








生物技术通报 ›› 2023, Vol. 39 ›› Issue (12): 90-98.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0129
李奕雅1( ), 吴一凡1, 丁能水2, 范小萍2, 陈凡1(
), 吴一凡1, 丁能水2, 范小萍2, 陈凡1( )
)
收稿日期:2023-02-15
出版日期:2023-12-26
发布日期:2024-01-11
通讯作者:
陈凡,男,博士,副教授,研究方向:细胞生物学;E-mail: cf@bio.mnnu.edu.cn作者简介:李奕雅,女,硕士研究生,研究方向:蛋白质功能与应用;E-mail: lyy@bio.mnnu.edu.cn
基金资助:
LI Yi-ya1( ), WU Yi-fan1, DING Neng-shui2, FAN Xiao-ping2, CHEN Fan1(
), WU Yi-fan1, DING Neng-shui2, FAN Xiao-ping2, CHEN Fan1( )
)
Received:2023-02-15
Published:2023-12-26
Online:2024-01-11
摘要:
建立基于萤火虫荧光素酶的大肠杆菌超声破碎辅助定量方法,利用荧光素酶灵敏度高、检测迅速的特点,对靶细胞的破碎效果进行表征。以表达了萤火虫荧光素酶的大肠杆菌作为内标,在破碎前与靶蛋白表达菌按一定比例混合,考察不同破碎条件下荧光素酶和靶蛋白活性向胞外释放的情况,对辅助定量效果进行评价。结果表明,将表达了萤火虫荧光素酶的大肠杆菌按1∶500(体积比)同待破碎菌悬液混合,能够通过破碎后上清液中荧光素酶活性的变化,正确反映靶细胞的破碎程度,并为破碎过程中蛋白质的活性保存提供参考。破碎产物中引入的荧光素酶,对后续靶蛋白的镍珠法纯化没有影响。含有萤火虫荧光素酶的菌体,可在-80℃环境下保存至少90 d而不产生酶活性的显著下降,且细胞对超声破碎的耐受性也不因冻存改变,可在临用前直接解冻并与靶细胞混合,起到辅助定量作用。因此,利用萤火虫荧光素酶对大肠杆菌超声破碎的程度进行辅助定量,是简便、稳定且高效的。
李奕雅, 吴一凡, 丁能水, 范小萍, 陈凡. 荧光素酶辅助定量大肠杆菌破碎效果的方法[J]. 生物技术通报, 2023, 39(12): 90-98.
LI Yi-ya, WU Yi-fan, DING Neng-shui, FAN Xiao-ping, CHEN Fan. Establishment of a Luciferase-assisted Quantitative Method for Measuring Ultrasonic Disruption of Escherichia coli Cells[J]. Biotechnology Bulletin, 2023, 39(12): 90-98.
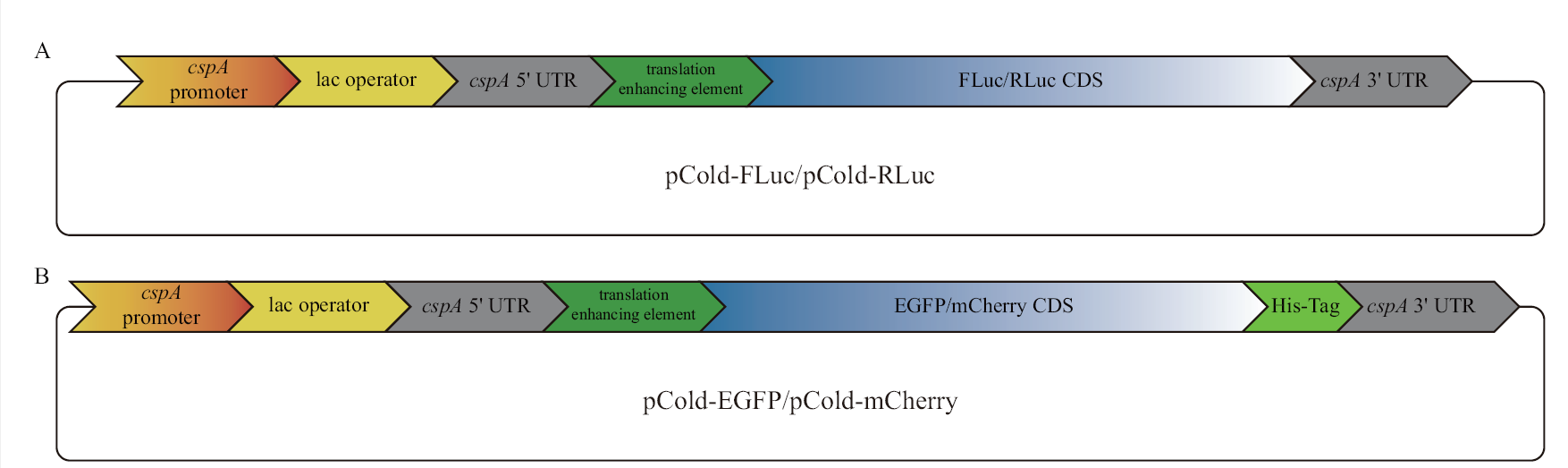
图1 本研究使用的载体结构示意图 A:在pCold III DNA载体翻译增强子下游插入萤火虫或海肾荧光素酶CDS(coding sequences),二者均不携带亲和纯化标签;B:在pCold III DNA载体翻译增强子下游插入EGFP或mCherry CDS,二者C端均携带组氨酸亲和纯化标签(His-Tag)。所构建的载体中,cspA启动子(cspA promoter)的作用受乳糖操纵序列的抑制,向培养基中添加IPTG(isopropyl β-D-thiogalactoside)后,抑制被解除,目的基因得到表达
Fig. 1 Schematic representation of the plasmid structures used in this study A: Firefly or Renilla luciferase CDS was inserted into the downstream of the translation enhancing element of pCold III DNA plasmids without affinity tags for purification;B: EGFP or mCherry CDS was inserted into the downstream of the translation enhancing element of pCold III DNA plasmids with a C-terminal His-Tag for purification. Among the functional elements of each plasmid, the function of cspA promoter is inhibited by lac operator and the inhibition is released after IPTG(isopropyl β-D-thiogalactoside)is added, and target gene is expressed

图3 三种菌悬液超声破碎的萤火虫荧光素酶辅助定量结果图 以破碎样品中活性最强的平均值为100%,计算菌体破碎程度
Fig. 3 Firefly luciferase-assisted quantification of ultrasonic disruption for three bacterial suspensions The degree of body fragmentation was calculated using the mean value of the strongest activity in the disrupted sample as 100%

图4 不同处理温度下4种蛋白质活性的变化 各图中以0℃处理样品的活性为100%,无相同小写字母的两组差异显著(P<0.05)
Fig. 4 Activity changes of four proteins incubated under different temperatures The remaining sample activity incubated under 0℃ in each plot was considered 100%. Groups do not share any lower letter indicate significant difference(P<0.05)

图5 不同超声破碎功率对破碎后荧光素酶活性的影响 各图中FLuc和RLuc对应的最大读数均值(100%活性)已标注在图中
Fig. 5 Effects of different ultrasonic power on luciferase activity after sonication The means of maximum readings(100% activity)corresponding to FLuc and RLuc are labeled in each plot

图6 萤火虫荧光素酶的添加对靶蛋白后续纯化的影响 A:EGFP表达菌破碎上清液的梯度荧光照片;B:mCherry表达菌破碎上清液的梯度荧光照片;C:FLuc表达菌破碎上清液的梯度荧光照片;D:镍珠纯化前后混合样品的SDS-PAGE电泳图谱
Fig. 6 Effect of firefly luciferase addition on the purification of target proteins A: Gradient fluorescence intensity graph of EGFP-expressing bacterial supernatant sample after sonication. B: Gradient fluorescence intensity graph of mCherry-expressing bacterial supernatant sample after sonication. C: Gradient fluorescence intensity graph of FLuc-expressing bacterial supernatant sample after sonication. D: SDS-PAGE image of mixed samples before and after nickel bead purification

图7 不同冻存时间对萤火虫荧光素酶辅助定量效果的影响 每条曲线后3个数据点的平均值作为菌体100%破碎的指标已直接标注于图中,且每条曲线3 min处所对应数据点的平均值和该数值对应的破碎率也已标注
Fig. 7 Effect of different cryo-storage time on firefly luciferase-assisted quantification For each curve, the mean readings at 15, 18, and 21 min were averaged and considered as the value(labeled directly in each figure)indicating a complete disruption of specific bacterial cells. The mean reading at 3 min and its ratio against complete disruption were also marked for comparison
| [1] | 张磊, 唐永凯, 李红霞, 等. 促进原核表达蛋白可溶性的研究进展[J]. 中国生物工程杂志, 2021, 41(S1): 138-149. |
| Zhang L, Tang YK, Li HX, et al. Advances in promoting solubility of prokaryotic expressed proteins[J]. China Biotechnol, 2021, 41(S1): 138-149. | |
| [2] |
Li SY, Zuo DY, Cheng HL, et al. Glutathione S-transferases GhGSTF1 and GhGSTF2 involved in the anthocyanin accumulation in Gossypium hirsutum L[J]. Int J Biol Macromol, 2020, 165(Pt B): 2565-2575.
doi: 10.1016/j.ijbiomac.2020.10.101 URL |
| [3] |
Bai YJ, Guo JR, Reiter RJ, et al. Melatonin synthesis enzymes interact with ascorbate peroxidase to protect against oxidative stress in cassava[J]. J Exp Bot, 2020, 71(18): 5645-5655.
doi: 10.1093/jxb/eraa267 pmid: 32474586 |
| [4] | 李航, 戚睿斌, 陈宗艳, 等. 外源蛋白表达系统及其应用的研究进展[J]. 黑龙江畜牧兽医, 2021(7): 34-37, 47. |
| Li H, Qi RB, Chen ZY, et al. Progress in research on foreign protein expression system and its application[J]. Heilongjiang Anim Sci Vet Med, 2021(7): 34-37, 47. | |
| [5] | Martins M, Ooi CW, Neves MC, et al. Extraction of recombinant proteins from Escherichia coli by cell disruption with aqueous solutions of surface-active compounds[J]. J Chem Technol Biotechnol, 2018, 93(7): 1864-1870. |
| [6] | 游思亮, 王裔娜, 田瑞平, 等. 响应面法优化高压均质破碎重组大肠杆菌的条件[J]. 江苏农业科学, 2021, 49(17): 71-74. |
| You SL, Wang YN, Tian RP, et al. Optimization of high-pressure homogeneous disruption of recombinant Escherichia coli by response surface methodology[J]. Jiangsu Agric Sci, 2021, 49(17): 71-74. | |
| [7] | 吴蕾, 雷鸣, 洪建辉, 等. 超声破碎重组大肠杆菌释放包含体的过程研究[J]. 化学工业与工程, 2002, 19(6): 422-425. |
|
Wu L, Lei M, Hong JH, et al. Studies of ultrasonic disruption process to release inclusion body in recombinant E. coli[J]. Chem Ind Eng, 2002, 19(6): 422-425.
doi: 10.1021/ie50207a031 URL |
|
| [8] |
Falgenhauer E, von Schönberg S, Meng C, et al. Evaluation of an E. coli cell extract prepared by lysozyme-assisted sonication via gene expression, phage assembly and proteomics[J]. Chembiochem, 2021, 22(18): 2805-2813.
doi: 10.1002/cbic.202100257 pmid: 34240805 |
| [9] | Kuduğ H, Ataman B, İmamoğlu R, et al. Production of red fluorescent protein(mCherry)in an inducible E. coli expression system in a bioreactor, purification and characterization[J]. Int Adv Res Eng J, 2019(1): 20-25. |
| [10] |
Branchini BR, Southworth TL, Fontaine DM, et al. A firefly luciferase dual color bioluminescence reporter assay using two substrates to simultaneously monitor two gene expression events[J]. Sci Rep, 2018, 8(1): 5990.
doi: 10.1038/s41598-018-24278-2 pmid: 29662072 |
| [11] | 薛秀花, 黄晨西. 荧光素酶基因的研究进展[J]. 生物学通报, 2013, 48(9): 1-4. |
| Xue XH, Huang CX. Research progress of luciferase gene[J]. Bull Biol, 2013, 48(9): 1-4. | |
| [12] |
Close DM, Xu TT, Sayler GS, et al. In vivo bioluminescent imaging(BLI): noninvasive visualization and interrogation of biological processes in living animals[J]. Sensors, 2011, 11(1): 180-206.
doi: 10.3390/s110100180 pmid: 22346573 |
| [13] |
Zhang YQ, Dai YP, Wang JX, et al. Mouse circulating extracellular vesicles contain virus-derived siRNAs active in antiviral immunity[J]. EMBO J, 2022, 41(11): e109902.
doi: 10.15252/embj.2021109902 URL |
| [14] |
Amadeo F, Plagge A, Chacko A, et al. Firefly luciferase offers superior performance to AkaLuc for tracking the fate of administered cell therapies[J]. Eur J Nucl Med Mol Imaging, 2022, 49(3): 796-808.
doi: 10.1007/s00259-021-05439-4 |
| [15] |
Saito-Moriya R, Nakayama J, Kamiya G, et al. How to select firefly luciferin analogues for in vivo imaging[J]. Int J Mol Sci, 2021, 22(4): 1848.
doi: 10.3390/ijms22041848 URL |
| [16] |
Ihssen J, Jovanovic N, Sirec T, et al. Real-time monitoring of extracellular ATP in bacterial cultures using thermostable luciferase[J]. PLoS One, 2021, 16(1): e0244200.
doi: 10.1371/journal.pone.0244200 URL |
| [17] |
Pozzo T, Akter F, Nomura Y, et al. Firefly luciferase mutant with enhanced activity and thermostability[J]. ACS Omega, 2018, 3(3): 2628-2633.
doi: 10.1021/acsomega.7b02068 pmid: 30023842 |
| [18] |
Bjerga GEK, Williamson AK. Cold shock induction of recombinant Arctic environmental genes[J]. BMC Biotechnol, 2015, 15: 78.
doi: 10.1186/s12896-015-0185-1 pmid: 26286037 |
| [19] |
Duellman T, Burnett J, Yang J. Quantitation of secreted proteins using mCherry fusion constructs and a fluorescent microplate reader[J]. Anal Biochem, 2015, 473: 34-40.
doi: 10.1016/j.ab.2014.12.010 pmid: 25542417 |
| [20] |
Rahman MM, Lamsal BP. Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties[J]. Compr Rev Food Sci Food Saf, 2021, 20(2): 1457-1480.
doi: 10.1111/crf3.v20.2 URL |
| [21] |
Ohmuro-Matsuyama Y, Gomi K, Shimoda T, et al. Improving the stability of protein-protein interaction assay FlimPIA using a thermostabilized firefly luciferase[J]. Front Bioeng Biotechnol, 2021, 9: 778120.
doi: 10.3389/fbioe.2021.778120 URL |
| [22] |
Koksharov MI, Ugarova NN. Thermostabilization of firefly luciferase by in vivo directed evolution[J]. Protein Eng Des Sel, 2011, 24(11): 835-844.
doi: 10.1093/protein/gzr044 pmid: 21900306 |
| [23] |
Zhou FF, Zhang L, Gong K, et al. LEF-1 activates the transcription of E2F1[J]. Biochem Biophys Res Commun, 2008, 365(1): 149-153.
doi: 10.1016/j.bbrc.2007.10.138 URL |
| [24] |
Li TT, Kong AN T, Ma ZQ, et al. Protein arginine methyltransferase 1 may be involved in pregnane x receptor-activated overexpression of multidrug resistance 1 gene during acquired multidrug resistant[J]. Oncotarget, 2016, 7(15): 20236-20248.
doi: 10.18632/oncotarget.7752 pmid: 26934120 |
| [25] |
Yang L, Chen Y, Liu N, et al. CircMET promotes tumor proliferation by enhancing CDKN2A mRNA decay and upregulating SMAD3[J]. Mol Cancer, 2022, 21(1): 23.
doi: 10.1186/s12943-022-01497-w pmid: 35042525 |
| [26] | Simpson RJ. Stabilization of proteins for storage[J]. Cold Spring Harb Protoc, 2010, 2010(5): 1-14. |
| [27] |
Golovanov AP, Hautbergue GM, Wilson SA, et al. A simple method for improving protein solubility and long-term stability[J]. J Am Chem Soc, 2004, 126(29): 8933-8939.
doi: 10.1021/ja049297h pmid: 15264823 |
| [1] | 陈彩萍, 任昊, 龙腾飞, 何冰, 鲁兆祥, 孙坚. 大肠杆菌Nissle 1917对炎症性肠病治疗作用的研究进展[J]. 生物技术通报, 2023, 39(6): 109-118. |
| [2] | 吴莉丹, 冉雪琴, 牛熙, 黄世会, 李升, 王嘉福. 猪源致病性大肠杆菌基因组比较与毒力因子分析[J]. 生物技术通报, 2023, 39(12): 287-299. |
| [3] | 侯炜辰, 叶柯, 李洁, 张洋子, 许文涛, 朱龙佼, 李相阳. 基于抗体-适配体夹心生物传感器检测大肠杆菌O157: H7[J]. 生物技术通报, 2023, 39(12): 81-89. |
| [4] | 唐瑞琪, 赵心清, 朱笃, 汪涯. 大肠杆菌对木质纤维素水解液抑制物的胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 205-216. |
| [5] | 李仁瀚, 张乐乐, 刘春立, 刘秀霞, 白仲虎, 杨艳坤, 李业. 基于紫色杆菌素生物合成途径的L-色氨酸生物传感器的构建[J]. 生物技术通报, 2023, 39(10): 80-92. |
| [6] | 高伟欣, 黄火清, 赵晶, 张鑫, 杨宁, 杨浩萌. 应用于基因编辑的核糖核蛋白复合体的构建与活性验证[J]. 生物技术通报, 2022, 38(8): 60-68. |
| [7] | 孙曼銮, 葛赛, 卜佳, 朱壮彦. 大肠杆菌核糖核酸酶调控机制研究[J]. 生物技术通报, 2022, 38(3): 234-245. |
| [8] | 李晓芳, 刘慧燕, 潘琳, 艾治宇, 李一鸣, 张恒, 方海田. 常温常压等离子体诱变选育高产L-异亮氨酸大肠杆菌[J]. 生物技术通报, 2022, 38(1): 150-156. |
| [9] | 吴蓉, 曹佳睿, 曹君, 刘飞翔, 杨猛, 苏二正. 南极假丝酵母脂肪酶B基因在大肠杆菌中的表达和发酵优化[J]. 生物技术通报, 2021, 37(2): 138-148. |
| [10] | 王凯凯, 王晓璐, 苏小运, 张杰. 大肠杆菌双质粒CRISPR-Cas9系统的优化及应用[J]. 生物技术通报, 2021, 37(12): 252-264. |
| [11] | 刘文浩, 王瑞丰, 刘琬琳, 许杰. 不同调控元件及组合对烟草外源蛋白瞬时表达的效果分析[J]. 生物技术通报, 2020, 36(7): 62-71. |
| [12] | 陈桥, 吴海英, 王宗寿, 谢雨康, 李宜青, 孙俊松. 聚羟基丁酸酯合成引发的高密度生长大肠杆菌的多位点突变分析[J]. 生物技术通报, 2020, 36(7): 112-118. |
| [13] | 项明源, 廖倡宇, 江地科, 张鹏飞, 王印, 罗燕, 杨泽晓, 姚学萍. PRRSV NADC30-like SYBR Green I qPCR检测方法的建立与应用[J]. 生物技术通报, 2020, 36(5): 80-85. |
| [14] | 张春晨, 胡双艳, 阮海华. 人源溶菌酶在大肠杆菌中的表达与复性研究[J]. 生物技术通报, 2020, 36(3): 153-161. |
| [15] | 王琦, 颜春蕾, 高洪伟, 吴薇, 杨庆利. 基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||