烯焦磷酸,在植物、动物、真菌中位于类异戊二烯物质、甾醇类等物质合成途径的上游,在生物体的生长与代谢活动中起着重要作用
Fig. 1 MVA pathway metabolic synthesis diagram MVA pathway is formed by acetyl-CoA under the catalysis of acetyl-CoA acetyltransferase, 3-hydroxy-3-methylglutaryl-CoA synthase, 3-hydroxy-3-methylglutaryl-CoA reductase, mevalonate kinase, phosphomevalonate kinase, mevalonate 5-pyrophosphate decarboxylase to generate isopentenyl-5-pyrophosphate. In plants, animals,and fungi, it is located in the upstream of isoprenoids and sterols, playing an important role in the growth and metabolic activities of organisms
| 物种名 Species name | 序列号GI No. |
|---|---|
| 黄花蒿Artemisia annua | gi|1387810006 |
| 人参Panax ginseng | gi|555431957 |
| 黄花蒿Artemisia annua | gi|1387770975 |
| 丹参Salvia miltiorrhiza | gi|374639353 |
| 番茄Solanum lycopersicum | gi|1480008164 |
| 海枣Phoenix dactylifera | gi|672109111 |
| 短花药野生稻Oryza brachyantha | gi|1002850285 |
| 玉米Zea mays | gi|1126132679 |
| 卷柏Selaginella moellendorffii | gi|1376963038 |
| 地钱Marchantia polymorpha | gi|1376841057 |
| 小立碗藓Physcomitrium patens | gi|1373905490 |
| 高卢蜜环菌Armillaria gallica | gi|1243522655 |
| 米曲霉Aspergillus oryzae | gi|391866384 |
| 酿酒酵母Saccharomyces cerevisiae | gi|171479 |
| 植物乳杆菌Lactiplantibacillus plantarum | gi|935445216 |
| 金黄色葡萄球菌Staphylococcus aureus | gi|897312969 |
| 欧洲熊蜂Bombus terrestris | gi|526117733 |
| 家蚕Bombyx mori | gi|526117733 |
| 智人Homo sapiens | gi|1294782 |
| 小鼠Mus musculus | gi|32363399 |
表1 不同物种Erg8序列号
Table 1 Erg8 numbers of different species
| 物种名 Species name | 序列号GI No. |
|---|---|
| 黄花蒿Artemisia annua | gi|1387810006 |
| 人参Panax ginseng | gi|555431957 |
| 黄花蒿Artemisia annua | gi|1387770975 |
| 丹参Salvia miltiorrhiza | gi|374639353 |
| 番茄Solanum lycopersicum | gi|1480008164 |
| 海枣Phoenix dactylifera | gi|672109111 |
| 短花药野生稻Oryza brachyantha | gi|1002850285 |
| 玉米Zea mays | gi|1126132679 |
| 卷柏Selaginella moellendorffii | gi|1376963038 |
| 地钱Marchantia polymorpha | gi|1376841057 |
| 小立碗藓Physcomitrium patens | gi|1373905490 |
| 高卢蜜环菌Armillaria gallica | gi|1243522655 |
| 米曲霉Aspergillus oryzae | gi|391866384 |
| 酿酒酵母Saccharomyces cerevisiae | gi|171479 |
| 植物乳杆菌Lactiplantibacillus plantarum | gi|935445216 |
| 金黄色葡萄球菌Staphylococcus aureus | gi|897312969 |
| 欧洲熊蜂Bombus terrestris | gi|526117733 |
| 家蚕Bombyx mori | gi|526117733 |
| 智人Homo sapiens | gi|1294782 |
| 小鼠Mus musculus | gi|32363399 |
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| rH-F | GACAACATCCAGGGTATCACTAAGC |
| rH-R | GGTCTCCTCGTAGATCATGGCA |
| qRT-AoErg8-F | GGCTCTAGTAACTGCCCTAGTA |
| qRT-AoErg8-R | AGCCTGTGCCAAGTTATGAA |
| pEX2B-AoErg8-F | TTCACGTGCCCGTGCTTAAGATGTCTTATCCGCCATCCGGGAG |
| pEX2B-AoErg8-R | GAGGCCATGATATCCTTAAGAAGCCAACCGGCATATTGGTC |
| pYES2.0-AoErg8-F | CTATAGGGAATATTAAGCTTATGTCTTATCCGCCATCCGGG |
| pYES2.0-AoErg8-R | GATGGATATCTGCAGAATTCAAGCCAACCGGCATATTGGTC |
表2 供试引物
Table 2 Primers used in this study
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| rH-F | GACAACATCCAGGGTATCACTAAGC |
| rH-R | GGTCTCCTCGTAGATCATGGCA |
| qRT-AoErg8-F | GGCTCTAGTAACTGCCCTAGTA |
| qRT-AoErg8-R | AGCCTGTGCCAAGTTATGAA |
| pEX2B-AoErg8-F | TTCACGTGCCCGTGCTTAAGATGTCTTATCCGCCATCCGGGAG |
| pEX2B-AoErg8-R | GAGGCCATGATATCCTTAAGAAGCCAACCGGCATATTGGTC |
| pYES2.0-AoErg8-F | CTATAGGGAATATTAAGCTTATGTCTTATCCGCCATCCGGG |
| pYES2.0-AoErg8-R | GATGGATATCTGCAGAATTCAAGCCAACCGGCATATTGGTC |
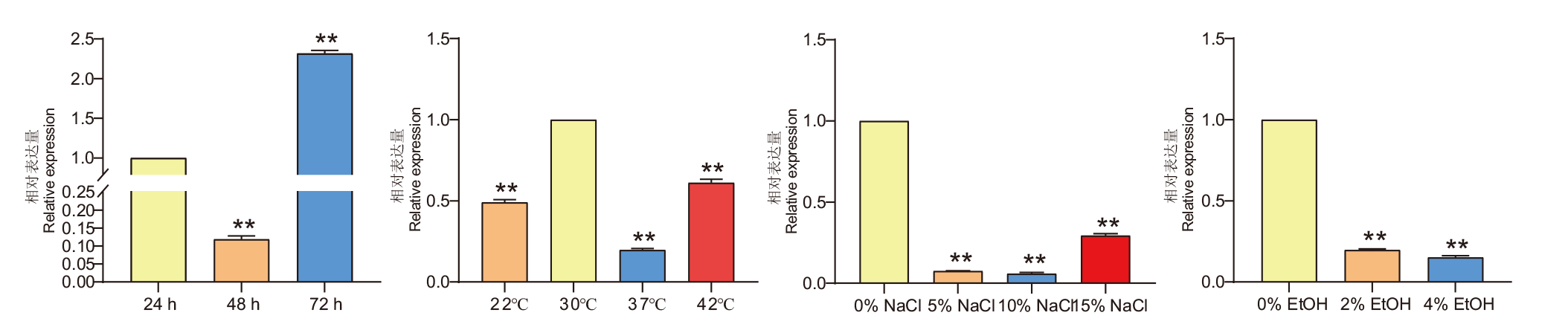
图3 AoErg8在琼脂CD培养基上不同生长时间和不同非生物胁迫下的表达水平 A:生长24、48和72 h AoErg8的表达;B:不同温度下AoErg8的表达;C:不同盐胁迫条件下AoErg8的表达;D:不同浓度乙醇(EtOH)胁迫条件下AoErg8的表达。将野生型米曲霉孢子悬浮液接种在单独的CD琼脂培养基或补充有NaCl或乙醇的CD琼脂培养基上,并在30℃下孵育(温度应激除外)。为了测定不同生长时间的mRNA水平,在24、48和72 h获菌丝体;对于其他测试,在72 h收获菌丝体。使用24 h、30℃下和在0% NaCl/乙醇中的AoErg8表达水平作为相应的参考。数值代表3个独立实验的平均值±标准差。通过GraphPad的t检验进行统计分析,*:P<0.05,**:P <0.01,下同
Fig.3 Expressions of AoErg8 on agar CD medium under different growth time and different abiotic stress A: Expression of AoErg8 at 24, 48, and 72 h of growth. B: Expression of AoErg8 at different temperatures. C: Expression of AoErg8 under different salt stress conditions.D: Expression of AoErg8 under different ethanol(EtOH)stress. In order to determine the level of mRNA at different growth times, Mycelium was harvested at 24, 48 and 72 h. For other tests, harvest Mycelium at 72 h. The expression levels of AoErg8 at 24 h, 30℃, and in 0% NaCl/ethanol were used as corresponding references. The numerical value represents the mean ± standard deviation of three independent experiments. Statistical analysis using GraphPad’s t-test, *: P<0.05, **: P <0.01, the same below
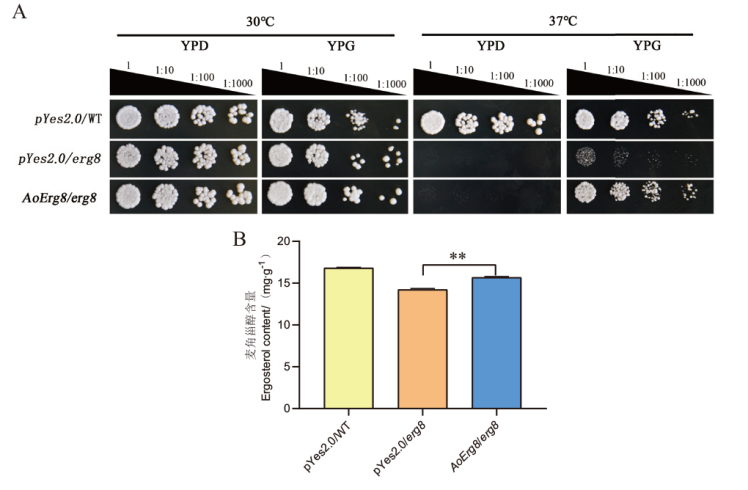
图4 AoErg8可以恢复酿酒酵母erg8突变体的表型 A:野生型生长、erg8突变体(Y40835)、AoErg8转化子在30℃和37℃的YPD和YPG培养基上的生长;B:测量所有转化子中的麦角甾醇含量,将对照和转化子在液体YPG中在30℃下培养2 d,并收集酵母以测定麦角甾醇的含量。将AoErg8转化子的麦角甾醇含量与相应培养基中的pYes2.0/erg8突变体进行比较
Fig. 4 AoErg8 may restore the phenotype of Saccharomyces cerevisiae erg8 mutant A: The growth of wild-type growth, erg8 mutant(Y40835), and AoErg8 transformant on YPD and YPG media at 30℃ and 37℃. B: Measure the ergosterol content in all transformants, incubate the control and transformants in liquid YPG at 30℃ for 2 d, and collect yeast to determine the ergosterol content. Compare the ergosterol content of AoErg8 transformant with the pYES2.0/erg8 mutant in the corresponding culture medium

图5 AoErg8过表达菌株的表型及麦角甾醇的含量 A:培养72 h后,CK(野生型米曲霉转化的pEX2B载体)、AoErg8过表达菌株在PDA培养基上的菌落形态;B:不同转基因菌株菌落的孢子数;C:不同转基因菌株的菌落直径;D:AoErg8过表达菌株的麦角甾醇含量。对照和转化子在30℃的DPY中培养3 d,并收集以测定麦角甾醇的含量。将每个实验组与相应的对照组进行比较
Fig. 5 Phenotype and ergosterol content of AoErg8 overexpressing strain A: After 72 h of cultivation, the colony morphology of CK(pEX2B vector transformed by wild-type A. oryzae)and AoErg8 overexpressing strains on PDA medium. B: Spore count of different transgenic strains. C: Colony diameter of different transgenic strains. D: Ergosterol content of AoErg8 overexpressing strains. The control and transformants were cultured in DPY at 30℃ for 3 d and collected for determination of ergosterol content. Compare each experimental group with the corresponding control group
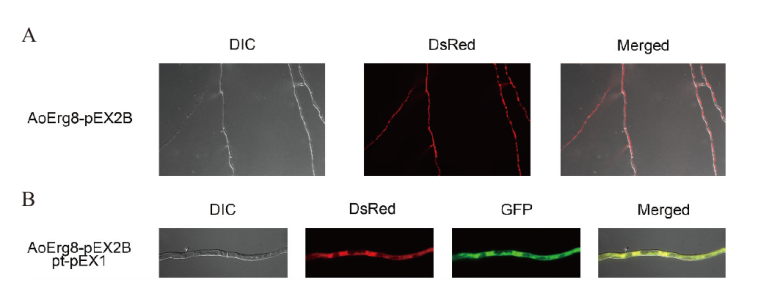
图6 AoErg8的亚细胞定位 A:用AoErg8-DsRed转化米曲霉3.042 ΔpyrG菌丝体;从左到右:DIC、DsRed荧光图像以及DsRed和DIC的合并图像;B:AoErg8-DsRed与细胞质的共定位;用AoErg8-DsRed和pt-pEX1-GFP载体共转化米曲霉3.042 ΔpyrG的菌丝体;从左到右:DIC,DsRed、GFP的荧光图像,以及DsRed、GFP和DIC的合并图像
Fig. 6 Subcellular localization of AoErg8 A: The mycelium of A. oryzae 3.042 ΔpyrG transformed with AoErg8-DsRed. Left to right: bright field(DIC), fluorescent images of DsRed and merged image of DsRed and bright field. B: Co-localization of AoErg8 DsRed with cytoplasm. The A. oryzae 3.042 ΔpyrG mycelium co-transformed of AoErg8-DsRed and pt-pEX1-GFP carriers. From left to right: bright field(DIC), fluorescent images of DsRed, GFP, and merged images of DsRed, GFP, and DIC
| [1] |
Lombard J, Moreira D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life[J]. Mol Biol Evol, 2011, 28(1): 87-99.
doi: 10.1093/molbev/msq177 pmid: 20651049 |
| [2] |
Sun YL, Niu YL, Huang H, et al. Mevalonate diphosphate decarboxylase MVD/Erg19 is required for ergosterol biosynthesis, growth, sporulation and stress tolerance in Aspergillus oryzae[J]. Front Microbiol, 2019, 10: 1074.
doi: 10.3389/fmicb.2019.01074 URL |
| [3] | Huang GC, Cai WX, Xu BJ. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms marketed in China and increased vitamin D2 content following UV-C irradiation[J]. Int J Vitam Nutr Res, 2017, 87(5/6): 1-10. |
| [4] |
Karpova NV, Andryushina VA, Stytsenko TS, et al. A search for microscopic fungi with directed hydroxylase activity for the synthesis of steroid drugs[J]. Prikl Biokhim Mikrobiol, 2016, 52(3): 324-332.
pmid: 29509389 |
| [5] |
Kobori M, Yoshida M, Ohnishi-Kameyama M, et al. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells[J]. Br J Pharmacol, 2007, 150(2): 209-219.
doi: 10.1038/sj.bjp.0706972 URL |
| [6] |
Kitchawalit S, Kanokmedhakul K, Kanokmedhakul S, et al. A new benzyl ester and ergosterol derivatives from the fungus Gymnoascus reessii[J]. Nat Prod Res, 2014, 28(14): 1045-1051.
doi: 10.1080/14786419.2014.903478 pmid: 24708569 |
| [7] |
Houten SM, Waterham HR. Nonorthologous gene displacement of phosphomevalonate kinase[J]. Mol Genet Metab, 2001, 72(3): 273-276.
pmid: 11243736 |
| [8] |
Andreassi JL, Leyh TS. Molecular functions of conserved aspects of the GHMP kinase family[J]. Biochemistry, 2004, 43(46): 14594-14601.
pmid: 15544330 |
| [9] |
Zhang ZH, Li CH, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis[J]. eLife, 2015, 4: e06322.
doi: 10.7554/eLife.06322 URL |
| [10] |
Lange BM, Ghassemian M. Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism[J]. Plant Mol Biol, 2003, 51(6): 925-948.
doi: 10.1023/A:1023005504702 URL |
| [11] |
Sando T, Takaoka C, Mukai Y, et al. Cloning and characterization of mevalonate pathway genes in a natural rubber producing plant, Hevea brasiliensis[J]. Biosci Biotechnol Biochem, 2008, 72(8): 2049-2060.
doi: 10.1271/bbb.80165 URL |
| [12] |
Ma YM, Yuan LC, Wu B, et al. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza[J]. J Exp Bot, 2012, 63(7): 2809-2823.
doi: 10.1093/jxb/err466 URL |
| [13] | 郑汉, 虞慕瑶, 濮春娟, 等. 香樟甲羟戊酸-5-磷酸激酶基因CcPMK的克隆和表达分析[J]. 中国中药杂志, 2020, 45(1): 78-84. |
| Zheng H, Yu MY, Pu CJ, et al. Cloning and expression analysis of 5-phosphomevalonate kinase gene(CcPMK)in Cinnamomum camphora[J]. China J Chin Mater Med, 2020, 45(1): 78-84. | |
| [14] |
Niu MY, Xiong YP, Yan HF, et al. Cloning and expression analysis of mevalonate kinase and phosphomevalonate kinase genes associated with the MVA pathway in Santalum album[J]. Sci Rep, 2021, 11(1): 16913.
doi: 10.1038/s41598-021-96511-4 |
| [15] |
Tsay YH, Robinson GW. Cloning and characterization of ERG8, an essential gene of Saccharomyces cerevisiae that encodes phosphomevalonate kinase[J]. Mol Cell Biol, 1991, 11(2): 620-631.
doi: 10.1128/mcb.11.2.620-631.1991 pmid: 1846667 |
| [16] |
Wang SX, Liu ZC, Wang XT, et al. Cloning and characterization of a phosphomevalonate kinase gene from Sanghuangporus baumii[J]. Biotechnol Biotechnol Equip, 2021, 35(1): 934-942.
doi: 10.1080/13102818.2021.1938678 URL |
| [17] |
Doun SS, Burgner JW, Briggs SD, et al. Enterococcus faecalis phosphomevalonate kinase[J]. Protein Sci, 2005, 14(5): 1134-1139.
doi: 10.1110/ps.041210405 URL |
| [18] | Dairi T. Studies on biosynthetic genes and enzymes of isoprenoids produced by actinomycetes[J]. Jpn J Antibiot, 2005, 58(1): 87-98. |
| [19] |
Kitamoto K. Cell biology of the Koji mold Aspergillus oryzae[J]. Biosci Biotechnol Biochem, 2015, 79(6): 863-869.
doi: 10.1080/09168451.2015.1023249 URL |
| [20] |
Hu ZH, Li GH, Sun YL, et al. Gene transcription profiling of Aspergillus oryzae 3.042 treated with ergosterol biosynthesis inhibitors[J]. Braz J Microbiol, 2019, 50(1): 43-52.
doi: 10.1007/s42770-018-0026-1 |
| [21] |
Sun YL, Niu YL, He B, et al. A dual selection marker transformation system using Agrobacterium tumefaciens for the industrial Aspergillus oryzae 3.042[J]. J Microbiol Biotechnol, 2019, 29(2): 230-234.
doi: 10.4014/jmb.1811.11027 URL |
| [22] |
Huang H, Niu YL, Jin Q, et al. Identification of six thiolases and their effects on fatty acid and ergosterol biosynthesis in Aspergillus oryzae[J]. Appl Environ Microbiol, 2022, 88(6): e0237221.
doi: 10.1128/aem.02372-21 URL |
| [23] |
Jin Q, Li GH, Qin KH, et al. The expression pattern, subcellular localization and function of three sterol 14α-demethylases in Aspergillus oryzae[J]. Front Genet, 2023, 14: 1009746.
doi: 10.3389/fgene.2023.1009746 URL |
| [24] |
Nguyen KT, Ho QN, Do LTBX, et al. A new and efficient approach for construction of uridine/uracil auxotrophic mutants in the filamentous fungus Aspergillus oryzae using Agrobacterium tumefaciens-mediated transformation[J]. World J Microbiol Biotechnol, 2017, 33(6): 107.
doi: 10.1007/s11274-017-2275-9 URL |
| [25] | 黄慧, 王立, 胡志宏, 等. 培养基组分及培养条件对米曲霉麦角甾醇含量的影响[J]. 江西科技师范大学学报, 2021(6): 87-92. |
| Huang H, Wang L, Hu ZH, et al. Effect of medium composition and culture conditions on ergosterol content in Aspergillus oryzae[J]. J Jiangxi Sci Technol Norm Univ, 2021(6): 87-92. | |
| [26] |
Ma SM, Garcia DE, Redding-Johanson AM, et al. Optimization of a heterologous mevalonate pathway through the use of variant HMG-CoA reductases[J]. Metab Eng, 2011, 13(5): 588-597.
doi: 10.1016/j.ymben.2011.07.001 pmid: 21810477 |
| [27] |
Hu ZH, He B, Ma L, et al. Recent advances in ergosterol biosynthesis and regulation mechanisms in Saccharomyces cerevisiae[J]. Indian J Microbiol, 2017, 57(3): 270-277.
doi: 10.1007/s12088-017-0657-1 URL |
| [28] |
Hu ZH, Huang H, Sun YL, et al. Effects on gene transcription profile and fatty acid composition by genetic modification of mevalonate diphosphate decarboxylase MVD/Erg19 in Aspergillus oryzae[J]. Microorganisms, 2019, 7(9): 342.
doi: 10.3390/microorganisms7090342 URL |
| [29] | 何海. 茯苓磷酸甲羟戊酸激酶基因克隆及功能分析[D]. 武汉: 华中农业大学, 2016. |
| He H. Cloning and characterization of phosphomevalonate kinase gene from Poria cocos[D]. Wuhan: Huazhong Agricultural University, 2016. | |
| [30] | 赵乐, 马利刚, 杨方方, 等. 独行菜磷酸甲羟戊酸激酶LaPMK基因克隆、生物信息学分析及原核表达[J]. 中草药, 2016, 47(17): 3087-3093. |
| Zhao L, Ma LG, Yang FF, et al. Cloning, bioinformatic analysis, and prokaryotic expression of LaPMK gene in Lepidium apetalum[J]. Chin Tradit Herb Drugs, 2016, 47(17): 3087-3093. | |
| [31] |
李辉, 温春秀, 刘灵娣, 等. 紫苏甲羟戊酸-5-磷酸激酶基因PfPMK的克隆与表达分析[J]. 华北农学报, 2022, 37(2): 49-55.
doi: 10.7668/hbnxb.20192609 |
| Li H, Wen CX, Liu LD, et al. Cloning and expression analysis of 5-phosphomevalonate kinase PfPMK gene in Perilla frutescens L[J]. Acta Agric Boreali Sin, 2022, 37(2): 49-55. |
| [1] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [2] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [3] | 滕梦鑫, 徐亚, 何静, 汪奇, 乔飞, 李敬阳, 李新国. 香蕉MaMC6的克隆及原核表达分析[J]. 生物技术通报, 2023, 39(12): 179-186. |
| [4] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [5] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [6] | 史亚楠, 王德培, 王一川, 周昊, 薛鲜丽. 敲除msn2对米曲霉生长和发酵产曲酸的影响[J]. 生物技术通报, 2022, 38(8): 188-197. |
| [7] | 杨佳宝, 周至铭, 张展, 冯丽, 孙黎. 向日葵HaLACS1的克隆、表达及酵母功能互补鉴定[J]. 生物技术通报, 2022, 38(6): 147-156. |
| [8] | 镐青青, 姚圣, 刘佳禾, 陈佩珍, 张梦洋, 季孔庶. 马尾松NAC转录因子基因PmNAC8的克隆及表达分析[J]. 生物技术通报, 2022, 38(4): 202-216. |
| [9] | 赵婷婷, 王俊刚, 王文治, 冯翠莲, 冯小艳, 张树珍. 甘蔗单糖转运蛋白基因ShSTP7序列分析及组织表达特征测定[J]. 生物技术通报, 2022, 38(4): 72-78. |
| [10] | 党瑗, 李维, 苗向, 修宇, 林善枝. 山杏油体蛋白基因PsOLE4克隆及其调控油脂累积功能分析[J]. 生物技术通报, 2022, 38(11): 151-161. |
| [11] | 骆鹰, 谭智, 王帆, 刘晓霞, 罗小芳, 何福林. 银杏GbR2R3-MYB1基因的克隆及非生物胁迫应答分析[J]. 生物技术通报, 2022, 38(10): 184-194. |
| [12] | 孙瑞芬, 张艳芳, 牛素清, 郭树春, 李素萍, 于海峰, 聂惠, 牟英男. 向日葵HaACO1基因的表达分析及功能验证[J]. 生物技术通报, 2021, 37(9): 114-124. |
| [13] | 范亚朋, 芮存, 张悦新, 陈修贵, 陆许可, 王帅, 张红, 徐楠, 王晶, 陈超, 叶武威. 陆地棉耐碱基因GHZAT12的克隆、表达及生物信息学分析[J]. 生物技术通报, 2021, 37(8): 121-130. |
| [14] | 山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155. |
| [15] | 孙小倩, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子FtMYBF的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2021, 37(3): 10-17. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||